Abstract
Acrolein is a highly electrophilic alpha, beta-unsaturated aldehyde to which humans are exposed in many situations and has been implicated in neurodegenerative diseases, such as Alzheimer’s disease. Lithium is demonstrated to have neuroprotective and neurotrophic effects in brain ischemia, trauma, neurodegenerative disorders, and psychiatric disorders. Previously we have found that acrolein induced neuronal death in HT22 mouse hippocampal cells. In this study, the effects of lithium on the acrolein-induced neurotoxicity in HT22 cells as well as its mechanism(s) were investigated. We found that lithium protected HT22 cells against acrolein-induced damage by the attenuation of reactive oxygen species and the enhancement of the glutathione level. Lithium also attenuated the mitochondrial dysfunction caused by acrolein. Furthermore, lithium significantly increased the level of phospho-glycogen synthase kinase-3 beta (GSK-3β), the non-activated GSK-3β. Taken together, our findings suggest that lithium is a protective agent for acrolein-related neurotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipid peroxidation, which increases in neurodegenerative disorders such as Alzheimer’s disease (AD) [1, 2], leads to the formation of a number of aldehyde by-products. 4-hydroxynonenal and malondialdehyde are the most abundant aldehydes, while acrolein is the most reactive one [3]. In Alzheimer’s brain, acrolein was found to be elevated in hippocampus and temporal cortex where oxidative stress is high [4]. Very recently, we have shown that chronic administration of acrolein to rats induced AD-like pathologies such as mild cognitive impairment, hippocampal atrophy, and an upregulation of beta-site APP cleaving enzyme 1 [5].
Acrolein was reported to increase the phosphorylation of tau, which is considered as a major pathologic hallmark of several neurodegenerative disorders, including AD, both in SH-SY5Y neuroblastoma cells and in primary cultures of mouse embryo cortical neurons. The basal phosphorylation of tau protein seems to be largely mediated by glycogen synthase kinase-3β (GSK-3β) [6, 7]. The activity of GSK-3β has been associated with both pathological features of AD, namely the buildup of Amyloid-β deposits and the formation of neurofibrillary tangles [8, 9]. We also have confirmed that activated GSK-3β is involved in acrolein-induced AD-like pathologies in HT22 murine hippocampal cells [10]. Due to these roles of GSK-3β in promoting AD, GSK-3β inhibitors may have positive therapeutic effects on AD patients and are currently in the early stage of testing [11].
Lithium, a specific inhibitor of GSK-3β, is a fundamental component of therapy for patients with bipolar disorders for over 50 years [12]. Meanwhile, lithium is demonstrated to be of benefit to the underlying pathology of AD [13, 14], as well as an array of other common neuronal disorders, including stroke, Parkinson’s disease, and Huntington’s disease [11, 15]. However, its effect on acrolein-induced neurotoxicity remains unknown. In this study, the effects of lithium on the acrolein-induced neurotoxicity in HT22 cells as well as its mechanism(s) were investigated.
Materials and Methods
Materials
Acrolein was purchased from Gelei Xiya Chemical Co. (Chengdu, China). Dullbecco’s modified Eagle’s medium (DMEM), and fetal bovine serum (FBS) were purchased from Gibico-BRL (Grand Island, NY, USA). 2′,7′-dichlorofluorescin diacetate (H2-DCF-DA), 3-(3,4-dimehylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), lithium chloride, Rhodamine 123 and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless stated otherwise. Glutathione (GSH) assay kit was purchased from Jiancheng Biochemical (Nanjing, China). Lithium chloride was dissolved in distilled water and stored at −20 °C. Rabbit polyclonal anti-phospho-GSK-3β (p-GSK-3β-Ser9) antibody and anti-total-GSK-3β antibody were obtained from Santa Cruz Biotechnology Inc. (CA, USA). Mouse anti-β-actin antibody was purchased from Sigma-Aldrich (St. Louis, MO, USA). Rabbit and Mouse horseradish peroxidase were purchased from Santa Cruz Biotechnology Inc. (CA, USA) and Promega (Medison, WI, USA), respectively.
