Abstract
Glioblastoma, the most common and aggressive primary brain tumors, carry a bleak prognosis and often recur even after standard treatment modalities. Emerging evidence suggests that deregulation of the Wnt/β-catenin/Tcf signaling pathway contributes to glioblastoma progression. Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit tumor cell proliferation by suppressing Wnt/β-catenin/Tcf signaling in various human malignancies. In this study, we sought to inhibit Wnt/β-catenin/Tcf signaling in glioblastoma cells by the NSAIDs diclofenac and celecoxib. Both diclofenac and celecoxib significantly reduced the proliferation, colony formation and migration of human glioblastoma cells. Diclofenac and celecoxib downregulated β-catenin/Tcf reporter activity. Western and qRT-PCR analysis showed that diclofenac and celecoxib reduced the expression of β-catenin target genes Axin2, cyclin D1 and c-Myc. In addition, the cytoplasmic accumulation and nuclear translocation of β-catenin was significantly reduced following diclofenac and celecoxib treatment. Furthermore, diclofenac and celecoxib significantly increased phosphorylation of β-catenin and reduced the phosphorylation of GSK3β. These results clearly indicated that diclofenac and celecoxib are potential therapeutic agents against glioblastoma cells that act by suppressing the activation of Wnt/β-catenin/Tcf signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been widely used for prophylaxis against cardiovascular disease, injuries and to some extent to headaches, as well as in the treatment of various cancers and other anomalies, including rheumatoid arthritis [1, 2]. NSAIDs exert anti-neoplastic and other therapeutic activities through specific inhibition of prostaglandin synthesis. This inhibition occurs by inactivation of the cyclooxygenase enzymes COX-1 and COX-2 [3, 4]. Traditional NSAIDs, such as diclofenac, aspirin, indomethacin, and sulindac inhibit COX-1 and COX-2 activity, whereas new-generation drugs, for example celecoxib and rofecoxib, selectively inhibit COX-2 activity. Despite convincing evidence from clinical investigations and from animal studies that the selective and non selective COX-2 inhibitors exert anti-neoplastic properties by targeting the enzyme COX-2, the underlying molecular mechanisms remain poorly understood. This is presumably because many studies described the anti-neoplastic activity of NSAIDs in the absence of any apparent involvement of COX-2 [5]. Several reports have suggested that NSAIDs may also inhibit tumor cell growth by acting on multiple COX-independent targets [6–9]. Among these, the most important pathway affected by NSAIDs is the Wnt/β-catenin/Tcf pathway [10–15]. In the absence of stimulation, β-catenin is captured in a destruction complex composed of APC, GSK3β and Axin, and phosphorylated by GSK3β and CK1α. Phosphorylated β-catenin is ubiquitinated and further degraded in 26S proteasomes. Upon stimulation β-catenin is released into the cytosol and subsequently translocated to nucleus, where it binds to transcriptional counterparts Lef1 and Tcf4 and activates the expression of their target genes [16, 17].
Glioblastoma (GBM) are the devastating primary tumors of the central nervous system that arise from the transformation of glia or their precursors [18]. Patients presenting with GBM have a dismal prognosis despite a variety of treatment options, including surgeries, chemotherapy and radiotherapy. GBM develop as a result of the stepwise accumulation of genetic alterations that may either disrupt cell cycle arrest pathways or activate various signal transduction pathways [19]. Recently, we reported on the deregulation of the Wnt/β-catenin/Tcf signaling pathway in both human [20, 21] and ENU-induced rat gliomas [22] and positively correlated pathway activation with histological malignancy. Several recent reports have also provided evidence that the Wnt/β-catenin/Tcf signaling pathway is aberrantly activated in gliomas and that knockdown of this pathway inhibits cell proliferation and induces apoptosis in glioma cell lines [23].
In this study, we examined the efficacy of two NSAIDs, diclofenac and celecoxib, on the Wnt/β-catenin/Tcf pathway in human GBM cells. Our results provide the evidence that diclofenac and celecoxib suppression of GBM cell growth parallels inhibition of Wnt/β-catenin/Tcf pathway activation.
