Abstract
Astrocyte-rich primary cultures (APCs) are frequently used as a model system for the investigation of properties of brain astrocytes. However, as APCs contain a substantial number of microglial and oligodendroglial cells, biochemical parameters determined for such cultures may at least in part reflect also the presence of the contaminating cell types. To lower the potential contributions of microglial and oligodendroglial cells on properties of the astrocytes in APCs we prepared rat astrocyte-rich secondary cultures (ASCs) by subculturing of APCs and compared these ASCs with APCs regarding basal metabolic parameters, specific enzyme activities and the accumulation of iron oxide nanoparticles. Immunocytochemical characterization revealed that ASCs contained only minute amounts of microglial and oligodendroglial cells. ASCs and APCs did not significantly differ in their specific glucose consumption and lactate production rates, in their specific iron and glutathione contents, in their specific activities of various enzymes involved in glucose and glutathione metabolism nor in their accumulation of iron oxide nanoparticles. Thus, the absence or presence of some contaminating microglial and oligodendroglial cells appears not to substantially modulate the investigated metabolic parameters of astrocyte cultures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astrocytes are the most abundant cell type in the brain [1]. Their passive character as “glue” has long been out of date and an increasing number of functions have been reported for astrocytes over the past decades [2]. Since the end feet of astrocytes are in direct contact with the blood capillaries, astrocytes are considered to supply neurons and other glial cell types with nutrients and to maintain the homeostasis of water and ions in brain [3, 4]. Furthermore, astrocytes can modulate the concentration of transmitters by uptake of neuro- and release of gliotransmitters [2, 3]. Astrocytes are considered to be involved in the exchange of energy substrates [5–8] and supply neurons with precursors for glutathione (GSH) synthesis [9, 10]. In addition, astrocytes have been discussed to protect neurons against the toxicity of metals [11, 12] and metal-containing nanoparticles [13].
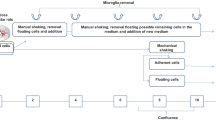
Astrocyte-rich cultures have frequently been used as a model system for the investigation of astrocyte functions [14]. The majority of studies on cultured astrocytes were performed on astrocyte-rich primary cultures (APCs) that are prepared from cerebral parts of neonatal rodent brain [15]. However, due to the cerebral origin, APCs are often contaminated with a proportion of other glial cell types, mainly by microglial cells [16–18]. In APCs, the presence of contaminating glial cell types could affect the overall specific metabolic properties determined for the culture either by their contribution to the metabolism of the culture and/or by the release of factors that may modulate the metabolic properties of the astrocytes present in the cultures. Several methods have been described to reduce the number of microglia in astrocyte-rich cultures, such as subculturing of APCs with a low re-seeding cell density, application of microglia toxins, shaking of the cultures to remove the microglial cells present on the top of the astroglial layer or magnetic separation [17, 18].
To test whether contaminating microglial and oligodendroglial cells in APCs affect the basal metabolic properties of the astrocytes, which represent the majority of cells in these cultures, we generated astrocyte-rich secondary cultures (ASCs) by subculturing APCs and confirmed that these ASCs contain only minute numbers of contaminating microglial and oligodendroglial cells. These ASCs were compared with APCs regarding glucose consumption and lactate production, the export of GSH, the specific activities of several important metabolic enzymes and the accumulation of iron oxide nanoparticles (IONPs). The data obtained revealed that ASCs and APCs did not significantly differ in the metabolic parameters investigated, suggesting that the contaminating microglial and oligodendroglial cells in APCs do not affect the investigated metabolic parameters of these cultures.
Materials and Methods
Material
Bovine serum albumin, NADH, NADPH, NAD+ and NADP+ were from Applichem (Darmstadt, Germany). Fetal calf serum (FCS) and penicillin/streptomycin solution were obtained from Biochrom (Berlin, Germany) and Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Gibco (Karlsruhe, Germany). All enzymes as well as glutathione (GSH) and glutathione disulfide (GSSG) were obtained from Roche (Mannheim, Germany). Dimercaptosuccinate-coated iron oxide nanoparticles (IONPs) were prepared and characterized as recently described [19]. Mouse anti-rat CD11b was from AbD Serotec (Düsseldorf, Germany). Goat Cy2-conjugated anti-rabbit immunoglobulin (IgG) F(ab)2, goat Cy3-conjugated anti-mouse IgG and donkey anti-sheep IgG conjugated with Cy3 were obtained from Dianova (Hamburg, Germany). Mouse anti-glutamine synthetase (GS) was purchased from BD Transduction Laboratories (Heidelberg, Germany) and rabbit anti-glial fibrillary acidic protein (GFAP) from DakoCytomation (Hamburg, Germany). Sheep anti-myelin associated glycoprotein (MAG) [20] was kindly provided by Dr. Frank Dietz (University of Bremen). Other chemicals of the highest purity available were obtained from Merck (Darmstadt, Germany), Sigma-Aldrich (Steinheim, Germany), Roth (Karlsruhe, Germany) or Janssen (Geel, Belgium). 96-well microtiter plates were purchased from Nunc (Wiesbaden, Germany) and sterile 24-well cell culture plates were from Sarstedt (Nümbrecht, Germany).