Cells Culture and Treatment
HT22 murine hippocampal neuronal cells (a gift from Prof. Jun Liu, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, China) [16] were maintained in DMEM supplemented with 10 % (v/v) FBS and incubated at 37 °C under 5 % CO2. The fresh solution of acrolein (1 M in distill water) was diluted in DMEM supplemented with 0.5 % (v/v) FBS immediately before adding to each well at the desired final concentrations. Cells in the control group were treated with vehicle alone. For the experiments, cells were pre-incubated with lithium for 30 min and followed by treatment with acrolein, or co-incubated with lithium and acrolein, or pre-incubated with acrolein for 30 min and followed by treatment with lithium for indicated times.
MTT Assay
Cell viability was determined as we described previously [5] based on conversion of MTT to MTT-formazan by mitochondrial enzymes. Briefly, cells were seeded into a 96-well plate at a density of 4 × 103 cells/well in growth medium and cultured to about 60–70 % confluence, prior to the initiation of experimental treatment. Photomicrographs of each culture were taken by phase contrast microscopy (IX71, Olympus, Tokyo, Japan) prior to the MTT assay. Cells were washed three times with PBS and 10 μL of MTT solution (5 mg/mL stock) was added to the cells, and then incubated for 1 h at 37 °C. The medium was removed carefully and 150 μL of DMSO was then added to resolve the blue formazan in living cells. Finally, the absorbance at 570 nm was read with an ELISA reader (Bio-Tek). Results were expressed as the percentage of MTT reduction and the absorbance of control cells was set as 100 %.
Measurement of Intracellular Reactive Oxygen Species Accumulation
Intracellular reactive oxygen species (ROS) formation was measured by fluorescence using H2-DCF-DA. The non-fluorescent dye freely permeates into cells, where it de-esterifies to form the ionizedfree acid (dichlorofluorescein), which reacts with ROS to form the fluorescent 2′,7′-dichlorofluorescein [17]. After the drug treatment, cells were washed with PBS, loaded with 10 μM H2DCF-DA in serum-free DMEM for 30 min at 37 °C, and then washed again with PBS and kept at room temperature for an additional 30 min to allow for the complete de-esterification of the dye. 2′,7′-dichlorofluorescein fluorescence was analyzed using a fluorescence plate reader (Flex Station3, Molecular Devices, USA) at excitation and emission wavelengths of 490 and 533 nm, respectively, or taken images using a fluorescence microscope (IX71, Olympus, Tokyo, Japan).
Estimation of Intracellular GSH
A GSH assay kit was used to measure intracellular GSH concentration. Cells were collected and sonicated in 50 μL of ice-cold lysis buffer. After centrifuging at 10,000×g for 15 min at 4 °C, the supernatant was collected. 50 μL of TEAM reagent was added to 1 mL supernatant. 50 μL of sample or the standard solution provided in the kit was applied to each sample wells. 150 μL of the freshly prepared assay mixture was added to each of the wells containing standards and samples. The plate was incubated in the dark on an orbital shaker at room temperature. Absorbance was measured at 405 nm using a plate reader (Bio-Tek) after 25 min incubation. All GSH values were normalized to per mg protein of each sample.
Determination of Mitochondrial Membrane Potential
HT22 cell mitochondrial membrane potential was monitored using the cell permeable cationic fluorescent dye Rhodamine 123, which preferentially enters mitochondria due to the highly negative mitochondrial membrane potential as previously reported [18, 19]. Briefly, after different treatment, HT22 cells were washed twice with PBS and then incubated with Rhodamine 123 solution (5 μg/mL) for 15 min at 37 °C in the dark. Then, cells were washed once with PBS, resuspended in 1 mL PBS and analyzed using flow cytometer. Mean fluorescence intensity of 10,000 cells/sample was presented.