Materials and Methods
Cell Culture and Reagents
Human glioblastoma cell line U87 was purchased from the National Center for Cell Science (Pune, India) and U251 was obtained from American Type Culture Collection (ATCC). Cells were grown in DMEM medium supplemented with 10 % fetal bovine serum and antibiotics (100 IU/ml penicillin, 100 μg/ml streptomycin) and were maintained in a humidified atmosphere containing 5 % CO2 at 37 °C. Diclofenac Sodium was obtained from Sigma Chemicals (St. Louis, USA), and celecoxib was kindly provided by Prof. P. Reddanna (University of Hyderabad, Hyderabad, India). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA). Luciferase assay system was obtained from Promega (Madison, WI). The pRL-TK plasmid was obtained from Upstate Biotech (Lake Placid, NY). Primary antibodies against β-catenin, phospho-β-catenin (Thr41, Ser45), c-Myc, cyclin D1, GSK3β, phospho-GSK3β, GAPDH, Lamin B and β-actin were purchased from Cell Signaling Technology (Beverly, MA). Secondary antibodies (anti-rabbit and anti-mouse IgG conjugated to HRP) were obtained from Sigma Chemical Co (St. Louis, MO). The Tcf-responsive reporter constructs pTOPFLASH and pFOPFLASH were kindly provided by H. Clevers (University of Utrecht, The Netherlands).
Cell Proliferation and Clonogenic Assays
Inhibition of cell proliferation was assessed by the reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) to formazan. U87 and U251 cells were seeded (2 × 103 cells/well) in 96-well plates. After overnight incubation, cells were treated with different concentrations of diclofenac and celecoxib or vehicle (0.1 % DMSO) alone for 24 h. Next, MTT was added to each well and incubated for 4 h. Finally, formazan crystals were solubilized in acidic isopropanol (0.04 N), and absorbance at 540 nm was measured on a multi-well microplate reader. Clonogenic assays were performed by plating 500 cells in a 100-mm dish and treating with diclofenac and celecoxib or vehicle (0.1 % DMSO) for 24 h. Cells were then grown for 8 days and stained with 0.5 % methylene blue in 50 % methanol. Colonies that contain ≥50 cells were counted. All treatments were performed in triplicate, and the results were expressed as mean ± SEM.
Cell Migration Assay
The cell migration was determined using QCM chemotaxis cell migration assay kit (Millipore) using the manufacturer’s protocol. Briefly, 1 × 105 cells of control, diclofenac, and celecoxib treated U87 cells per 300 μl of serum-free medium were seeded onto the upper chamber and 0.75 ml of complete growth medium containing 10 % FBS was added to each well in the lower chamber. At the end of the incubation period, nonmigrating cells on the inside of the filter were removed carefully with a cotton swab, and subjected to staining and absorbance was measured on a multi-well microplate reader.
Reporter Gene Assays
Reporter gene assays were performed as described [24]. Briefly, U87 and U251 GBM cells were seeded in 24-well plates and transiently transfected with pTOPFLASH, or pFOPFLASH along with pRL-TK using Lipofectamine. pRL-TK was used as a control to normalize transfection efficiency. After 6 h of transfection, cells were treated with diclofenac and celecoxib or vehicle (0.1 % DMSO) for 24 h and then stimulated with Wnt3a for additional 12 h. Cells were then washed with PBS and lysed with Passive Lysis Buffer, after which Dual-Luciferase reporter assays were performed according to the manufacturer’s instructions.
Quantitative RT-PCR Analysis
Total RNA was isolated from U87 and U251 glioblastoma cells using Trizol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Reverse transcription (RT) reactions were performed by using the Superscript III reverse transcriptase reagent kit (Invitrogen, Carlsbad, CA). Real-time PCR was done by using specific real-time PCR primers for following genes: Axin2: (F) TTATGCTTTGCACTACGTCCCTCCA and (R) CGCAACATGGTCAACCCTCAGAC; Cyclin D1: (F) TCCAGAGTGATCAAGTGTGA and (R) GATGTCCACGTCCCGCACGT; and β-Actin: (F) GTGGGCATGGGTCAGAAG and (R) TCCATCACGATGCCAGTG. Results were normalized to the β-actin transcript levels and the difference in fold expression was calculated by using delta–delta-CT method.