Cell Culture
Astrocyte-rich primary cultures (APCs) were prepared from brains of neonatal Wistar rats as described earlier [21, 22]. The harvested cells were seeded into wells of 24 well-plates with or without coverslips (300,000 cells/well) or in 25 cm2-flasks (3 million cells/flask). The culture medium (DMEM containing 10 % FCS, 25 mM glucose, 1 mM pyruvate, 20 units/mL penicillin G and 20 μg/mL streptomycin sulfate) was renewed every seventh day and the APCs were used for experiments at a culture age of 14–21 days. After 2 weeks in culture, the APCs grown in 25 cm2-flasks were used to prepare ASCs. Loosely attached cells were detached by hitting the flask, removing the culture medium and washing the attached cells with sterile phosphate buffered saline (PBS; 10 mM potassium phosphate buffer pH 7.4 containing 150 mM sodium chloride). The remaining confluent cell layer was exposed to 1.5 mL 0.25 % (w/v) trypsin in PBS for 7 min at 37 °C. Subsequently, the trypsinization was stopped by addition of 3 mL DMEM with 10 % FCS and the cells were centrifuged (400 g, 5 min, 4 °C). The cell pellet was resuspended in DMEM with 10 % FCS, the cell viability was determined by nigrosine exclusion [23] and 10,000 viable cells were seeded per well of a 24-well plate (with or without coverslips). The cells in ASCs were grown for up to 3 weeks and the culture medium was renewed every seventh day. After 3 weeks in culture, ASCs had reached confluence.
Immunocytochemical Staining
Cells of APCs and ASCs were washed with cold PBS and fixed with 400 μL 3.5 % (w/v) paraformaldehyde in PBS for 10 min at room temperature (RT). Cells were washed thrice with cold PBS in intervals of 5 min prior to application of 400 μL 0.1 % (w/v) glycine in PBS for 10 min at RT and a subsequent permeabilization with 400 μL 0.3 % (w/v) Triton X-100 in 0.1 % glycine for 10 min at RT. After washing three times with PBS, cells which should be stained for GS were incubated for 1 h at RT with 5 % bovine serum albumin in PBS. Cells were washed thrice with PBS and incubated with primary antibodies (rabbit anti-GFAP 1:200, mouse anti-GS 1:200, mouse anti-CD11b 1:100, sheep anti-MAG 1:500) over night at 4 °C, washed and incubated with secondary antibodies (Cy 3-labeled anti-mouse 1:200, Cy 2-labeled anti-rabbit 1:200, Cy 3-labeled anti-sheep 1:500) for 30 min at RT. After incubation with the secondary antibody, nuclei were counterstained with DAPI (1 μg/mL in PBS) for 5 min at RT. Cells were washed thrice with PBS and dehydrated by an ethanol serial (70–100 %) before embedding in DPX mounting medium. Cellular fluorescence was monitored by an Eclipse TE-2000U fluorescence microscope with a DS-QilMc camera and imaging software NIS-Elements BR (Nikon, Düsseldorf, Germany) using appropriate filter sets for Cy2 (excitation: 465–495 nm, emission: 505–515 nm, dichromatic mirror: 505 nm), Cy3 (excitation: 510–560 nm, emission: 590 nm, dichromatic mirror: 575 nm) and DAPI (excitation: 330–380 nm, emission: 420 nm, dichromatic mirror: 400 nm).
Experimental Incubations
To determine glucose consumption, lactate production and glutathione export from cultured astrocytes, the cells were washed with 1 mL incubation buffer (IB; 20 mM HEPES, 5 mM glucose, 1.8 mM CaCl2, 1 mM MgCl2, 5.4 mM KCl, 145 mM NaCl, pH 7.4) and incubated with 1 mL (glutathione export) or 200 μL (lactate release, glucose consumption) IB for up to 6 h at 37 °C. At the indicated time points, the media were collected to determine the extracellular contents of glutathione, lactate and glucose and the cells were washed with 1 mL ice-cold PBS before analysis of cellular protein and glutathione contents.
The accumulation of iron oxide nanoparticles by cells in ASCs and APCs was investigated by incubation for 4 h with 0.75 or 1.5 mM iron as IONPs at 37 or 4 °C. Media were collected for determination of extracellular lactate dehydrogenase (LDH) activity as indicator for a loss of cell viability. The cells were washed with cold PBS and either stored at −20 °C for determination of protein and iron contents or used directly for Perls’ staining to visualize cellular iron.
Determination of Metabolic Parameters and Enzyme Activities
The cellular protein content per well was determined by the Lowry method [24] using bovine serum albumin as a standard. Cellular iron contents were quantified by a modification [25] of a previously published colorimetric method in microtiter plates [26]. Cytochemical staining for iron was done as recently described [19, 25]. The amount of total glutathione (GSx = GSH + 2 GSSG) and glutathione disulfide (GSSG) in media samples and cell lysates were determined by the colorimetric Tietze assay [27]. The concentrations of lactate and glucose in media were determined by coupled enzymatic assays as previously described [22, 28, 29].