Western Blotting
Cells were washed twice with ice-cold PBS, and then suspended in 100 μL of lysis buffer (20 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, 1 % Triton X-100, protease inhibitor cocktail, 2 mM Na3VO4, and 10 mM NaF, pH 7.5). The protein concentration was determined using BCA assay kit (Beyotime, Jiangsu, China). An equal amount of protein (20 μg) was loaded in each lane. Proteins were separated using SDS-PAGE gel electrophoresis and electrically transferred to a PVDF membrane (Millipore). After blocking the membrane with 5 % skim milk, target proteins were immunodetected using specific antibodies. After incubation with the secondary antibody, bands were visualized using an ECL plus kit (Pierce) and exposed to autoradiographic films according to the manufacturer’s protocol. The intensities of bands were performed using Quantity One Software (Bio-Rad, Hercules, CA).
Statistical Analysis
All experiments described in this study were repeated at least three times. Data were presented as mean ± SD of multiple independent experiments. Statistics were analyzed with one-way ANOVA followed by a least significant difference test (SPSS 17.0 software). Statistical significance was considered at P < 0.05.
Results
Lithium Prevents Acrolein-Induced Cell Death in HT22 Cells
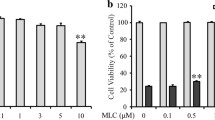
To identify lithium capable of exerting neuronal protection against acrolein, we investigated the effects of lithium on HT22 cells. Exposure of HT22 cells to acrolein for 24 h reduced cell viability in a concentration-dependent manner (Fig. 1a). Acrolein (25 μM) was applied in following experiments because the concentration corresponds to that in AD patients’ brains (hippocampus 5.0 ± 1.6 nmol/mg protein and amygdala 2.5 ± 0.9 nmol/mg protein) [20, 21], which is calculated based on average protein content of each culture dish in our experiments. Next, HT22 cells were pre-incubated with various concentrations of lithium for 30 min, followed by acrolein treatment for 24 h. The results suggested that lithium protected HT22 cells against acrolein-induced cell death, with 1 mM as the most potent concentration (Fig. 1b). Under a phase-contrast microscope, cell morphology was significantly damaged by acrolein exposure, which was markedly attenuated by lithium (Fig. 1c).
Effect of lithium on acrolein-induced neurotoxicity. Acrolein resulted in significant cell death in a concentration-dependent manner. Lithium treatment significantly attenuated acrolein-induced cell death. Cell viability was measured by MTT assay. Experiments were repeated at least three times. Data are expressed as mean ± SD. a Effects of acrolein treatment for 24 h on HT22 cell viability. **P < 0.01 versus control group. b Concentration course of lithium on acrolein-induced neurotoxicity for 24 h. ## P < 0.01 versus control group. *P < 0.05, **P < 0.01 versus acrolein-treated cells in the absence of lithium. c Phase contrast microphotographs (×100) of HT22 cells pretreated for 30 min with lithium then incubation with 25 μM acrolein for 24 h. d HT22 cells were co-incubated with acrolein and lithium, or incubated with acrolein firstly for 30 min and followed by post-treatment with lithium for additional 24 h. *P < 0.05 versus acrolein-treated cells in the absence of lithium
To clarify whether lithium could play neuronal protection role at other conditions, HT22 cells were co-incubated with acrolein and lithium, or incubated with acrolein for 30 min and post-treated with lithium for 24 h. The results showed that lithium (both co-treatment and post-treatment) protected HT22 cells against acrolein-induced cell death (Fig. 1d).
Lithium Attenuates Acrolein-Induced ROS Production and GSH Depletion
It is well-known that the toxicity of acrolein is mediated by the production of ROS and oxidative stress-induced damage [22]. Hence, we investigated whether the neuroprotective effects of lithium blocked acrolein-induced oxidative stress in HT22 cells. Acrolein at 25 μM increased the intracellular ROS production by about 1.8-fold after 12 h in HT22 cells. Lithium at the tested concentrations (0.3 and 1 mM) profoundly reduced the acrolein-induced ROS accumulation (Fig. 2a, b) without affecting basal level of ROS.