Preparation of Cytosolic and Nuclear Extracts
Cytosolic and nuclear fractions were performed as reported previously [25]. Briefly, U87 and U251 cells were pretreated with different concentrations of diclofenac and celecoxib or vehicle (0.1 % DMSO) and then harvested and washed in ice-cold PBS, lysed in 400 μl cold buffer A (HEPES 10 mmol/l (pH 7.9), KCl 10 mmol/l, 1 mmol/l EDTA, phenylmethanesulfonyl fluoride (PMSF) 1 mmol/l, 1 mmol/l EGTA, dithiothreitol (DTT) 1 mmol/l, aprotinin 1 mg/l, leupeptin 1 mg/l, and pepstatin A 1 mg/l). After 15 min incubation on ice, 0.1 % NP-40 was added to the lysates and the tubes were vigorously rocked for 1 min and centrifuged (20,800g, 5 min) at 4 °C. The supernatant was collected as cytosolic fraction. Nuclear pellets were then washed with cold buffer A, then resuspended in 50 μl cold buffer B (HEPES 20 mmol/l (pH 7.9), NaCl 420 mmol/l, edetic acid 0.1 mmol/l, egtazic acid 0.1 mmol/l, PMSF 1 mmol/l, DTT 1 mmol/l, aprotinin 1 mg/l, leupeptin 1 mg/l and pepstatin A 1 mg/l) and vigorously rocked for 30 min at 4 °C followed by centrifugation at 20,800g for 5 min and the supernatant was collected as the nuclear fraction.
Western Blotting
U87 and U251 GBM cells were seeded in 100 mm culture dishes and after treatment with diclofenac and celecoxib or vehicle (0.1 % DMSO) cells were lysed in modified RIPA buffer (150 mM NaCl, 50 mM Tris–HCl, 50 mM NaF, 5 mM EDTA, 0.5 % [wt/vol] sodium deoxycholate and 1 % Triton X-100) containing phosphatase and protease inhibitors. Lysates were run on SDS-PAGE followed by Western blotting using indicated antibodies and immunoreactivity was visualized by using ECL kit.
Immunocytochemistry
U87 and U251 cells were seeded onto glass cover slips in 12-well plates and treated with diclofenac and celecoxib or vehicle (0.1 % DMSO) alone for 24 h. Cells were then washed with PBS and fixed with 4 % paraformaldehyde, followed by permeabilization with 0.2 % Triton X-100. After blocking with 5 % goat serum for 1 h, cells were incubated with β-catenin (1:200) primary antibody for 1 h at room temperature. After 3 washes with PBS, cells were incubated with anti-FITC conjugated secondary antibodies (Invitrogen, Carlsbad, CA) for 1 h at room temperature. Cells were washed in PBS and cover slips were mounted with VECTASHIELD mounting medium (Vector Labs, CA, USA). Fluorescent images were captured with a Leica confocal microscope.
Statistical Analysis
SPSS software was used for all statistical analyses. A Student’s t test was used to assess statistical differences between control and treatment groups. The level of significance was set at p < 0.05. Statistical differences among groups were analyzed with ANOVA.
Results
Diclofenac and Celecoxib Inhibit the Proliferation, Colony Formation and Migration of GBM Cells
To determine the effect of diclofenac and celecoxib on proliferation and colony formation, MTT assay and clonogenic assays were performed respectively. The MTT assay showed that both diclofenac and celecoxib significantly reduced the proliferation of U87 and U251 GBM cells in a dose-dependent manner (Fig. 1a). In clonogenic assays, compared to vehicle (0.1 % DMSO) treated cells the number of the colonies formed was significantly (p < 0.05) reduced following treatment of the cells with diclofenac and celecoxib (Fig. 1b). Further, cell migration assays showed that the diclofenac and celecoxib significantly reduced the migration of U87 GBM cells (Fig. 1c, d). Taken together, these results clearly demonstrated that both diclofenac and celecoxib exhibited anti-neoplastic activities on GBM cells.
Effect of diclofenac and celecoxib on proliferation, colony formation and migration of GBM cells. a U87 and U251 GBM cells were treated with vehicle (0.1 % DMSO) or indicated concentrations of diclofenac and celecoxib for 24 h and subjected to an MTT assay. b A total of 500 cells were plated in 100-mm culture dishes and treated with vehicle (0.1 % DMSO) or diclofenac and celecoxib for 24 h. After 8 days, colonies were stained with methylene blue and those colonies containing ≥50 cells were counted. c, d U87 cells were treated with vehicle, or diclofenac and celecoxib for 12 h and subjected to QCM cell migration assay as described in “materials and methods” section. All data presented are the mean ± SEM and are representative of three independent experiments. ‘0’ denotes the vehicle treated control, p < 0.05, t test
Diclofenac and Celecoxib Inhibit Wnt/β-Catenin/Tcf Reporter Activation
The activity of Wnt signaling is mediated by the binding of the β-catenin/Tcf complex to its specific promoter elements. To investigate whether administration of diclofenac and celecoxib downregulated β-catenin/Tcf signaling, we used a set of reporter constructs which contained either three copies of the optimal Tcf motif (CCTTTGATC) or three copies of the mutant motif (CCTTTGGCC), both of which were upstream of a minimal c-Fos promoter driving luciferase expression (pTOPFLASH and pFOPFLASH respectively). Reporter assays revealed that both diclofenac and celecoxib downregulated β-catenin/Tcf signaling in a dose-dependent manner in U87 (Fig. 2a) and U251 GBM cells (Fig. 2b). To exclude any nonspecific effects of diclofenac and celecoxib on gene expression, total pTOPFLASH activity is corrected by subtracting any effect of the drugs on pFOPFLASH activity, where a mutation renders Tcf binding sites inactive.
