Abstract
The aim of this study was to investigate the neuroprotective effects of (2S)-5, 2′, 5′-trihydroxy-7-methoxyflavanone (TMF), a natural product from Abacopteris penangiana (Hook.) Ching, in oxidative stress-induced neurodegeneration models in vitro and in vivo. In PC12 cells, preincubation of TMF (3–20 μM) for 24 h decreased the dopamine-induced toxicity and attenuated the redox imbalance in PC12 cells through regulating the ratio of reduced glutathione/oxidized glutathione (GSH/GSSG), which is a sensitive marker of oxidative stress. Additionally, long-term intraperitoneal (i.p.) injection of TMF (4 or 8 mg/kg/day) for 2 weeks significantly improved the behavioral performance of d-galactose (d-gal) treated mice in a Morris water maze test. Biochemical analysis revealed that TMF inhibited the activation of AP-1 (activator protein-1) and upregulated the level of BDNF (brain derived neurophic factor) as well as the ratio of GSH/GSSG in the hippocampus of d-gal treated mice. Furthermore, western blotting analysis indicated that TMF increased phosphorylation of cAMP-response element-binding protein (CREB). Therefore, the natural product TMF possessed a potential for the treatment of neurodegenerative diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the progress of aging of population, high incidence of Parkinson’s disease (PD) and Alzheimer’s disease (AD) has attracted researchers’ attention. Studies have revealed that oxidative stress plays an important role in the pathogenesis of PD and AD, proved by the decreased levels of reduced glutathione (GSH), elevated levels of oxidized glutathione (GSSG) and increased production of reactive oxygen species (ROS) detected in PD and AD patients and animal models [1, 2].
PD is characterized by a progressive degeneration of dopaminergic neurons in the substantia nigra. Previous studies have proved that PD has an association with dopamine toxicity and antioxidant system [2]. Dopamine is thought to be a major source of oxidative stress in dopaminergic neuron [3]. Increased turnover of dopamine and a consequent dopamine oxidation have been suggested to cause neurodegeneration [4]. The enzymatic or nonenzymatic oxidation of dopamine liberates free radicals and quinone, which cause alteration of the mitochondrial membrane permeability, leading to neuronal cell death [5–7]. d-galactose (d-gal) is a reducing sugar and can be metabolized at normal concentration. At high levels, reversely, it reacts with the free amines of amino acids in proteins and peptides in vivo to form advanced glycation end products, which induce ROS production and lead to neuronal death in a variety of age-related neurodegenerative disorders including AD [8–10].
In the past decades, many flavonoids have received a great attention owing to their biological properties, including antioxidative, radical scavenging, immunoregulative and anti-inflammatory effects [11, 12]. More interestingly, evidences have suggested that some flavonoids are highly effective in reversing age-related declines in neuro-cognitive performance through their ability to interact with the cellular and molecular architecture of the brain responsible for memory and by reducing neuronal loss due to neurodegenerative processes [13].
(2S)-5, 2′, 5′-trihydroxy-7-methoxyflavanone (TMF, Fig. 1) is a natural product isolated from Abacopteris penangiana (Hook.) Ching, which is a folk medicine plant commonly used by Tujia nationality in China [14, 15]. A series of comparative pharmacological studies among derivatives of TMF have suggested that the rare 2, 5-substituted B ring of TMF is responsible for its powerful active oxygen radicals scavenging activity and protective effects on primary cultured hepatocytes against lipid peroxidation [16–18].
The purpose of this study was to investigate the neuroprotective effects of TMF in oxidative stress-induced neurodegeneration models in vitro and in vivo. Furthermore, the underlying mechanisms involved in the neuroprotective effects of TMF were explored.
Materials and Methods
Chemicals and Reagents
Dopamine, d-gal, N-acetyl-l-cysteine (NAC), 3-(4, 5-Dimethylthiazol)-2, 5-diphenyltetrazolium-bromid (MTT) and nerve growth factor (NGF) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum and horse serum were purchased from Gibco BRL (Gaithersburg, MD, USA). The antibodies used for western blot were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). All solvents and chemicals used in the study were of analytical grade and purchased from Sinopharm chemical Reagent Co. Ltd. (Shanghai, China).
Plant Material and Isolation of TMF
Dried rhizomes of A. penangiana were collected in June 2009 from Jiujiang, Jiangxi province, China and authenticated by Prof. Ce-Ming Tan, Jiujiang Forest Plants Specimen Mansion. The voucher specimen (PZX0311) has been deposited in School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology (HUST).