The specific activities of metabolic enzymes in astrocyte-rich cultures were determined after lysis of the cultures in 200 μL 20 mM potassium phosphate buffer (pH 7.4) containing 1 % (w/v) Triton X-100. The activities of citrate synthase (CS), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), glucose-6-phosphate dehydrogenase (G6PDH), glutathione reductase (GR) and LDH were determined as previously described [29, 30] and the activities obtained were normalized to the protein content per well to calculate the specific activities.
Presentation of the Data
The data presented were obtained in at least three experiments performed on independently prepared cultures. Results are presented as mean values ± standard deviation. Significance of differences between multiple sets of data was analysed by ANOVA followed by the Bonferroni post hoc-test, while differences between two sets of data were analysed by the t test. P values >0.05 were considered as not significant. The microscopical images shown are derived from one culture but are representative for at least three experiments performed on independently prepared cultures.
Results
Morphology and Immunocytochemical Characterization of ASCs and APCs
Passaging of the cells in APCs and re-seeding in a cell density of 10,000 cells/well generated within 3 weeks a confluent ASC. The morphology of the cells in ASCs was rather flat giving a low phase contrast (Fig. 1a, c). In contrast, cells in APCs were more round and some had long processes (Fig. 1b, d). In addition, the contrast-rich cells on top of the basal cell layer in APCs (Fig. 1b, d) were not detectable anymore in the ASCs (Fig. 1a, c). Immunocytochemical analysis for the presence of marker proteins of different glial cell types revealed that both ASCs and APCs were strongly enriched in astrocytes, as deduced from the large number of cells that expressed the astrocyte marker proteins GFAP and GS (Fig. 2a, b, c, d). However, the staining intensity and the staining pattern for GFAP differed between the two types of cultures. While in APCs the majority of cells were strongly stained for GFAP (Fig. 2b), most cells of ASCs were only weakly stained for GFAP (Fig. 2a; Table 1). However, some patches of cells in ASCs were also positive for both GFAP and GS (Fig. 2a, c), while such patches were not observed in APCs (Fig. 2b, d). Figure 3 shows an example for the immunocytochemical discrimination in ASCs between strongly and weakly GFAP-positive astrocytes. Cells strongly positive for GFAP revealed an intensive fluorescence signal after a microscopical exposure time of 800 ms (Fig. 3a), while under such exposure conditions weakly stained GFAP-positive cells were not detectable. Clear signals for cells that were weakly stained for GFAP were obtained after an exposure time of 2 s (Fig. 3b). Omission of the GFAP antibody revealed a very low unspecific background staining for both exposure times (Fig. 3c, d).
Immunocytochemical staining of a 21 d-old ASC and a 14 d-old APC. Astrocyte cultures were stained for the astrocytic marker proteins GFAP (a, b) and GS (c, d), for the microglial marker protein CD11b (e, f) or for the oligodendrocyte marker protein MAG (g, h). The scale bar in (g) represents 50 μm and applies to all panels
Identification of cells in ASCs that are strongly and weakly GFAP-positive. ASCs were immunocytochemically stained for GFAP and monitored on the fluorescence microscope by either a short (800 ms; a, c) or a long (2 s; b, d) exposure time. To identify the level of unspecific background fluorescence, the anti-GFAP antibody was omitted in the staining procedure (c, d)
Presence of microglial cells and oligodendrocytes was investigated by staining of ASCs and APCs for CD11b (Fig. 2e, f) and MAG (Fig. 2g, h). Immunocytochemical staining of APCs revealed a large number of cells that were strongly GFAP-positive (Fig. 2b), some GS-positive cells (Fig. 2d) and a large number of small fluorescent elements that were positive after staining for the microglial marker CD11b (Fig. 2f) and the oligodendroglial marker MAG (Fig. 2h). In contrast, ASCs contained predominately cells that were weakly stained for GFAP and hardly any cells that were positive for the microglial marker CD11b (around 1 %) and the oligodendroglial marker MAG (around 3 %) (Fig. 2e, g; Table 1). We also tried to quantify in APCs the number of cells that were strongly GFAP-positive (65 ± 24 %), weakly GFAP-positive (3 ± 5 %), CD11b-positive (26 ± 4 %) and MAG-positive (8 ± 4 %). However, with our equipment it was not possible to reliably connect in the confluent APCs a given nucleus with all stained microglial or oligodendroglial elements that belong to the respective cell. Thus, the results obtained in our attempt to quantify in APCs the number of CD11b-positive cells and MAG-positive cells are likely to overestimate the real number of microglial and oligodendroglial cells.
Protein Content and Viability of Astroglia-Rich Cultures
To investigate whether the presence or almost absence of contaminating glial cells may affect overall basal metabolic parameters of the cultures, we compared ASCs and APCs regarding their glucose and glutathione metabolism as well as for their ability to accumulate IONPs. The protein content of the 3 weeks old ASCs (114 ± 26 μg/well) was almost identical to that of APCs (109 ± 5 μg/well). In contrast, the protein content of 2 weeks old ASCs (68 ± 15 μg/well) was significantly lower (p < 0.01) than that of 3 weeks old ASCs (114 ± 26 μg/well), demonstrating that ASCs have to be cultured for 3 weeks before they have a protein content that is almost identical to that of APCs.