Diclofenac and celecoxib attenuated Tcf reporter activity. a, b U87 and U251 GBM cells were transfected with TOPFLASH or FOPFLASH reporter plasmids together with pRL-TK plasmid. Following a 6 h transfection, cells were treated for 24 h with vehicle or indicated concentrations of diclofenac and celecoxib and followed by Wnt3a stimulation for 12 h and luciferase values were measured in luminometer. TOPFLASH and FOPFLASH activities were corrected for transfection efficiency using the pRL-TK activity. All data presented are the mean ± SEM and are representative of three independent experiments. ‘0’ denotes the vehicle treated control. *p < 0.05, t test
Diclofenac and Celecoxib Downregulated the Expression of β-Catenin Target Genes
To confirm the β-catenin response transcription (CRT) inhibition data obtained from the luciferase assays, we determined if diclofenac and celecoxib affect the expression of Axin2, cyclin D1 and c-Myc a known targets of β-catenin signaling. qRT-PCR analysis demonstrated that both diclofenac and celecoxib significantly reduced the expression of Axin2 and cyclin D1 in U87 (Fig. 3a) and U251 GBM cells (Fig. 3b). Further, western blot analysis showed that both diclofenac and celecoxib significantly decreased the protein levels of cyclin D1 and c-Myc in U87 and U251 GBM cells (Fig. 3c). This result implies that both diclofenac and celecoxib reduced β-catenin/Tcf signaling in GBM cells.
Effect of diclofenac and celecoxib on β-catenin target genes. Total RNA was isolated from vehicle-, diclofenac- or celecoxib-treated U87 (a) and U251 (b) cells and subjected to RT-qPCR using the primers specific for Axin2 and cyclin D1. All data presented are the mean ± SEM. *p < 0.05, t test. c Whole cell lysates were isolated from vehicle-, diclofenac- or celecoxib-treated U87 and U251 cells and subjected Western blot analysis with cyclin D1 and c-Myc antibodies. β-actin used as an internal control. ‘0’ denotes the vehicle-treated control. Representative blots were chosen from among the three individual experiments
Diclofenac and Celecoxib Attenuated the Nuclear Translocation of β-Catenin
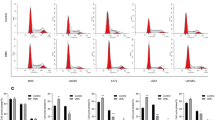
To confirm whether the inhibition of β-catenin signaling by diclofenac and celecoxib results from an alteration in the localization β-catenin, cytoplasmic and nuclear extracts were prepared from U87 and U251 GBM cells and subjected to western analysis. Compared to vehicle-treated cells, the cytoplasmic accumulation of β-catenin was significantly reduced following celecoxib treatment in both U87 and U251 GBM cells (Fig. 4a). However, no substantial decrease in cytoplasmic β-catenin was observed in diclofenac treated cells (Fig. 4a). Further, the levels of nuclear β-catenin were significantly reduced in both diclofenac and celecoxib treated U87 and U251 cells (Fig. 4b). To further confirm whether diclofenac and celecoxib alter the subcellular localization of β-catenin immunocytochemical analyses were performed. In vehicle-treated cells, β-catenin was localized both in the cytosol and the nucleus. However, diclofenac and celecoxib treatment significantly reduced the localization of nuclear β-catenin (Fig. 4c). These results clearly demonstrated that diclofenac and celecoxib downregulated β-catenin/Tcf signaling by inhibiting the nuclear translocation of β-catenin.
