Abstract
This study investigated the neuroprotection and potential mechanism of carbon monoxide (CO) against perinatal hypoxic–ischemic brain damage in rats by electrical acupuncture (EA). Animal behavior, morphological changes, cystathionine beta-synthase (CBS), hypoxia-inducible factor-1α (HIF-1α), and heme oxygenase-1 (HO-1) expression levels, and CO content in rat cortex cells were determined. Results demonstrated that EA treatment decreased the slope behavior and increased the overhang behavior of perinatal rats. The treatment also decreased the number of positive cells. The activator and inhibitor of CBS aggravated and remitted the hypoxic damage in cortex cells, respectively. EA treatment decreased CBS expression level and increased HO-1 and HIF-1α expression levels in perinatal rat cortex cells. Compared with the control groups, the CO content of cortex cells in the EA treatment group significantly increased (**p < 0.01). We hypothesized that EA treatment increases cortical CO content to protect against hypoxic damage via the hydrogen sulfide/CBS–CO/HO-1–HIF-1α system. This study provided a significant reference for EA therapy and cued a novel protective mechanism for cerebral palsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral palsy (CP) is a neurological disorder that appears in infancy or early childhood [1]. It is often complicated by mental retardation, epilepsy, perceptions of barriers, obstacles to communication behavior, and other abnormalities. CP in children has a serious effect on their quality of life and increases the burden on the society and family [2]. CP morbidity in Chinese children is 1.8–4.0 ‰ and shows an increasing trend each year [3]. It is the most common disorder among pediatric neurological diseases and disabilities [4]. However, an effective treatment for CP is unavailable to date. With the rapid development of Traditional Chinese Medicine, acupuncture and its curative effect on CP have received considerable attention worldwide [5].
Acupuncture, an original and no-drug therapy medical system, has curative effects on central and peripheral nerve injuries [6]. Electrical acupuncture (EA) can restrain the aspartate transaminase hyperplasia in the ischemic region to facilitate synapsis formation. It can also regulate electrical activity, improve brain blood circulation, increase cerebral blood oxygen supply, and enhance recession neuron metabolism [7, 8]. As our study, areas of increased and decreased signal intensities in children with CP significantly differed from those of normal children after being acupunctured at the Taichong acupoint. Areas of decreased signal intensity in children with CP were found to be mainly distributed in the frontal lobe (BA 10, BA 8, BA 21, and BA 46), anterior central gyrus (BA 4), and parahippocampal gyrus [9]. Meanwhile, novel gas signal molecules, such as hydrogen sulfide (H2S) [10] and nitric oxide (NO), have significant neuroprotection against hypoxic–ischemic brain damage (HIBD) in rats subjected to EA. EA treatment can decrease NO generation via the nNOS/NO system in the cerebral cortex to protect against HIBD, and also decrease H2S generation by down-regulating cystathionine beta-synthase (CBS) expression in the cerebral cortex to protect against HIBD [11]. Therefore, we investigated whether carbon monoxide (CO) is another mediator of EA in perinatal HIBD and explored its neuroprotective mechanism.
Materials and Methods
Experimental Animals and Groups
The 96 Sprague–Dawley pregnant rats (specific pathogen-free) were purchased and raised in the Laboratory Animal Center of the Academy of Military Medical Sciences until delivery. The rats (7 day, 13.9–22 g) were randomly divided into eight groups (n = 12) [Sham, Sham + EA, HIBD, HIBD + EA, HIBD + SAM (S-adenosyl-l-methionine, an activator of CBS, Sigma-Aldrich, USA), HIBD + SAM + EA, HIBD + HA (Hydroxylamine, an inhibitor of CBS, Sigma-Aldrich, USA), and HIBD + HA + EA] and subjected to HIBD. Wherein, the total of 12 animals in each group was used for the behavioural testing and further divided into two groups (n = 6). The total of six animals was used for HE and IHC staining, and the other 6 animals were used for CO content and Western blot assay as following. This experiment was approved by the Experiment and the Chinese people’s liberation army general hospital ethics committee.