For determining the glucose and glutathione metabolism of the different types of astrocyte-rich cultures, the cells were incubated in IB for up to 6 h. Microscopical inspection revealed that during this time frame, no obvious detachment of cells nor any loss in cell viability were observed (data not shown) which was confirmed by the almost constant protein content (Fig. 4a) and by the absence of any significant increase in the extracellular LDH activity (Fig. 4b) during the incubation of ASCs or APCs.
Glucose and glutathione metabolism of ASCs and APCs. ASCs (black symbols) and APCs (white symbols) were incubated for up to 6 h at 37 °C and the cellular protein content (a), the extracellular LDH activity (b), the specific extracellular lactate content (c), the specific glucose consumption (d), as well as the extracellular (e) and cellular (f) contents of GSx (circles) and GSSG (triangles) were determined. Significant differences between the data obtained for ASCs and APCs (n = 3) were not observed
Glucose Metabolism of ASCs and APCs
Incubation of ASCs or APCs in glucose-containing medium caused an almost linear increase in the extracellular concentration of lactate (Fig. 4c) and an almost linear decrease in extracellular glucose concentration (data not shown) which is reflected by the calculated almost linear increases in cellular glucose consumption (Fig. 4d). The almost linear increases in extracellular lactate accumulation and in glucose consumption within the first 4 h of incubation were used to calculate the rates of lactate production and glucose consumption for both ASCs and APCs. The specific lactate production rates of 0.8 ± 0.2 μmol/(h mg) (ASCs) and 1.0 ± 0.2 μmol/(h mg) (APCs) and the specific glucose consumption rates of 0.5 ± 0.2 μmol/(h mg) (ASCs) and 0.5 ± 0.1 μmol/(h mg) (APCs) did not differ significantly (Table 2). Also the ratio of the lactate production rate to the glucose consumption rate of ASCs (1.8 ± 0.2) and APCs (1.9 ± 0.3) was almost identical for both culture types. In addition, the specific activities of some key enzymes in glucose and energy metabolism, i.e., G6PDH, GAPDH, LDH and CS, did not differ significantly in ASCs and APCs (Table 3).
Glutathione Metabolism of ASCs and APCs
Quantification of the basal GSx contents of untreated ASCs and APCs revealed that these cultures contained almost identical levels of GSx with 48 ± 13 and 51 ± 7 nmol GSx/mg protein, respectively. Only minute amounts of GSSG contributed to these GSx contents as the specific GSSG contents amounted to only 1.2 ± 0.3 (ASCs) and 1.4 ± 0.1 nmol GSx/mg (APCs).
Incubation of ASCs or APCs in IB for up to 6 h caused an almost linear increase in the extracellular contents of GSx (Fig. 4e) which was accompanied with a small, but not significant loss in cellular GSx (Fig. 4f). Some of the extracellular GSx accounted for GSSG, while in the cells the GSSG levels were in the range of the detection limit of the method applied (Fig. 4f). Calculation of the rates of extracellular accumulation of GSx revealed that ASCs [2.6 ± 0.8 nmol/(h mg)] and APCs [2.4 ± 0.1 nmol/(h mg)] had almost identical rates of GSH export (Table 2). In addition, the specific activity of GR, which reduces GSSG to GSH, was almost identical for both types of astrocyte cultures (Table 3).
Iron Content and Accumulation of Iron Oxide Nanoparticles by ASCs and APCs
Untreated ASCs and APCs had almost identical basal iron contents of 8 ± 4 and 10 ± 2 nmol/mg, respectively. Perls’ staining of untreated APCs for iron revealed that a few cells in these cultures were strongly positive for iron (Fig. 5b), while such cells were not detectable in ASCs (Fig. 5a). The basal iron contents of ASCs or APCs were not significantly elevated during incubation for 4 h at 37 °C or at 4 °C in the absence of IONPs (Table 4). In contrast, presence of 0.75 or 1.5 mM iron as IONPs during the incubation at 37 °C strongly increased the specific cellular iron content of both ASCs and APCs to values of around 1,500 and 2,500 nmol iron/mg protein, respectively (Table 4). The IONP-treated cells displayed a strong staining for iron which did not differ between ASCs and APCs (Fig. 5c, d). Compared to the 37 °C incubation condition, the cellular iron accumulation was significantly lowered by around 50–60 %, if ASCs or APCs had been incubated with IONPs at 4 °C (Table 4). For these conditions, only a low intensity of the Perls’ iron staining was observed for ASCs (Fig. 5e), while some Perls’-positive cells were found in APCs that had been exposed to IONPs at 4 °C (Fig. 5f). None of these incubation conditions did lower the cell viability as demonstrated by the absence of a significant increase in extracellular LDH activity and by the almost constant cellular protein content compared to the respective control incubations (0 μM) (Table 4).
Discussion
Astrocyte-rich primary cultures are a frequently used model system to investigate properties and functions of brain astrocytes [14, 15, 22], since these cultures are enriched for astrocytes. However, APCs contain also other types of glial cells which contribute to and/or may affect the basal metabolic properties determined for the cultures. To address this question we prepared and immunocytochemically characterized ASCs and compared their basal metabolic parameters and properties with those of APCs.