Effects of lithium on acrolein-induced ROS accumulation and GSH depletion. Twelve hours of acrolein (25 μM) treatment resulted in significant increase of intracellular ROS. Intracellular GSH level was depleted by acrolein at 12 h. Lithium dramatically scavenged intracellular ROS and reversed GSH level. Experiments were repeated at least three times. a Fluorescence microphotographs (×100) of HT22 cells pretreated for 30 min with lithium then incubation with acrolein. b Fluorescence intencity of H2-DCF-DA were measured by a fluorimetric plate reader. ## P < 0.01 versus control group; **P < 0.01 versus acrolein-treated cells in the absence of lithium. c Intracellular GSH level pretreated for 30 min with lithium then incubation with acrolein for 12 h. ## P < 0.01 versus control group; **P < 0.01 versus acrolein-treated cells in the absence of lithium
It has been suggested that acrolein induces GSH depletion and then damages cells [22]. The effect of lithium on GSH level, an essential endogenous antioxidant, was examined on acrolein-treated HT22 cells. After 12 h of acrolein treatment, the total GSH level was determined spectrophotometrically. In the hippocampal neuronal cells, treatment with acrolein led to a rapid decrease of intracellular GSH level within 12 h (from (51.2 ± 9.4) nmol/mg protein to (9.5 ± 3.6) nmol/mg protein, P < 0.01). Treatment with lithium (0.3 and 1 mM) dramatically restored the GSH depletion (Fig. 2c).
Lithium Attenuates Acrolein-Induced Mitochondrial Damage
It was found that mitochondria is one of the main intracellular targets of acrolein [23]. Mitochondrial damage could induce an increase of ROS and a subsequent decrease in GSH content [24]. To explore whether lithium has effect on acrolein-induced mitochondrial damage, we further determined mitochondrial membrane potential in HT22 cells. As shown in Fig. 3a, lithium alone didn’t affect the basal mitochondrial function in HT22 cells. 25 μM of acrolein treatment alone for 6 h in HT22 cells decreased mitochondrial membrane potential to about 80 % as compared to control cells (Fig. 3b). Lithium significantly reversed the mitochondrial membrane potential reduction induced by acrolein (Fig. 3c).
Effect of lithium on acrolein-induced mitochondrial membrane potential reduction. After 6 h exposure to acrolein in the absence or presence of lithium, HT22 cells were stained with the mitochondrial dye rhodamine 123 and analyzed using a flow cytometer. Experiments were repeated at least three times. a Lithium alone treated group (blue line), b Acrolein alone treated group (red line), and c lithium pretreated for 30 min and then incubated with acrolein (green line) was compared with untreated control group (solid gray). d Mean fluorescence intensity of 10,000 cells/sample were presented. *P < 0.05 versus control group, # P < 0.05 versus acrolein-treated cells in the absence of lithium (Color figure online)
Lithium Boosts the Protective Inactivation (Phosphorylation) of GSK-3β
Several studies directly link activation of GSK-3β to the neuropathology of neurodegenerative diseases including AD [25, 26]. Inactivation (phosphorylation) of GSK-3β exerts beneficial therapeutic effects on neuronal protection in AD and other central nervous system diseases [7, 15]. To examine whether GSK-3β was involved in the protective mechanisms of lithium, the phosphorylated protein level of GSK-3β was assayed by Western blotting. Our results showed the phosphorylation of GSK-3β slightly decreased at 6 h but significantly increased at 12 h after exposure to acrolein in HT22 cells. Interestingly, lithium potently boosted the phosphorylation of GSK-3β from 6 to 12 h after acrolein exposure (Fig. 4).