Construction of HIBD Model
The HIBD model was established according to literature [12], and the detailed procedures are as follows. The new born rats were narcotized by diethyl ether, and the four limbs were fixed to allow incision along the neck midline. The thyroid, vein, and nerve tissues were stripped to expose the left carotid artery communis, ligated with 5/0 surgical line, and then sutured. After 2 h, the rats were placed in a lower oxygen tank to maintain an appropriate environmental temperature under continuous hypoxia with 8 % oxygen and 92 % nitrogen gas in the container for 2 h. Then, the baby rats were returned to their mothers in the cage. The Sham-operated groups were subjected to surgery, with their left carotid artery communis exposed, but they did not undergo ligation.
Electric Acupuncture Intervention Experiment and Intraperitoneal Injection
The rats in the EA group were acupunctured at the BaiHui (crossing point before and after the skull and the linkline of the two ears) and DaZhui (below the detail of the cervical spine) acupoints with an electric acupuncture (frequency: 2/100 Hz, intensity: 3 mA, 30 min/day/time) for 14 day. The limbs of the rats in the control group were only fixed, and was not performed EA.
The young rats in the HIBD + HA and HIBD + HA + EA groups were injected with 12.5 mg/kg/day HA (dissolved in saline) via the peritoneal cavity 20 min prior to the acupuncture procedure or fixation. Similarly, the young rats in the HIBD + SAM and HIBD + SAM + EA groups were injected with 50 mg/kg/d SAM (dissolved in saline) via the peritoneal cavity 20 min prior to the EA procedure or fixation. The control group was injected with an equal volume of normal saline.
Behavioral Testing
For overhang experiment, the front legs of the rats were grasped in a horizontal glass rod (diameter 0.6 cm). These rats were suspended on the table with a distance of 45 mm, and the overhanging time was recorded. For slope experiment, the rats were placed headdown on a plane to an angle of 45°inclination. The time was measured when the rat was in the up-headed position.
Hematoxylin and Eosin (HE) Staining
The total of six baby rats were after behavior testing was executed and further prepared wax block. The wax block was sectioned and the slides (3–4 μm) were deparaffinized and rehydrated, and over-stained with hematoxylin for 3 min followed with differentiation and destaining using acidic alcohol. Bicarbonate was applied (2 min) until the nuclei stand out sharply in blue. The sections were placed in 70 % ethanol (3 min) and then in eosin (2 min). The images of the cortex were captured using a microscope connected to a CCD camera at 200 × magnification at the same image acquisition settings.
Immunohistochemistry (IHC) Assay
The total of six baby rats were after behavior testing was executed and further prepared wax block. The wax block was sectioned and the slides (3–4 μm) were deparaffinized and rehydrated, and post-fixed with 4 % paraformaldehyde for 10 min, and then washed with phosphate-buffered saline (PBS). Endogenous peroxidase was inactivated by 3 % H2O2 (30 min), and incubated with 10 % normal goat serum in PBS for 30 min at room temperature. Then, the sections were incubated with rabbit anti-CBS (SC-67154, 1:200; Santa Cruz, the United States), anti-HIF-1α (AH-337-1, 1:100; Biyuntian Company, China), and anti-HO-1 (ab13243, 1:300; Abcam, England) monoclonal in PBS containing 0.3 % Triton X-100 overnight at 4 °C and washed with PBS, and then incubated in peroxidase-conjugated goat anti-rabbit IgG (1:200; ZYMED, the United States) for 1 h at room temperature. Finally, the sections were developed with diaminobenzidine (Sigma, the United States) in PBS containing 0.001 % H2O2 for 30 min. The images were captured at the same image acquisition settings, and the amount of immuno-positive cells and total positive area in the assigned subregions was measured by Image-Pro Plus 7.0 software (http://www.mediacy.com/index.aspx?page=IP_Premier ) and histogram analysis using Origin 9.0 software (http://www.originlab.com/).