Immunocytochemical staining of APCs confirmed the presence of microglial cells and oligodendrocytes in cultures prepared by our [21, 22, 31] or other protocols [32, 33]. Subculturing and seeding of a low number of viable cells to obtain ASCs lowered the number of microglia and oligodendrocytes substantially, confirming literature data [34, 35]. Likely reason for the enrichment in astrocytes in ASCs is that the subculturing prevents contact between the contaminating cells and/or that certain growth factors are missing which would be required for the proliferation of the contaminating cells [35].
In ASCs, the morphology of astrocytes and the expression of astrocyte marker proteins were different to those observed for APCs. The majority of the cells in ASCs were only weakly GFAP-positive, whereas most cells in APCs were strongly stained for GFAP. Furthermore, the GS staining pattern of astrocytes in rat ASCs was similar to their GFAP-staining, while in rat APCs mainly the GFAP-negative astrocytes were positive for GS. These data obtained for rat cultures contrast literature data observed for APCs [36] and ASCs [37] from mouse brain where most astrocytes express both GFAP and GS. Differences in the expression of GFAP and/or GS in cultured astrocytes may reflect the known heterogeneity of astrocytes in brain [38, 39] and/or an enrichment of certain subtypes of astrocytes during culturing and passaging. Furthermore, the expression of astrocytic markers is known to be affected by subculturing, although the literature data are not consistent as subculturing has been reported to increase [40], not affect [37] or decrease [41] GFAP protein levels. This discrepancy may be a consequence of differences in the culturing conditions which are known to strongly affect the expression of cell type specific markers [42–44]. The low expression of GFAP in the astrocytes of ASCs may be a direct consequence of the low number of microglial cells in these cultures, as the culture medium of ASCs is likely to be deprived of microglia-derived factors which have been reported to induce GFAP expression in astrocytes [45, 46]. The low GFAP expression and/or absence of non-astrocytic cell types may also contribute to the flat and contrast-low appearance of astrocytes in the ASCs, contrasting the morphology of the phase-dark and process bearing astrocytes in APCs [47].
Absence or presence of a substantial amount of contaminating microglial cells and oligodendrocytes may affect metabolic parameters that are investigated for astrocyte-rich cultures as the contaminant cell types may differ in their metabolism from astrocytes, thereby affecting the overall parameters determined for the sum of all cells in a given culture. In addition, contact of astrocytes to other cell types in the cultures investigated as well as compounds released by these contaminating cells may activate or suppress metabolic processes in astrocytes. To address such questions we compared APCs with ASCs concerning basal metabolic parameters such as the release of glutathione and lactate as well as the accumulation of IONPs. Remarkably, ASCs and APCs did not differ significantly in specific enzyme activities, specific metabolite levels, glycolytic flux, GSH export or in the accumulation of IONPs, clearly demonstrating that removal of most of the contaminating microglial and oligodendroglial cells from the APCs by generating ASCs did not affect the overall metabolic parameters investigated. These data are consistent with recent findings showing similar metabolism of amino acids in primary and passaged mice astrocyte cultures [37].
Cultured astrocytes are known to have a high glycolytic capacity [48–50]. The data obtained here for glucose consumption and lactate release by ASCs or APCs and the high ratio of lactate release to glucose consumption are similar to those previously reported for cultured astrocytes [51, 52] though lower ratios were also observed for astroglial cultures [53]. The almost identical specific activities of the enzyme GAPDH, G6PDH and LDH in ASCs and APCs support the view that the basal glucose metabolism of these cultures does not differ. The specific activities of GAPDH, LDH and CS determined here were lower as described previously by us [29, 30, 54] or others [55–57], which may be a consequence of the culturing and/or lysis conditions, as we have used for our present study a detergent to lyse the cells for enzyme activity assays.
Astrocytes play an important role in the GSH metabolism of the brain and supply precursors for GSH to neurons in a process that is initiated by GSH export [9, 10, 58]. The basal GSx content of ASCs and APCs determined here (around 55 nmol/mg protein) is similar to values reported previously for such cultures [59, 60]. The very low cellular GSSG content in both APCs and ASCs revealed that the cells in these cultures do not suffer from severe oxidative stress [9]. The high potential of both ASCs and APCs to efficiently maintain low cellular GSSG levels is consistent with the almost identical specific activities of GR, the enzyme which continuously reduces GSSG to GSH in a NADPH-dependent reaction [61], and of G6PDH, the rate limiting enzyme of the NADPH-providing pentose phosphate pathway [62]. The rates of GSH export from ASCs and APCs of around 2.5 nmol/(h mg protein) is similar to literature data for cultured rat or mouse astrocytes [60, 63]. The transporter predominantly responsible for the observed basal GSH export in ASCs and APCs is most likely the multidrug resistance protein 1, as this transporter has been reported to be the primary mediator of GSH export from rat and mouse astrocyte cultures [63, 64].
Astrocytes are considered to have important functions in the distribution of iron in the brain [11] and in the handling of iron oxide nanoparticles [13]. The basal iron contents of ASCs and APCs (8 ± 4 and 10 ± 2 nmol/mg protein, respectively) did not differ from each other and were similar to those previously reported for astrocyte cultures [19, 26, 65]. Thus, the presence of contaminating oligodendrocytes and microglia which have higher iron contents as astrocytes [66–70] had no influence on the basal cellular iron content of these cultures, probably due to their in general smaller size in comparison to astrocytes. However, cytochemical iron staining revealed for untreated APCs some cells that were strongly iron-positive which may just be microglial or oligodendroglial cells as these cells contain high amounts of iron [66–70]. This view is supported by the observation that such iron-positive cells were not found in untreated ASCs.