Effects of lithium on GSK-3β inactivation. HT22 cells were pretreated with lithium (1 mM) for 30 min and then incubation with 25 μM of acrolein for additional 6 h or 12 h. a Phosphorylation of GSK-3β slightly decreased at 6 h but significantly increased at 12 h after exposure to acrolein in HT22 cells. Lithium promoted the phosphorylation of GSK-3β from 6 h to 12 h of acrolein exposure. b Densitometric measurements of band intensity were performed using Quantity One Software. Blots represent one of three independent experiments and bar graph represents quantitative results of the ratio between p-GSK-3β and β-actin used as protein loading control. Results are expressed as ratio of control (considered as 1). *P < 0.05, **P < 0.01 versus control group. CT control, Li lithium, Acr acrolein
Discussion
Our results demonstrated that lithium efficiently protects the mouse hippocampal HT22 cells against acrolein-induced neurotoxicity. The protective effects were mediated by the ROS scavenging ability of lithium, modulation of mitochondrial function and survival pathway of GSK-3β. These findings are of great interest because acrolein has been shown to accumulate in specific brain regions from AD patients [4, 27], and acrolein could induce AD-like pathologies in vitro and in vivo [5]. On the other hand, lithium might represent a promising treatment for acrolein-related diseases.
Recent studies suggested an important pathologic role of acrolein-induced oxidative stress in the development of AD [27, 28], and we also have showed that oral administration of acrolein induced AD-like pathologies in rats [5]. The present data demonstrate again that acrolein induced ROS production, depleted intracellular GSH and damaged mitochondrial function in HT22 hippocampal neuronal cells (Figs. 2, 3). These results are consistent with our previous data in rat primary astrocytes and SK-N-SH cells [28, 29]. Strategies to scavenge ROS had yielded benefits to oxidative stress-induced neurodegenerative diseases [2]. Lithium, as a specific GSK-3β inhibitor, dramatically inhibited the ROS accumulation and blocked the GSH depletion in HT22 cells exposed to acrolein. The concentration of lithium used in current study is similar or even smaller to that used in other studies [30, 31]. In addition, oxidative stress is accompanied by a decrease in mitochondrial membrane potential, which is widely considered as an indicator of mitochondrial functionality [32]. Our previous data have showed that acrolein triggered mitochondrial apoptotic process at 6 h of exposure [10], and the present study found that lithium could prevent the process of mitochondrial damage (Fig. 3).
Moreover, we discovered that lithium promoted promotes the inactivation of GSK-3β, and boosted boosts the anti-oxidative ability of HT22 cells (Fig. 4). GSK-3β is thought to induce apoptosis by activating caspase-3 [33]. Interestingly, our study found that acrolein mildly activated activates GSK-3β at 6 h while inactivated it at 12 h. This result is consistent with our previous apoptosis result indicating that acrolein elevates the activity of caspase-3 at 6 h but not at 12 h of exposure [10]. We chose GSK-3β as a target kinase of oxidative stress resistance in HT22 cells since data showed GSK-3β activity plays very important roles in determining the fate of oxidative stress-inflicted neuronal cells [34]. In HT22 cells, Schafer et al. [30] showed that GSK-3β activity is directly involved in pathways leading to oxidative HT22 cells death since its inhibition by lithium could be shown to lead to an increased resistance of neurons to the oxidative stressors glutamate and H2O2. In our present study, we also found lithium could profoundly attenuate the acrolein-induced oxidative damage in HT22 neuronal cells.
Conclusion
Taken together, to the best of our knowledge, our findings for the first time show that lithium potently prevents acrolein-induced neuronal death through attenuating ROS accumulation, GSH depletion, mitochondrial dysfunction and GSK-3β activation. Our results also suggest that lithium may provide a promising approach for the treatment of acrolein-related neurodegenerative diseases, such as AD.