CO Content Assay
The other six baby rats were after behavior testing was executed and the rat brain cortex tissues (about 50 mg) were ground and added to nine volumes of normal saline. The homogenate was centrifuged at 2,500 rpm for 10 min, and 200 µL of supernatant was obtained to measure A568 and A581. Then, the absolute absorbance value (A568 − A581) was determined. Subsequently, HbCO % and Hb contents (g/L) were calculated according to the functions HbCO % = 0.822x + 0.001 (x: A568 − A581) and Hb = A540nm × 367.7, respectively. Then, CO content was calculated according to the function: CO = HbCO % × Hb × 106 × 4/(64456x) (x: protein concentration, g/L). Histogram analysis was carried out by Origin 9.0 software (http://www.originlab.com/).
Western Blot Assay
The other six baby rats were after behavior testing was executed and the rat brain cortex tissues were ground, and added 50 μl cell lysate buffer (50 mM Tris–Cl pH 6.8, 15 mM NaCl, 5 mM EDTA, 0.5 % NP-40, 1 mM PMSF). The homogenate was centrifuged at 10,000 rpm for 10 min and added 50 μl 2 × loading buffer to boil at 95 °C for 10 min. Approximately 35 mg of protein samples was fractionated by 12 % polyacrylamide gels and transferred to a polyvinylidene difluoride membrane following the manufacturers’ instructions. The membrane was probed with the first antibody, rabbit-derived anti-HO-1 antibody (1:2,000 in TBST; Abcam, England), rabbit-derived anti-HIF-1α antibody (1:1,000 in TBST; Biyuntian Company, China), rabbit-derived anti-CBS antibody (1:500 in TBST; Santa Cruz, the United States), and mouse-derived anti-β-actin antibody (1:1,500 in TBST; Beijing Zhongshan Biotechnology, China) for 1.5 h at room temperature. Then, the membrane was incubated with horseradish peroxidase-conjugated goat anti-mouse secondary antibody (1:5,000 in TBST; Beijing Zhongshan Biotechnology, China) at room temperature for 1 h. Chemiluminescence substrate luminol reagent (GE Healthcare, the United States) and exposure to X-ray film were used to examine the immunolabeled bands. The optical density of the bands was scanned and quantified using the ImageJ 1.46 software (http://rsb.info.nih.gov/ij/download.html).
Statistical Analysis
All data were expressed as mean ± SD. Statistical analysis was performed using SPSS software (version 21.0, SPSS; http://spss.en.softonic.com/). In Student’s t test, p < 0.05 and p < 0.01 were considered to indicate significant difference and highly significant difference, respectively.
Results
EA Improves Animal Slope and Overhang Behavior
The results of behavioral assessment demonstrated that EA decreased the slope time in the Sham + EA (4.3720 ± 0.5263 s), HIBD + EA (6.2718 ± 1.2698 s), HIBD + SAM + EA (6.2854 ± 0.6759 s), and HIBD + HA + EA (5.6669 ± 0.6710 s) groups compared with the control groups, including Sham (4.8058 ± 0.9104 s), HIBD (8.5850 ± 1.3375 s), HIBD + SAM (7.5076 ± 1.2875 s), and HIBD + HA (6.6476 ± 1.0800 s). This result indicates that hypoxia can increase the slope time in HIBD in contrast to the Sham-operated group (Table 1).
In addition, EA increased the overhang time in the Sham + EA (32.3611 ± 11.7491 s), HIBD + EA (16.5948 ± 2.8045 s), HIBD + SAM + EA (17.2709 ± 2.8763 s), and HIBD + HA + EA (19.8972 ± 3.3812 s) groups compared with the control groups, including Sham (31.5280 ± 2.5603 s), HIBD (10.6621 ± 2.1383 s, **p < 0.01), HIBD + SAM (11.7773 ± 3.4761 s, **p < 0.01), and HIBD + HA (13.0923 ± 2.7124 s, **p < 0.01). This finding indicates that hypoxia can significantly decrease the overhang time in HIBD in contrast to the Sham-operated group (Table 1, **p < 0.01).