On application of IONPs, both ASCs and APCs efficiently accumulated the particles in a temperature dependent process, confirming literature data for APCs [19, 71]. The accumulation of IONPs at 37 °C was similarly strong in ASCs and APCs. Although among the different types of brain cells especially microglia are considered to accumulate large amounts of magnetic IONPs [72–74], no difference in IONP accumulation was observed between the microglia-containing APCs and the ASCs that hardly contained microglial cells. These results suggest that the high amounts of iron found in IONP-treated ASCs and APCs reflect predominately the accumulation of IONPs by astrocytes and that contaminating microglial and oligodendroglial cells in APCs do not overproportional contribute to the overall IONP accumulation in APCs.
In summary, the preparation of rat ASCs by subculturing of APCs resulted in a strong enrichment of astrocytes and a substantial decrease in number of contaminating other glial cell types. Compared to APCs, subculturing of astrocytes to generate ASCs affected the morphology of astrocytes and the expression of the astrocytic markers GFAP and GS, but did not alter the basal metabolic parameters investigated, including glycolytic flux, GSH export and the accumulation of IONPs. Thus, the presence of a moderate number of contaminating glial cells in APCs appears not to substantially affect the contribution of astrocytes in the metabolic pathways investigated. Potential differences in metabolic parameters in ASCs and APCs appear to be in the same range as differences between individual preparations of either type of astrocyte-rich culture.
References
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A (2012) Glial cells in (patho)physiology. J Neurochem 121:4–27
Kettenmann H, Verkhratsky A (2011) Neuroglia—living nerve glue. Fortschr Neurol Psychiatr 79:588–597
Kimelberg HK (2010) Functions of mature mammalian astrocytes: a current view. Neuroscientist 16:79–106
Barros LF, Deitmer JW (2010) Glucose and lactate supply to the synapse. Brain Res Rev 63:149–159
Belanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14:724–738
Mangia S, Simpson IA, Vannucci SJ, Carruthers A (2009) The in vivo neuron-to-astrocyte lactate shuttle in human brain: evidence from modeling of measured lactate levels during visual stimulation. J Neurochem 109:55–62
Zielke HR, Zielke CL, Baab PJ, Tildon JT (2007) Effect of fluorocitrate on cerebral oxidation of lactate and glucose in freely moving rats. J Neurochem 101:9–16
Hirrlinger J, Dringen R (2010) The cytosolic redox state of astrocytes: maintenance, regulation and functional implications for metabolite trafficking. Brain Res Rev 63:177–188
Schmidt MM, Dringen R (2012) Glutathione (GSH) synthesis and metabolism. In: Choi IY, Gruetter R (ed) Neural metabolism in vivo, 4th volume, Springer, pp 1029–1050
Dringen R, Bishop GM, Koeppe M, Dang TN, Robinson SR (2007) The pivotal role of astrocytes in the metabolism of iron in the brain. Neurochem Res 32:1884–1890
Scheiber IF, Dringen R (2013) Astrocyte functions in the copper homeostasis of the brain. Neurochem Int 62:556–565
Hohnholt MC, Geppert M, Luther EM, Petters C, Bulcke F, Dringen R (2013) Handling of iron oxide and silver nanoparticles by astrocytes. Neurochem Res 38:227–239
Lange SC, Bak LK, Waagepetersen HS, Schousboe A, Norenberg MD (2012) Primary cultures of astrocytes: their value in understanding astrocytes in health and disease. Neurochem Res 37:2569–2588
de Vellis J, Cole R (2012) Preparation of mixed glial cultures from postnatal rat brain. Methods Mol Biol 814:49–59
Crocker SJ, Frausto RF, Whitton JL, Milner R (2008) A novel method to establish microglia-free astrocyte cultures: comparison of matrix metalloproteinase expression profiles in pure cultures of astrocytes and microglia. Glia 56:1187–1198
Losciuto S, Dorban G, Gabel S, Gustin A, Hoenen C, Grandbarbe L, Heuschling P, Heurtaux T (2012) An efficient method to limit microglia-dependent effects in astroglial cultures. J Neurosci Methods 207:59–71
Saura J (2007) Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation 4:26
Geppert M, Hohnholt MC, Thiel K, Nurnberger S, Grunwald I, Rezwan K, Dringen R (2011) Uptake of dimercaptosuccinate-coated magnetic iron oxide nanoparticles by cultured brain astrocytes. Nanotechnology 22:145101
Dauth S, Schmidt MM, Rehders M, Dietz F, Kelm S, Dringen R, Brix K (2012) Characterisation and metabolism of astroglia-rich primary cultures from cathepsin K-deficient mice. Biol Chem 393:959–970
Hamprecht B, Loffler F (1985) Primary glial cultures as a model for studying hormone action. Methods Enzymol 109:341–345