Effect of diclofenac and celecoxib on cytosolic accumulation and nuclear translocation of β-catenin. U87 and U251 cells were seeded in 100-mm dishes and treated with vehicle (0.1 % DMSO) or different concentrations of diclofenac and celecoxib for 24 h. a Cytoplasmic and b nuclear extracts were prepared from U87 and U251 cells respectively, and subjected to western blotting with β-catenin. GAPDH and Lamin B were used as loading controls for cytoplasmic and nuclear fractions, respectively. The blots were representatives of three independent experiments. ‘0’ denotes the vehicle-treated control. c U87 and U251 cells were treated with vehicle (0.1 % DMSO), diclofenac or celecoxib for 24 h and subjected to immunocytochemical analysis as described in the “materials and methods” section. Fluorescent images were captured with a Leica confocal microscope
Diclofenac and Celecoxib Increased the S/T Phosphorylation of β-Catenin and Reduced the Phosphorylation of GSK3β
Several previous reports have demonstrated that NSAIDs downregulated β-catenin/Tcf signaling by selectively increasing levels of phosphorylated β-catenin [14, 26]. To further understand the mechanism underlying the downregulation of β-catenin reporter activity, we investigated whether reduced β-catenin/Tcf4 activity was a result of an increase in β-catenin phosphorylation. Phosphorylated β-catenin (Thr41/Ser45) levels were significantly increased in a dose-dependent manner after diclofenac and celecoxib treatment (Fig. 5). In addition we also found that phosphorylation of GSK3β which is phosphorylated and inactivated by PI3K/Akt signaling was significantly reduced following diclofenac and celecoxib treatment (Fig. 5). These results suggest that diclofenac and celecoxib reduced the β-catenin/Tcf4 signaling activity by increasing the phosphorylation of β-catenin and reducing the GSK3β phosphorylation.
Effect of diclofenac and celecoxib on phosphorylation of β-catenin and GSK3β. Cell lysates of U87 and U251 cells treated with vehicle (0.1 % DMSO) or different concentrations of diclofenac and celecoxib were subjected to western blot analysis against phospho-β-catenin (Thr41/Ser45) and phospho-GSK3β. ‘0’ denotes the vehicle-treated control and β-actin used as loading control. Representative blots were chosen from among the three individual experiments
Discussion
Chemopreventive effects of NSAIDs are mainly mediated through COX-2 inhibition. However, prior studies indicate that alternative targets are also implicated in the tumor suppressive effects of NSAIDs, including Bcl-2, NF-κB, PPARγ and NAG-1 suggesting that a number of distinct molecular pathways may play a role in the anti-tumor effects of these drugs. The present study demonstrate that the Wnt/β-catenin/Tcf signaling pathway is attenuated by NSAIDs diclofenac and celecoxib and suppression of GBM cell growth is accomplished partly through the inhibition of Wnt/β-catenin/Tcf pathway activation.
NSAIDs have been proven to possess significant anti-proliferative potential in various cancer cells in vitro and in vivo. Increasing evidences suggest that long-term low dose intake of NSAIDs may prevent certain types of cancers [27, 28]. Although observational study results are also conflicting with regard to cancer risks, many studies consistently demonstrate that NSAIDs use is associated with a reduced risk of colon cancer. Most importantly, recent case–control studies suggested an inverse relationship between NSAIDs use and glioma risk [29–31]. To shed some light on the potential mechanisms involved in the NSAID mediated anti-proliferative effects in GBM we examined the alteration in the Wnt/β-catenin pathway following diclofenac and celecoxib treatment. Diclofenac, one of the oldest NSAIDs inhibits cyclooxygenases non-selectively. Diclofenac reduces 94 % of COX-2 and 49 % of COX-1 activity [32] in humans and reduces the risk of squamous cell carcinoma and malignant melanoma [33]. Recent studies have shown that diclofenac reduces lactate production in GL261 murine glioma cells both in vitro and in vivo [34] in a Cox-independent manner. Further, diclofenac used at nontoxic concentrations significantly decreased lactate production