EA Alleviates Cortex Damage in Perinatal HIBD in Rats
For acupuncture images exhibiting (Fig .1a), the new born rats was fixed on the table to performed acupuncture.
EA alleviated the cortex damage in perinatal HIBD in rats and decreased the number of positive cells in the Sham + EA, HIBD + EA, HIBD + SAM + EA, and HIBD + HA + EA groups compared with the control groups, including Sham, HIBD, HIBD + SAM, and HIBD + HA (Fig. 1b). Hypoxia can also trigger severe damage of the cortex cells. The CBS activator SAM aggravated this damage, whereas the CBS inhibitor HA alleviated this damage (Fig. 1b).
EA Decreases CBS Expression Level in Rat Cortex
EA decreased CBS expression level in the cortex cells compared with the control groups (Fig. 2a). A significant difference in CBS expression level was found between the Sham + EA and Sham groups (Fig. 2b, **p < 0.01). Similarly, significant difference in CBS expression level was also found between the other EA groups and control groups, including HIBD (Fig. 2b, **p < 0.01), HIBD + SAM (Fig. 2b, **p < 0.01), and HIBD + HA (Fig. 2b, **p < 0.01). As expected, the CBS activator SAM and the CBS inhibitor HA significantly increased its expression level in the cortex cells, respectively.
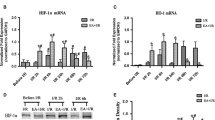
EA Increases HIF-1α Expression Level in Rat Cortex
EA increased HIF-1α expression level in the cortex cells compared with the control groups (Fig. 3a). A significant difference in HIF-1α expression level was found between the Sham + EA and Sham groups (Fig. 3b, **p < 0.01). Similarly, a significant difference in HIF-1α expression level was found between the other EA groups and control groups, including HIBD (Fig. 3b, **p < 0.01), HIBD + SAM (Fig. 3b, *p < 0.05), and HIBD + HA (Fig. 3b, **p < 0.01). Unexpectedly, HIF-1α expression level slightly decreased in the HIBD group compared with that in the Sham group.
EA Increases HO-1 Expression Level to Up-Regulate CO Content in Rat Cortex
EA increased HO-1 expression level in the cortex cells compared with the control groups (Fig. 4a). A significant difference in HO-1 expression level was found between the Sham + EA and Sham groups (Fig. 4b, **p < 0.01). Similarly, a significant difference in HO-1 expression level was also found between the other EA groups and the control groups, including HIBD (Fig. 4b, **p < 0.01), HIBD + SAM (Fig. 4b, **p < 0.01), and HIBD + HA (Fig. 4b, **p < 0.01). Subsequently, the CO content in the cortex cells significantly increased with EA compared with the control groups (Fig. 5). A significant difference in CO content in the cortex cells was found between the Sham + EA and Sham groups (**p < 0.01). Similarly, a significant difference in the CO content in the cortex cells was also found between the other EA groups and the control groups, including HIBD (**p < 0.01), HIBD + SAM (**p < 0.01), and HIBD + HA (**p < 0.01). Additionally, hypoxia can reduce more CO in the HIBD group compared with the Sham group.
EA Down-Regulates CBS Expression Level to Up-Regulate HIF-1α and HO-1 in the Cortex of HIBD in Rats
EA down-regulated CBS expression level (**p < 0.01, compared with each control group) and up-regulated HIF-1α (**p < 0.01, compared with each control group) and HO-1 expression levels (*p < 0.05, **p < 0.01, compared with each control group). Hypoxia decreased CBS expression level and increased HIF-1α and HO-1 expression levels. Meanwhile, the CBS activator SAM increased CBS and HO-1expression levels and significantly decreased HIF-1α expression level. The CBS inhibitor HA decreased CBS and HIF-1α expression levels and increased HO-1 expression level (Fig. 6).