Tulpule K, Hohnholt MC, Hirrlinger J, Dringen R (2014) Primary cultures of astrocytes and neurons as model systems to study the metabolism and metabolite export from brain cells. In: Hirrlinger J, Waagepetersen H (ed) Neuromethods: Brain energy metabolism, Springer, Heidelberg (in press)
Kaltenbach JP, Kaltenbach MH, Lyons WB (1958) Nigrosin as a dye for differentiating live and dead ascites cells. Exp Cell Res 15:112–117
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Geppert M, Hohnholt M, Gaetjen L, Grunwald I, Baumer M, Dringen R (2009) Accumulation of iron oxide nanoparticles by cultured brain astrocytes. J Biomed Nanotechnol 5:285–293
Riemer J, Hoepken HH, Czerwinska H, Robinson SR, Dringen R (2004) Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal Biochem 331:370–375
Hirrlinger J, Dringen R (2005) Multidrug resistance protein 1-mediated export of glutathione and glutathione disulfide from brain astrocytes. Methods Enzymol 400:395–409
Dringen R, Gebhardt R, Hamprecht B (1993) Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res 623:208–214
Schmidt MM, Dringen R (2009) Differential effects of iodoacetamide and iodoacetate on glycolysis and glutathione metabolism of cultured astrocytes. Front Neuroenergetics 1:1–10
Minich T, Yokota S, Dringen R (2003) Cytosolic and mitochondrial isoforms of NADP+-dependent isocitrate dehydrogenases are expressed in cultured rat neurons, astrocytes, oligodendrocytes and microglial cells. J Neurochem 86:605–614
Gutterer JM, Dringen R, Hirrlinger J, Hamprecht B (1999) Purification of glutathione reductase from bovine brain, generation of an antiserum, and immunocytochemical localization of the enzyme in neural cells. J Neurochem 73:1422–1430
Sola C, Casal C, Tusell JM, Serratosa J (2002) Astrocytes enhance lipopolysaccharide-induced nitric oxide production by microglial cells. Eur J Neurosci 16:1275–1283
Walker AG, Chapman J, Bruce CB, Rumsby MG (1984) Immunocytochemical characterisation of cell cultures grown from dissociated 1-2-day post-natal rat cerebral tissue. A developmental study. J Neuroimmunol 7:1–20
McCarthy KD, de Vellis J (1980) Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 85:890–902
Richardson A, Hao C, Fedoroff S (1993) Microglia progenitor cells: a subpopulation in cultures of mouse neopallial astroglia. Glia 7:25–33
Knorpp T, Robinson SR, Crack PJ, Dringen R (2006) Glutathione peroxidase-1 contributes to the protection of glutamine synthetase in astrocytes during oxidative stress. J Neural Transm 113:1145–1155
Skytt DM, Madsen KK, Pajecka K, Schousboe A, Waagepetersen HS (2010) Characterization of primary and secondary cultures of astrocytes prepared from mouse cerebral cortex. Neurochem Res 35:2043–2052
Oberheim NA, Goldmann SA, Nedergaard M (2012) Heterogeneity of astrocytic form and function. Methods Mol Biol 814:23–45
Zhang Y, Barres BA (2010) Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol 20:588–594
Du F, Qian ZM, Zhu L, Wu XM, Qian C, Chan R, Ke Y (2010) Purity, cell viability, expression of GFAP and bystin in astrocytes cultured by different procedures. J Cell Biochem 109:30–37
Vanella A, Avola R, Condorelli DF, Campisi A, Costa A, Guiffrida Stella AM, Perez-Polo JR (1989) Antioxidant enzymatic activities and resistance to oxidative stress in primary and subcultured rat astroglial cells. Int J Dev Neurosci 7:233–241
Codeluppi S, Gregory EN, Kjell J, Wigerblad G, Olson L, Svensson CI (2011) Influence of rat substrain and growth conditions on the characteristics of primary cultures of adult rat spinal cord astrocytes. J Neurosci Methods 197:118–127
Goetschy JF, Ulrich G, Aunis D, Ciesielski-Treska J (1986) The organization and solubility properties of intermediate filaments and microtubules of cortical astrocytes in culture. J Neurocytol 15:375–387
Sen E, Basu A, Willing LB, Uliasz TF, Myrkalo JL, Vannucci SJ, Hewett SJ, Levison SW (2011) Pre-conditioning induces the precocious differentiation of neonatal astrocytes to enhance their neuroprotective properties. ASN Neuro 3:e00062
Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H (2007) Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci 25(3):649–658
Tilleux S, Berger J, Hermans E (2007) Induction of astrogliosis by activated microglia is associated with a down-regulation of metabotropic glutamate receptor 5. J Neuroimmunol 189:23–30
Passaquin AC, Schreier WA, de Vellis J (1994) Gene expression in astrocytes is affected by subculture. Int J Dev Neurosci 12:363–372
Bolanos JP, Almeida A, Moncada S (2010) Glycolysis: a bioenergetic or a survival pathway? Trends Biochem Sci 35:145–149
Figley CR, Stroman PW (2011) The role(s) of astrocytes and astrocyte activity in neurometabolism, neurovascular coupling, and the production of functional neuroimaging signals. Eur J Neurosci 33:577–588
Hertz L, Peng L, Dienel GA (2007) Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab 27:219–249