Dried rhizomes of A. penangiana (500 g) were extracted with 80 % ethanol (1:6, w/v). The solvent was evaporated under reduced pressure to yield the ethanol extract (90 g). The extract (15 g) was fractionated by macroporous resins HPD500 (Bonherb Technology Company, Hebei, China; EtOH–H2O, 20: 80, 40: 60, 60: 40, 80: 20, each 3,000 ml) to give fractions A (2.0 g), B (5.9 g), C (2.8 g) and D (1.5 g), respectively. Fraction D (800 mg) was repurified by silica gel column chromatography (300–400 mesh, Qingdao Marine Chemical Company, China) using CHCl3–MeOH (30:1) to yield fraction D1 (380 mg). Fraction D1 was subjected to Sephadex LH-20 (Fluka BioChemika, Switzerland) using CHCl3–MeOH (1:1) and then further purified on a silica gel (300–400 mesh) column, eluting with CHCl3–MeOH (30:1) to give a purified compound (58 mg). The structure was identified as TMF by Nuclear Magnetic Resonance (NMR, Bruker AM-400 spectrometer) and Circular Dichroism (CD, Jasco J-810) analysis [15, 19]. Purity of the compound was more than 98 % by HPLC analysis.
Cell Culture and Drug Treatment
Differentiated PC12 cells were used in experiments in vitro. Cell differentiation was conducted according to the reported method [20]. Briefly, PC12 cells were plated in 35 mm culture dishes at a density of 2.5 × 104 cells/dish in DMEM supplemented with 10 % fetal calf serum, 6 % horse serum, 100 U/ml Penicillin, and 100 μg/ml Streptomycin and were kept at 37 °C in humidified 5 % CO2/95 % air. After 24 h incubation, culture medium was replaced with NGF (50 ng/ml) containing medium. The medium was replaced with fresh NGF containing medium every 48 h. After 8 days, cell differentiation was finished. Differentiated PC12 cells were maintained in DMEM supplemented with 10 % heat inactivated fetal calf serum, 100 U/ml Penicillin, and 100 μg/ml Streptomycin and were kept at 37 °C in humidified 5 % CO2/95 % air. The drug treatment process was as follows: PC12 cells were preincubated for a given period of time with TMF (solvent: 0.1 % DMSO in DMEM) or N-acetyl-l-cysteine (NAC, positive control; solvent: 0.1 % DMSO in DMEM), respectively; cells treated with the same solvent alone as a negative control. Consequently, PC12 cells were treated with 0.5 mM dopamine (solvent: 0.1 % DMSO in DMEM) for 12 h.
Cell Viability Measurement
Cells were plated at a density of 1 × 104/100 μl in 96-well plates and grown for 24 h before treatment. The cell viability was determined by the conventional MTT reduction assay [21]. Briefly, 20 μl MTT (5 mg/ml) was added to the culture medium. After incubation for 4 h at 37 °C, culture medium was removed and the produced blue formazan crystals were solubilized with 100 μl DMSO. The absorbance was measured at 490 nm with a microplate reader (Thermo Multiskan Ascent V 1.23, Finland).
Animals and Administration
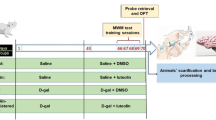
Thirty two 2-month old Kunming strain mice weighing 20–25 g were obtained from the Animal Center of Tongji Medical College, of HUST (Wuhan, China). Kunming strain mice were originated from outbred group of Swiss mice and introduced to Kunming, China, from Indian Haffkine institute in 1946 [22]. Presently, the mice are the most widely used colony in China. Kunming mice show high reproduction and survival rate, and possess strong disease resistance and resilience. Due to limited amount of TMF isolated from the plant and based on the reported researches [23–25], Kunming mice are chosen as the animal model in the present study. Prior to experiments, mice were allowed free access to food and water and were kept under conditions of constant temperature (25 ± 2 °C) and humidity (45 ± 5 %). Eight mice were housed per cage on a 12 h light/12 h dark schedule. After acclimatization to the laboratory conditions, the mice were randomly divided into four groups (eight in each group): vehicle control group, d-gal model group, TMF low dose group, and TMF high dose group. The mice in d-gal model group and two TMF-treated groups were daily subcutaneously injected with d-gal at the dose of 150 mg/kg (solvent: physiological saline) once daily for 7 weeks, while those of vehicle control group were treated with same volume of physiological saline. From the sixth week, the mice in TMF-treated groups were intraperitoneal (i.p.) injected with TMF at the dose of 4 or 8 mg/kg (solvent: physiological saline), respectively, after the injection of d-gal. At the same time, mice in the vehicle control and the d-gal model group were i.p. injected with same volume of physiological saline for 2 weeks. At the end of the treatment period, behavioral test was performed as follows. The behavioral test was conducted at the same time of the day (9:00 am–5:00 pm). All experiments were performed in compliance with the Chinese legislation on the use and care of laboratory animals and were approved by the Committee on Animal Care and Use of HUST.