in glioma cells and resulted in a significant delay of glioma growth. Using of NSAIDs in combination with additional chemotherapeutic compounds may increase the susceptibility to conventional cytotoxic drugs. Zerbini et al. [35], demonstrated that that combination of diclofenac and sulindac sulfide with NF-κB inhibitors led to enhanced apoptosis induction in ovarian cancer. Celecoxib combined with radiation plays a critical role in the suppression of growth of CD133positive glioblastoma stem-like cells and proven that radiosensitizing drug for clinical application in glioblastoma [36]. Even though celecoxib is a selective COX-2 inhibitor, accumulating evidence suggests that it exhibits anti-neoplastic activity in tumor cells that lack the COX-2 enzyme [37, 38]. Several researchers are currently conducting phase II clinical studies to determine the efficacy of celecoxib alone or combined with other drugs in the treatment of glioblastoma. It has been demonstrated that celecoxib aggravates ER stress in GBM in combination with bortezomib resulting in the killing of tumor cells [39]. Also, combined use of celecoxib and temozolomide in a rat orthotopic glioma model proven that these drugs are effective in treating gliomas [40]. It has recently been reported that anti-tumor activity is enhanced by a low-dose combination of the recombinant urokinase kringle domain and celecoxib in glioma models and that this combined treatment resulted in more potent inhibition of tumor growth than each monotherapy [41]. Most importantly, celecoxib could be safely administered to glioblastoma patients who received cranial irradiation, temozolomide, and dexamethasone for peritumoral brain edema [42]. These studies have stimulated the use of diclofenac and celecoxib and provide a desirable strategy for anti-glioma therapy. Although clinical trials on NSAIDs as a chemotherapeutic agent against glioblastoma are increasing, the underlying molecular mechanisms by which diclofenac and celecoxib exerts its antineoplastic activity are not completely understood. In this study, first we set out to investigate whether diclofenac and celecoxib has significant anti-proliferative effects on glioblastoma cell lines. MTT assay and clonogenic assays revealed that diclofenac and celecoxib have significant anti-proliferative effects on U87 and U251 GBM cell lines. Further, cell migration assays demonstrated that diclofenac and celecoxib reduced the migration of GBM cells.
The Wnt/β-catenin/Tcf signaling pathway is constitutively activated and contributed to the malignant progression of glioma. Several reports have demonstrated that a link exists between the chemopreventive effects of NSAIDs and β-catenin/Tcf4 activity in colon cancer cells [6, 12]. However, the effect of NSAIDs diclofenac and celecoxib on Wnt/β-catenin signaling in glioblastoma cells has not been studied. Because inhibition of Wnt/β-catenin activation has been linked with antitumor activity, we hypothesize that diclofenac and celecoxib mediates its effects at least partly through inhibition of Wnt/β-catenin activation. We therefore focused on β-catenin, a major regulator of Tcf-responsive transcription, as a potential target of diclofenac and celecoxib action. We first used a Tcf reporter assay to assess the effects of diclofenac and celecoxib on the activity of the pathway as a whole, and then checked the inhibitory effects of diclofenac and celecoxib on the expression of β-catenin targets. We observed a reduction of both β-catenin/Tcf reporter activity and their targets Axin-2, c-Myc and cyclin D1 that occurred in a concentration-dependent manner. In addition both the drugs inhibited the cytosolic accumulation and nuclear translocation of β-catenin in GBM cells and also enhanced the phosphorylation of β-catenin. In summary, we have demonstrated that the NSAIDs diclofenac and celecoxib exhibits anti-proliferative effects in GBM cells partly by suppressing Wnt/β-catenin/Tcf pathway activation. These findings might provide the rationale for initiating in vivo studies to examine the efficacy of NSAIDs as chemopreventive agents against glioblastoma and explain some features of NSAID-mediated chemoprevention.