Discussion
We demonstrated that EA can alleviate hypoxia-induced cortex cell damage in perinatal HIBD in rats. The number of positive cells significantly decreased with EA compared with each control. Additionally, EA can increase HIF-1α expression level, decrease CBS expression level, and increase HO-1 expression level in the perinatal rat cortex. The CO content in the rat cortex also increased with EA.
Gas biology is an emerging discipline that is receiving considerable attention [13–16]. Currently, several novel gas signal molecules (H2S, NO, and CO) involved in the regulation of the structure and function of the nervous system under physiological and pathological conditions have been explored [17–20]. These gas signal molecules are characterized by low molecular weight, continued generation, rapid diffusion, and extensive effect; these molecules can also freely transfer a variety of biomembranes to several organs.
In Traditional Chinese Medicine, acupuncture can regulate the gas signal molecules to protect an organism. For instance, acupuncture can increase NO local generation [21]; Acupuncture can increase NO generation on the skin surface of acupoints [22]; Acupuncture can reduce NO content in rat corpus striatum of transient middle cerebral artery occlusion and further alleviate brain damage [23]. Acupuncture can decrease the nNOS/NO system to recover the neuronal function in HIBD in rats [11]. All these results indicated that NO gas signal molecule had a significant neuroprotection against HIBD. However, no study has reported on the neuroprotection of the gas signal molecule CO in HIBD, especially in children with CP. Therefore, we constructed a perinatal HIBD model to determine it. This study validated that the over-expressed CO had a significant protection against perinatal HIBD in rats and that CBS, HO-1, and HIF-1α were involved in this process. On the one hand, acupuncture decreased CBS expression level in the cortex to reduce H2S content in our previous study [10]. On the other hand, the down-regulated CBS increased HIF-1α and HO-1 expression levels, and the over-expressed HO-1 can generate excessive CO in the cortex to protect against HIBD.
For behavior testing, the overhang experiment was used to measure the myodynamia, and further assessed the movement of new born rats. The slope experiment was used to measure the balance function of new born rats. Both of these experiments were used to estimate the curative effect of acupuncture after HIBD. By comparing, the EA treatment could decrease the slope time and increase the overhang time, indicating the EA is favor of improving the balance and the movement, especially after HIBD.
Taken together, the recent study revealed that the acupuncture could alleviate the HIBD of rats via up-regulating of the CO content in the cortex cells. It provided a significant reference for the acupuncture therapy in CP and cued a novel protective mechanism for CP.
References
Richards CL, Malouin F (2013) Cerebral palsy: definition, assessment and rehabilitation. Handb Clin Neurol 111:183–195
Hustad KC, Allison K, McFadd E, Riehle K (2014) Speech and language development in 2-year-old children with cerebral palsy. Dev Neurorehabil 17:167–175
Zivkovic Z, Golubovic S (2012) Tongue mobility in patients with cerebral palsy. Vojnosanit Pregl 69:488–491
Holmefur M, Kits A, Bergstrom J, Krumlinde-Sundholm L, Flodmark O, Forssberg H, Eliasson AC (2013) Neuroradiology can predict the development of hand function in children with unilateral cerebral palsy. Neurorehabil Neural Repair 27:72–78
Duncan B, Shen K, Zou LP, Han TL, Lu ZL, Zheng H, Walsh M, Venker C, Su Y, Schnyer R, Caspi O (2012) Evaluating intense rehabilitative therapies with and without acupuncture for children with cerebral palsy: a randomized controlled trial. Arch Phys Med Rehabil 93:808–815