References
Sultana R, Perluigi M, Allan Butterfield D (2013) Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic Biol Med 62:157–169. doi:10.1016/j.freeradbiomed.2012.09.027
Adibhatla RM, Hatcher JF (2010) Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 12(1):125–169. doi:10.1089/ARS2009.2668
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11(1):81–128
Williams TI, Lynn BC, Markesbery WR, Lovell MA (2006) Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol Aging 27(8):1094–1099. doi:10.1016/j.neurobiolaging.2005.06.004
Huang YJ, Jin MH, Pi RB, Zhang JJ, Ouyang Y, Chao XJ, Chen MH, Liu PQ, Yu JC, Ramassamy C, Dou J, Chen XH, Jiang YM, Qin J (2013) Acrolein induces Alzheimer’s disease-like pathologies in vitro and in vivo. Toxicol Lett 217(3):184–191. doi:10.1016/j.toxlet.2012.12.023
Gomez-Ramos A, Diaz-Nido J, Smith MA, Perry G, Avila J (2003) Effect of the lipid peroxidation product acrolein on tau phosphorylation in neural cells. J Neurosci Res 71(6):863–870. doi:10.1002/jnr.10525
Munoz-Montano JR, Moreno FJ, Avila J, Diaz-Nido J (1997) Lithium inhibits Alzheimer’s disease-like tau protein phosphorylation in neurons. FEBS Lett 411(2–3):183–188
Jope RS, Johnson GV (2004) The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci 29(2):95–102. doi:10.1016/j.tibs.2003.12.004
Jope RS, Yuskaitis CJ, Beurel E (2007) Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res 32(4–5):577–595. doi:10.1007/s11064-006-9128-5
Huang Y, Jin M, Pi R, Zhang J, Chen M, Ouyang Y, Liu A, Chao X, Liu P, Liu J, Ramassamy C, Qin J (2013) Protective effects of caffeic acid and caffeic acid phenethyl ester against acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Neurosci Lett 535:146–151. doi:10.1016/j.neulet.2012.12.051
Forlenza OV, de Paula VJ, Machado-Vieira R, Diniz BS, Gattaz WF (2012) Does lithium prevent Alzheimer’s disease? Drugs Aging 29(5):335–342. doi:10.2165/11599180-000000000-00000
Rowe MK, Wiest C, Chuang DM (2007) GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder. Neurosci Biobehav Rev 31(6):920–931. doi:10.1016/j.neubiorev.2007.03.002
Appleby BS, Cummings JL (2013) Discovering new treatments for Alzheimer’s disease by repurposing approved medications. Curr Top Med Chem 13(18):2306–2327
King MK, Pardo M, Cheng Y, Downey K, Jope RS, Beurel E (2014) Glycogen synthase kinase-3 inhibitors: rescuers of cognitive impairments. Pharmacol Ther 141(1):1–12. doi:10.1016/j.pharmthera.2013.07.010
Aghdam SY, Barger SW (2007) Glycogen synthase kinase-3 in neurodegeneration and neuroprotection: lessons from lithium. Curr Alzheimer Res 4(1):21–31
Liu J, Li L, Suo WZ (2009) HT22 hippocampal neuronal cell line possesses functional cholinergic properties. Life Sci 84(9–10):267–271. doi:10.1016/j.lfs.2008.12.008
Nijmeh J, Moldobaeva A, Wagner EM (2010) Role of ROS in ischemia-induced lung angiogenesis. Am J Physiol Lung Cell Mol Physiol 299(4):L535–L541. doi:10.1152/ajplung.00002.2010
Chazotte B (2011) Labeling mitochondria with rhodamine 123. Cold Spring Harb Protoc 2011(7):892–894. doi:10.1101/pdb.prot5640
Singh M, Murthy V, Ramassamy C (2010) Modulation of hydrogen peroxide and acrolein-induced oxidative stress, mitochondrial dysfunctions and redox regulated pathways by the Bacopa monniera extract: potential implication in Alzheimer’s disease. J Alzheimers Dis 21(1):229–247. doi:10.3233/JAD-2010-091729
Hamann K, Nehrt G, Ouyang H, Duerstock B, Shi R (2008) Hydralazine inhibits compression and acrolein-mediated injuries in ex vivo spinal cord. J Neurochem 104(3):708–718. doi:10.1111/j.1471-4159.2007.05002.x