References
Cha YI, DuBois RN (2007) NSAIDs and cancer prevention: targets downstream of COX-2. Annu Rev Med 58:239–252
Ulrich CM, Bigler J, Potter JD (2006) Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer 6:130–140
Thun MJ, Henley SJ, Patrono C (2002) Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst 94:252–266
Howe LR, Dannenberg AJ (2002) A role for cyclooxygenase-2 inhibitors in the prevention and treatment of cancer. Semin Oncol 29:111–119
Gupta RA, Dubois RN (1998) Aspirin, NSAIDS, and colon cancer prevention: mechanisms? Gastroenterology 114:1095–1098
Smith ML, Hawcroft G, Hull MA (2000) The effect of non-steroidal anti-inflammatory drugs on human colorectal cancer cells: evidence of different mechanisms of action. Eur J Cancer 36:664–674
Tegeder I, Pfeilschifter J, Geisslinger G (2001) Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J 15:2057–2072
Singh R, Cadeddu RP, Fröbel J, Wilk CM, Bruns I, Zerbini LF, Prenzel T, Hartwig S, Brunnert D, Schroeder T, Lehr S, Haas R, Czibere A (2011) The non-steroidal anti-inflammatory drugs Sulindac sulfide and Diclofenac induce apoptosis and differentiation in human acute myeloid leukemia cells through an AP-1 dependent pathway. Apoptosis 16:889–901
Mayorek N, Naftali-Shani N, Grunewald M (2010) Diclofenac inhibits tumor growth in a murine model of pancreatic cancer by modulation of VEGF levels and arginase activity. PLoS ONE 5(9):e12715
Xia JJ, Pei LB, Zhuang JP, Ji Y, Xu GP, Zhang ZP, Li N, Yan JL (2010) Celecoxib inhibits β-catenin-dependent survival of the human osteosarcoma MG-63 cell line. J Int Med Res 38:1294–1304
McDonald SL, Silver AR (2011) On target? Strategies and progress in the development of therapies for colorectal cancer targeted against WNT signalling. Colorectal Dis 13:360–369
Dihlmann S, Siermann A, von Knebel Doeberitz M (2001) The nonsteroidal anti-inflammatory drugs aspirin and indomethacin attenuate β-catenin/Tcf4 signaling. Oncogene 20:645–653
Gardner SH, Hawcroft G, Hull MA (2004) Effect of nonsteroidal anti-inflammatory drugs on β-catenin protein levels and catenin-related transcription in human colorectal cancer cells. Br J Cancer 91:153–163
Bos CL, Kodach LL, van den Brink GR, Diks SH, van Santen MM, Richel DJ, Peppelenbosch MP, Hardwick JCH (2006) Effect of aspirin on the Wnt/beta-catenin pathway is mediated via protein phosphatase 2A. Oncogene 25:6447–6456
Boon EMJ, Keller JJ, Wormhoudt TAM, Giardiello FM, Offerhaus GJA, van der Neut R, Pals ST (2004) Sulindac targets nuclear beta-catenin accumulation and Wnt signaling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. Br J Cancer 90:224–229
Yao H, Ashihara E, Maekawa T (2011) Targeting the Wnt/β-catenin signaling pathway in human cancers. Expert Opin Ther Targets 15:873–887
Saito-Diaz K, Chen TW, Wang X, Thorne CA, Wallace HA, Page-McCaw A, Lee E (2013) The way Wnt works: components and mechanism. Growth Factors 31:1–31
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432:396–401
Ohgaki H, Kleihues P (2009) Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci 100:2235–2241
Sareddy GR, Panigrahi M, Challa S, Mahadevan A, Babu PP (2009a) Activation of Wnt/beta-catenin/Tcf signaling pathway in human astrocytomas. Neurochem Int 55:307–317
Sareddy GR, Geeviman K, Panigrahi M, Challa S, Mahadevan A, Babu PP (2012) Increased β-catenin/Tcf signaling in pilocytic astrocytomas: a comparative study to distinguish pilocytic astrocytomas from low-grade diffuse astrocytomas. Neurochem Res 37:96–104
Sareddy GR, Challa S, Panigrahi M, Babu PP (2009) Wnt/beta-catenin/Tcf signaling pathway activation in malignant progression of rat gliomas induced by transplacental N-ethyl-N-nitrosourea exposure. Neurochem Res 34:1278–1288
Pu P, Zhang Z, Kang C, Jiang R, Jia Z, Wang G, Jiang H (2009) Downregulation of Wnt2 and beta-catenin by siRNA suppresses malignant glioma cell growth. Cancer Gene Ther 16:351–361
Sareddy GR, Nair BC, Gonugunta VK, Zhang QG, Brenner A, Brann DW, Tekmal RR, Vadlamudi RK (2012) Therapeutic significance of estrogen receptor β agonists in gliomas. Mol Cancer Ther 11:1174–1182
Kesanakurti D, Sareddy GR, Babu PP, Kirti PB (2009) Mustard NPR1, a mammalian IkappaB homologue inhibits NF-kappaB activation in human GBM cell lines. Biochem Biophys Res Commun 390:427–433