Morris Water Maze Test
The Morris water maze (MWM) test was performed as previously described [24, 26]. The experimental apparatus consisted of a circular water tank (120 cm in diameter, 50 cm in height), containing water (22 ± 1 °C) to a depth of 30 cm which was rendered opaque by adding black nontoxic carbon ink, and a video tracking system for automation of behavioral experiments (Ethovision, Noldus Information Technology bv, Wageningen, The Netherland). Four poles along the perimeter of the pool conceptually divided the maze into four equal quadrants. A platform (10 cm in diameter, 29 cm in height) was submerged 1 cm below the water surface and placed at the midpoint of one quadrant. The pool was located in a test room, which contained various prominent visual cues. Each mouse received three trials per day for five consecutive days. During the test trial, the mouse was placed in the water facing the wall at one of three randomized starting positions (in three different quadrants that did not contain the platform), with each trial having a ceiling time of 60 s and a trial interval of approximately 60 s. After climbing onto the platform, the animal remained there for 30 s before the next trial. If the mouse failed to reach the escape platform within 60 s, it was gently placed on the platform and allowed to remain there for 30 s. Latency to escape from the water maze (finding the submerged escape platform) was calculated for each trial. On the sixth day, a probe test was carried out by removing platform and allowing each mouse to swim freely for 60 s. The latency of first crossing the platform (the previous platform area), the time that a mouse spent in the target quadrant (where the platform was located) and the number of crossing the non-exits (the previously platform site) were recorded for each trial. All data were recorded and analyzed by the video tracking system.
Preparation of the Tissue Samples
All mice were deeply anaesthetized by chloral hydrate and sacrificed by decapitation after the MWM test. Brains were promptly dissected on ice to obtain the hippocampus. One half hippocampus tissues were homogenized in cold physiological saline. The homogenate (10 %) was centrifuged at 4,000×g at 4 °C for 10 min, and the supernatant was collected for GSH/GSSG assay and enzyme-linked immunosorbent assay. The other half of hippocampus tissues were stored at −80 °C for the western blot analysis.
Measurement of GSH/GSSG Ratio in PC12 Cells and Hippocampal Tissue
Treated PC12 cells were washed twice with PBS (0.1 M, pH = 7.4), and then scraped from the plates into ice-cooled PBS (containing 0.05 mM EDTA) followed by homogenization on ice. The yielded homogenate was immediately centrifuged at 4 °C for 30 min at 10,000×g. The resulting supernatant and homogenate of hippocampal tissue were subjected to the commercially available colorimetric kit for GSH/GSSG assay (Jiancheng Bioengineering Institute, Nanjing, China). According to the manufacturer’s guideline, the concentrations of GSH and GSSG have been normalized with the protein content in the homogenates of PC12 cells and hippocampal tissue.
Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of AP-1 (activator protein-1) and BDNF (brain derived neurophic factor) were measured using specific ELISA kits for AP-1 and BDNF (R&D systems Inc., Minneapolis, MN, USA). According to the manufacturer’s recommendations, levels of AP-1 and BDNF have been normalized with the protein content in the homogenate of hippocampal tissue.
Western Blot Analysis
The hippocampal expressions of CREB and phospho-CREB (p-CREB) were evaluated by western blot analysis according to the reported method [24]. Briefly, the tissue samples were ground in liquid nitrogen and protein concentrations were determined using the BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA). Protein samples (50 μg) were separated by 12 % SDS–polyacrylamide gel electrophoresis and then transferred to a PVDF membrane (Roche Diagnostics Corporation, Indianapolis, IN, USA) by electrophoretic transfer (Bio-Rad Laboratories, Inc., USA). Transferred membranes were blocked for 1 h at room temperature with 5 % nonfat milk in Tris-buffered saline containing 0.1 % Tween 20 (TBST), and then incubated overnight at 4 °C with different primary antibodies (anti-CREB (1:500), anti-p-CREB (1:400)). After three washes with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies in TBST with 3 % nonfat milk for 1 h at room temperature. Immunoblots were developed on films using the enhanced chemiluminescence technique (Super Signal West Pico; Pierce Biotechnology, Rockford, IL, USA). Quantification of bands was determined by densitometric analysis using Bio-Rad Quantity One. The data were normalized using GAPDH (1:1,000) as an internal control.