Lovell MA, Xie C, Markesbery WR (2001) Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging 22(2):187–194
Tomitori H, Nakamura M, Sakamoto A, Terui Y, Yoshida M, Igarashi K, Kashiwagi K (2012) Augmented glutathione synthesis decreases acrolein toxicity. Biochem Biophys Res Commun 418(1):110–115. doi:10.1016/j.bbrc.2011.12.143
Roy J, Pallepati P, Bettaieb A, Tanel A, Averill-Bates DA (2009) Acrolein induces a cellular stress response and triggers mitochondrial apoptosis in A549 cells. Chem Biol Interact 181(2):154–167. doi:10.1016/j.cbi.2009.07.001
Luo J, Robinson JP, Shi R (2005) Acrolein-induced cell death in PC12 cells: role of mitochondria-mediated oxidative stress. Neurochem Int 47(7):449–457. doi:10.1016/j.neuint.2005.07.002
Kanninen K, White AR, Koistinaho J, Malm T (2011) GSK/Nrf2—targeting glycogen synthase kinase-3beta for therapeutic benefit against oxidative stress in Alzheimer’s disease: involvement of the Nrf2-ARE pathway. Int J Alzheimers Dis 2011:985085. doi:10.4061/2011/985085
Hooper C, Killick R, Lovestone S (2008) The GSK3 hypothesis of Alzheimer’s disease. J Neurochem 104(6):1433–1439. doi:10.1111/j.1471-4159.2007.05194.x
Bradley MA, Markesbery WR, Lovell MA (2010) Increased levels of 4-hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free Radic Biol Med 48(12):1570–1576. doi:10.1016/j.freeradbiomed.2010.02.016
Nam DT, Arseneault M, Ramassamy C (2011) Regulation of redox-sensitive signaling pathways in rat primary astrocytes following acrolein exposure. J Alzheimers Dis 25(2):263–277. doi:10.3233/JAD-2011-102094
Doggui S, Belkacemi A, Paka GD, Perrotte M, Pi R, Ramassamy C (2013) Curcumin protects neuronal-like cells against acrolein by restoring Akt and redox signaling pathways. Mol Nutr Food Res 57(9):1660–1670. doi:10.1002/mnfr.201300130
Schafer M, Goodenough S, Moosmann B (1005) Behl C (2004) Inhibition of glycogen synthase kinase 3 beta is involved in the resistance to oxidative stress in neuronal HT22 cells. Brain Res 1–2:84–89. doi:10.1016/j.brainres.2004.01.037
Zamfirescu G, Buzgariu W, Uluiu M, Tesio C (1999) Lithium action upon Hep2 tumoral cells in vitro. Rom J Physiol 36(3–4):219–231
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS (2004) Calcium, ATP, and ROS: a mitochondrial love–hate triangle. Am J Physiol Cell Physiol 287(4):C817–C833. doi:10.1152/ajpcell.00139.2004
Chen G, Bower KA, Ma C, Fang S, Thiele CJ, Luo J (2004) Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death. FASEB J 18(10):1162–1164. doi:10.1096/fj.04-1551fje
Lee KY, Koh SH, Noh MY, Park KW, Lee YJ, Kim SH (2007) Glycogen synthase kinase-3beta activity plays very important roles in determining the fate of oxidative stress-inflicted neuronal cells. Brain Res 1129(1):89–99. doi:10.1016/j.brainres.2006.10.055
Acknowledgments
This study was supported by Guangdong Provincial International Cooperation Project of Science and Technology (No. 2012B050300015) to R. Pi and Guangdong Provincial International Cooperation Project of Science and Technology (No. 2012B050600019) to J. Qin.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yingjuan Huang and Jian Qin have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Huang, Y., Qin, J., Chen, M. et al. Lithium Prevents Acrolein-Induced Neurotoxicity in HT22 Mouse Hippocampal Cells. Neurochem Res 39, 677–684 (2014). https://doi.org/10.1007/s11064-014-1252-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1252-z