Dihlmann S, Klein S, Doeberitz Mv MK (2003) Reduction of beta-catenin/T-cell transcription factor signaling by aspirin and indomethacin is caused by an increased stabilization of phosphorylated beta-catenin. Mol Cancer Ther 2:509–516
Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW (2011) Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377:31–41
Wu CY, Wu MS, Kuo KN, Wang CB, Chen YJ, Lin JT (2010) Effective reduction of gastric cancer risk with regular use of nonsteroidal anti-inflammatory drugs in Helicobacter pylori-infected patients. J Clin Oncol 28:2952–2957
Ferris JS, McCoy L, Neugut AI, Wrensch M, Lai R (2012) HMG CoA reductase inhibitors, NSAIDs and risk of glioma. Int J Cancer 131:E1031–E1037
Scheurer ME, El-Zein R, Thompson PA, Aldape KD, Levin VA, Gilbert MR, Weinberg JS, Bondy ML (2008) Long-term anti-inflammatory and antihistamine medication use and adult glioma risk. Cancer Epidemiol Biomarkers Prev 17:1277–1281
Sivak-Sears NR, Schwartzbaum JA, Miike R, Moghadassi M, Wrensch M (2004) Case-control study of use of nonsteroidal anti-inflammatory drugs and glioblastoma multiforme. Am J Epidemiol 159:1131–1139
Van Hecken A, Schwartz JI, Depre M, De Lepeleire I, Dallob A, Tanaka W, Wynants K, Buntinx A, Arnout J, Wong PH, Ebel DL, Gertz BJ, De Schepper PJ (2000) Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteers. J Clin Pharmacol 40:1109–1120
Johannesdottir SA, Chang ET, Mehnert F, Schmidt M, Olesen AB, Sørensen HT (2012) Nonsteroidal anti-inflammatory drugs and the risk of skin cancer: a population-based case-control study. Cancer 118:4768–4776
Chirasani SR, Leukel P, Gottfried E, Hochrein J, Stadler K, Neumann B, Oefner PJ, Gronwald W, Bogdahn U, Hau P, Kreutz M, Grauer OM (2013) Diclofenac inhibits lactate formation and efficiently counteracts local immune suppression in a murine glioma model. Int J Cancer 132:843–853
Zerbini LF, Tamura RE, Correa RG, Czibere A, Cordeiro J, Bhasin M, Simabuco FM, Wang Y, Gu X, Li L, Sarkar D, Zhou JR, Fisher PB, Libermann TA (2011) Combinatorial effect of non-steroidal anti-inflammatory drugs and NF-κB inhibitors in ovarian cancer therapy. PLoS ONE 6:e24285
Ma HI, Chiou SH, Hueng DY, Tai LK, Huang PI, Kao CL, Chen YW, Sytwu HK (2011) Celecoxib and radioresistant glioblastoma-derived CD133 + cells: improvement in radiotherapeutic effects. Laboratory investigation. J Neurosurg 114:651–662
Grosch S, Maier TJ, Schiffmann S, Geisslinger G (2006) Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst 98:736–747
Crane CH, Mason K, Janjan NA, Milas L (2003) Initial experience combining cyclooxygenase-2 inhibition with chemoradiation for locally advanced pancreatic cancer. Am J Clin Oncol 26:S81–S84
Kardosh A, Golden EB, Pyrko P, Uddin J, Hofman FM, Chen TC, Louie SG, Petasis NA, Schonthal AH (2008) Aggravated endoplasmic reticulum stress as a basis for enhanced glioblastoma by bortezomib in combination with celecoxib or its non-coxib analogue, 2, 5-dimethyl-celecoxib. Cancer Res 68:843–851
Kang SG, Kim JS, Park K, Kim JS, Groves MD, Nam DH (2006) Combination celecoxib and temozolomide in C6 rat glioma orthotopic model. Oncol Rep 15:7–13
Kim CK, Joe YA, Lee SK, Kim EKOE, Kim HK, Oh BJ, Hong SH, Hong YK (2010) Enhancement of anti-tumor activity by low dose combination of the recombinant urokinase kringle domain and celecoxib in a glioma model. Cancer Lett 288:251–260
Grossman SA, Oslon J, Batchelor T, Peereboom D, Lesser G, Desideri S, Ye X, Hammour T, Supko JG (2008) Effect of phenytoin on celecoxib pharmacokinetics in patients with glioblastoma. Neuro Oncol 10:190–198
Acknowledgments
The authors acknowledge the financial support of CSIR (for providing fellowship to GRS), DBT, ICMR, DST, Government of India, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sareddy, G.R., Kesanakurti, D., Kirti, P.B. et al. Nonsteroidal Anti-inflammatory Drugs Diclofenac and Celecoxib Attenuates Wnt/β-Catenin/Tcf Signaling Pathway in Human Glioblastoma Cells. Neurochem Res 38, 2313–2322 (2013). https://doi.org/10.1007/s11064-013-1142-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1142-9