Tulpule K, Dringen R (2012) Formate generated by cellular oxidation of formaldehyde accelerates the glycolytic flux in cultured astrocytes. Glia 60:582–593
Cruz F, Wolf A (2001) Effects of the novel cyclosporine derivative PSC833 on glucose metabolism in rat primary cultures of neuronal and glial cells. Biochem Pharmacol 62:129–139
Zwingmann C, Richter-Landsberg C, Brand A, Leibfritz D (2000) NMR spectroscopic study on the metabolic fate of [3-13C]alanine in astrocytes, neurons, and cocultures: implications for glia-neuron interactions in neurotransmitter metabolism. Glia 32:286–303
Schmidt MM, Dringen R (2010) Fumaric acid diesters deprive cultured primary astrocytes rapidly of glutathione. Neurochem Int 57:460–467
Hazell AS, Desjardins P, Butterworth RF (1999) Increased expression of glyceraldehyde-3-phosphate dehydrogenase in cultured astrocytes following exposure to manganese. Neurochem Int 35:11–17
O’Brien J, Kla KM, Hopkins IB, Malecki EA, McKenna MC (2007) Kinetic parameters and lactate dehydrogenase isozyme activities support possible lactate utilization by neurons. Neurochem Res 32:597–607
Vasquez OL, Almeida A, Bolanos JP (2001) Depletion of glutathione up-regulates mitochondrial complex I expression in glial cells. J Neurochem 76:1593–1596
Dringen R, Pfeiffer B, Hamprecht B (1999) Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci 19:562–569
Arend C, Brandmann M, Dringen R (2013) The antiretrovial protease inhibitor ritonavir accelerates glutathione export from cultured primary astrocytes. Neurochem Res 38:732–741
Scheiber IF, Dringen R (2011) Copper-treatment increases the cellular GSH content and accelerates GSH export from cultured rat astrocytes. Neurosci Lett 498:42–46
Dringen R, Gutterer JM (2002) Glutathione reductase from bovine brain. Methods Enzymol 348:281–288
Dringen R, Hoepken HH, Minich T, Ruedig C (2007) Pentose phosphate pathway and NADPH metabolism. In: Dienel G, Gibson G (eds) Handbook of neurochemistry and molecular neurobiology, 5th volume: neural energy utilization. Springer, Heidelberg, pp 41–62
Minich T, Riemer J, Schulz JB, Wielinga P, Wijnholds J, Dringen R (2006) The multidrug resistance protein 1 (Mrp1), but not Mrp5, mediates export of glutathione and glutathione disulfide from brain astrocytes. J Neurochem 97:373–384
Hirrlinger J, Schulz JB, Dringen R (2002) Glutathione release from cultured brain cells: multidrug resistance protein 1 mediates the release of GSH from rat astroglial cells. J Neurosci Res 69:318–326
Geppert M, Hohnholt MC, Nurnberger S, Dringen R (2012) Ferritin up-regulation and transient ROS production in cultured brain astrocytes after loading with iron oxide nanoparticles. Acta Biomater 8:3832–3839
Bishop GM, Dang TN, Dringen R, Robinson SR (2011) Accumulation of non-transferrin-bound iron by neurons, astrocytes, and microglia. Neurotox Res 19:443–451
Hoepken HH (2005) Untersuchungen zum Eisenstoffwechsel neuraler Zellen. Dissertation, University of Tuebingen, Germany
Thorburne SK, Juurlink BH (1996) Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem 67:1014–1022
Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR (2009) Oligodendrocytes and myelination: the role of iron. Glia 57:467–478
Urrutia P, Aguirre P, Esparza A, Tapia V, Mena NP, Arredondo M, Gonzalez-Billault C, Nunez MT (2013) Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem 126:541–549
Lamkowsky MC, Geppert M, Schmidt MM, Dringen R (2012) Magnetic field-induced acceleration of the accumulation of magnetic iron oxide nanoparticles by cultured brain astrocytes. J Biomed Mater Res A 100A:323–334
Jenkins SI, Pickard MR, Furness DN, Yiu HH, Chari DM (2012) Differences in magnetic particle uptake by CNS neuroglial subclasses: implications for neural tissue engineering. Nanomedicine (Lond) 8:951–968
Pinkernelle J, Calatayud P, Goya GF, Fansa H, Keilhoff G (2012) Magnetic nanoparticles in primary neural cell cultures are mainly taken up by microglia. BMC Neurosci 13:32
Luther EM, Petters C, Bulcke F, Kaltz A, Thiel K, Bickmeyer U, Dringen R (2013) Endocytotic uptake of iron oxide nanoparticles by cultured brain microglial cells. Acta Biomater 9:8454–8465
Acknowledgments
The authors would like to thank Dr. Frank Dietz (University of Bremen) for providing the MAG- and anti-sheep-antibodies and Dr. Eva M. Luther for her help with the analysis of immunocytochemical stainings.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petters, C., Dringen, R. Comparison of Primary and Secondary Rat Astrocyte Cultures Regarding Glucose and Glutathione Metabolism and the Accumulation of Iron Oxide Nanoparticles. Neurochem Res 39, 46–58 (2014). https://doi.org/10.1007/s11064-013-1189-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1189-7