Statistical Analysis
The values were presented as mean ± SEM. Differences were considered as significant at P < 0.05. Results were statistically analyzed by performing one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests using SPSS 11.5 software for Windows (SPSS Inc., Chicago, IL). Group differences in the escape latency in the MWM training task were analyzed with two-way ANOVA with repeated measures, the factors being treatment and training day.
Results and Discussion
Protective Effect of TMF on Dopamine-Treated PC12 Cells
The effect of TMF on PC12 cells viability was assessed by the MTT reduction assay. The results in Fig. 2 showed that treatment with 0.5 mM Dopamine for 12 h reduced the viability of PC12 cells. Pretreatment with TMF (3, 10, 20 μM) and NAC (N-acetyl-l-cysteine, positive control, 20 μM) for 24 h induced a significant increase on cell survival compared with only dopamine-treated group (P < 0.05).
Protective effect of TMF on dopamine-induced toxicity in PC12 cells by MTT assay. Preincubation with TMF or NAC (positive control) for 24 h protected PC12 cells from dopamine toxicity (0.5 mM, for 12 h). Data are expressed as percent cell viability from vehicle control cultures and are mean ± SEM (n = 5). * p < 0.05 and ** p < 0.01 compared with the model group (only dopamine treated)
Studies in vitro have shown that cell death induced by dopamine associates with the production of ROS in variety of cell types including PC12 cells. NAC was employed as positive control for its powerful antioxidant activity. The result suggests that TMF reverses the decreased survival rate of PC12 cells by offsetting the oxidative stress caused by dopamine.
TMF Regulated the GSH/GSSG Ratio in Dopamine-Induced Redox Imbalance of PC12 Cells
GSH is an important protein thiol which coordinates body defense system against oxidative stress. It can scavenge free radicals and other reactive free oxygen species effectively. In such reactions, GSH is oxidized to form GSSG, which is then reduced to GSH by the NADPH-dependent glutathione reductase [27].
The GSH/GSSG system is the most important redox system in cells and the ratio of GSH/GSSG can be seen as an oxidative stress marker. There is evidence to suggest that the GSH/GSSG equilibrium may have an important modulatory role on the activity of certain key signal transduction proteins and a depletion of cellular GSH was recently hypothesized as a component of the signal transduction pathway that regulates the activation of the transcription factor NF-κB and AP-1 [28].
As results showed (Fig. 3a, b), dopamine-induced PC12 cells show reduced level of GSH and increased level of GSSG, leading to lower GSH/GSSG ratio than vehicle control group (P < 0.01), suggesting that dopamine treatment caused serious redox imbalance in PC12 cells. TMF exerted its neuroprotective effect by elevating GSH level and reducing GSSG level in PC12 cells, and inducing a significant increase of GSH/GSSG ratio (10 and 20 μM, P < 0.01) with a concentration-dependent manner. These results indicated that the TMF could restore the normal intracellullar redox balance which was impaired by dopamine.
Regulative effects of TMF on GSH/GSSG ratio in dopamine-induced PC12 cells and d-gal treated mice. a Levels of GSH and GSSG in PC12 cells. b GSH/GSSG ratios in PC12 cells. c Levels of GSH and GSSG in the hippocampus of d-gal treated mice. d GSH/GSSG ratios in the hippocampus of d-gal treated mice. Data are expressed as mean ± SEM (n = 3). *, # p < 0.05 and **, ## p < 0.01 compared with the model group (only dopamine/d-gal treated)
TMF Improved Performance of d-Gal Treated Mice in the MWM Test
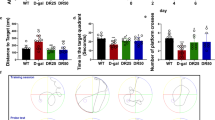
The MWM test was employed to assess the effect of TMF on impairment of spatial learning and memory. The performance of mice in all groups was improved with training by the shortened escape latencies across successive days (Fig. 4a). Tested mice displayed no difference in motor activity observed during the MWM test. The results showed significant difference in mean latency of the animal between training days [F(4, 140) = 7.260, P < 0.001] and between treatments [F(3, 140) = 5.949, P < 0.001], but no interaction between the factors day and treatment [F(12, 140) = 0.180, P > 0.05]. The d-gal treatment caused the significant cognitive impairment in the model mice, proved by the fact that the d-gal model mice had longer escape latency than mice in vehicle control group in all training days (P < 0.001). Reversely, the prolonged escape latency of mice in the d-gal model group was reduced by long-term administration of TMF in all five training days.