Shi B, Bu H, Lin L (1992) A clinical study on acupuncture treatment of pediatric cerebral palsy. J Tradit Chin Med 12:45–51
Cavazos JE, Golarai G, Sutula TP (1991) Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J Neurosci 11:2795–2803
Cavazos JE, Sutula TP (1990) Progressive neuronal loss induced by kindling: a possible mechanism for mossy fiber synaptic reorganization and hippocampal sclerosis. Brain Res 527:1–6
Wu Y, Jin Z, Li K, Lu ZL, Wong V, Han TL, Zheng H, Caspi O, Liu G, Zeng YW, Zou LP (2008) Effect of acupuncture on the brain in children with spastic cerebral palsy using functional neuroimaging (FMRI). J Child Neurol 23:1267–1274
Liu Y, Zou LP, Du JB, Wong V (2010) Electro-acupuncture protects against hypoxic-ischemic brain-damaged immature rat via hydrogen sulfide as a possible mediator. Neurosci Lett 485:74–78
Liu Y, Zou LP, Du JB (2011) Nitric oxide-mediated neuronal functional recovery in hypoxic-ischemic brain damaged rats subjected to electrical stimulation. Brain Res 1383:324–328
Rice JE 3rd, Vannucci RC, Brierley JB (1981) The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9:131–141
Semenza GL, Prabhakar NR (2012) Gas biology: small molecular medicine. J Mol Med (Berl) 90:213–215
Kajimura M, Nakanishi T, Takenouchi T, Morikawa T, Hishiki T, Yukutake Y, Suematsu M (2012) Gas biology: tiny molecules controlling metabolic systems. Respir Physiol Neurobiol 184:139–148
Hoshi T, Lahiri S (2004) Cell biology. Oxygen sensing: it’s a gas! Science 306:2050–2051
Suematsu M (2003) Quartet signal transducers in gas biology. Antioxid Redox Signal 5:435–437
Zhang Y, Tang ZH, Ren Z, Qu SL, Liu MH, Liu LS, Jiang ZS (2013) Hydrogen sulfide, the next potent preventive and therapeutic agent in aging and age-associated diseases. Mol Cell Biol 33:1104–1113
Zhang L, Yang G, Untereiner A, Ju Y, Wu L, Wang R (2013) Hydrogen sulfide impairs glucose utilization and increases gluconeogenesis in hepatocytes. Endocrinology 154:114–126
Zhang J, Chen S, Liu H, Zhang B, Zhao Y, Ma K, Zhao D, Wang Q, Ma H, Zhang Z (2013) hydrogen sulfide prevents hydrogen peroxide-induced activation of epithelial sodium channel through a PTEN/PI(3, 4, 5)P3 dependent pathway. PLoS One 8:e64304
Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, Wang R (2013) Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal 18:1906–1919
Tsuchiya M, Sato EF, Inoue M, Asada A (2007) Acupuncture enhances generation of nitric oxide and increases local circulation. Anesth Analg 104:301–307
Ma SX, Li XY, Sakurai T, Pandjaitan M (2007) Evidence of enhanced non-enzymatic generation of nitric oxide on the skin surface of acupuncture points: an innovative approach in humans. Nitric Oxide 17:60–68
Zhao P, Huang ZN, Chen G, Cheng JS (2000) Electro-acupuncture attenuates nitric oxide release from rat striatum after transient middle cerebral artery occlusion. Acupunct Electrother Res 25:101–107
Acknowledgments
This research projects is sponsored by Supported by: International Science and Technology Cooperation Fundation of the Ministry of Science and Technology of China, (No.2008DFA31850), International Cooperation of Science and Technique Foundation of Beijing (2007G05) and the Beijing Chinese medicine projects (Grant no. JJ2005-17).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yichen Liu is the first author.
Rights and permissions
About this article
Cite this article
Liu, Y., Li, Z., Shi, X. et al. Neuroprotection of Up-Regulated Carbon Monoxide by Electrical Acupuncture on Perinatal Hypoxic–Ischemic Brain Damage in Rats. Neurochem Res 39, 1724–1732 (2014). https://doi.org/10.1007/s11064-014-1366-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1366-3