Effects of TMF on the spatial learning and memory of mice in the MWM test. a Mean latency in the hidden platform test during five consecutive days training. b The latency of first crossing the platform in the probe trial. c The number of crossings over the exact location of the former platform in the probe trial. d Comparison of time spent in the target quadrant in the probe trial. All values are expressed as mean ± SEM (n = 7). * P < 0.05 and ** P < 0.01 compared with the d-gal model group
In the probe trial followed by the training days, showed in Fig. 4b–d, the d-gal model group took longer latency of first platform crossing (P < 0.05), made fewer platform crossings (P < 0.01) and spent less time in target zone (P < 0.05) than the vehicle control group. Significant differences were observed between the d-gal model group and the TMF high dose group (8 mg/kg/day; P < 0.05), but there was no significant difference between the d-gal model group and the TMF low dose group (4 mg/kg/day; P > 0.05).
The above results of the MWM test suggested that long-term injection of TMF improved spatial learning and memory in d-gal treated mice.
TMF Regulated the GSH/GSSG Ratio in the Hippocampus of d-Gal Treated Mice
Shown in Fig. 3c and d, d-gal treated mice displayed a significant decrease in the GSH/GSSG ratio (P < 0.01) compared with mice in the vehicle control group, suggesting injection of d-gal caused serious imbalanced redox state in hippocampal tissues of model mice. Meanwhile, TMF administration significantly reversed the decreased GSH/GSSG ratio caused by d-gal.
As mentioned before, GSH/GSSG ratio worked as a sensitive marker of oxidative stress which influenced the activity of some key signal transduction proteins and transcription factors. Therefore, a hypothesis can be drawn that TMF may also have effect on some important cell signals dominated by the glutathione redox system.
The Effects of TMF on the Levels of AP-1, BDNF and p-CREB in the Hippocampus of d-Gal Treated Mice
ROS can regulate the expression of different genes including immediate early response genes such as c-fos and c-jun. Heterodimer complex of their respective protein products forms the transcription factor activator protein (AP-1). It regulates gene expression in response to a variety of stimuli, including cytokines, growth factors, stress, and bacterial and viral infections [29]. AP-1 has been proven to depend on change of the GSH/GSSG ratio [30]. As shown in Fig. 5a, the concentration of AP-1 in the hippocampus of mice in model group was significantly higher than mice in the vehicle control group (P < 0.01). While TMF treatment (8 mg/kg/day) reversed the increased level of AP-1 in hippocampus induced by d-gal (P < 0.01), indicating that the TMF inhibited the activation process of AP-1 induced by the hippocampal redox imbalance status.
The effects of TMF on the levels of AP-1, BDNF and p-CREB in the hippocampus of d-gal treated mice. a Levels of AP-1 in the hippocampus of mice by ELISA. b Levels of BDNF in the hippocampus of mice by ELISA. c Representative western blot images for each of the treatment groups. d p-CREB/CREB ratios in hippocampus of mice. All values are expressed as mean ± SEM (n = 4). * P < 0.05 and ** P < 0.01 compared with the d-gal model group
In brain, particularly in hippocampus, stress evokes a large array of molecular effects, which produce structural, functional, molecular and behavioral changes [31]. The gene coding for the neurotrophin Brain Derived Neurotrophic Factor (BDNF) is a stress-responsive gene. Regulation of BDNF is interesting as this neurotrophin has been linked with the control of synaptic plasticity and long-term memory, and decreases in BDNF and p-BDNF have been reported in AD [13]. One of the most well known and immediate transcriptional regulator of BDNF gene expression is CREB. CREB is a transcription factor that regulates genes associated with neuronal survival, memory consolidation and synaptic plasticity [32].
The levels of BDNF and p-CREB in the hippocampus of mice in model group were significantly lower than mice in vehicle control group (P < 0.01), leading to the cognitive impairment in d-gal treated mice which was proved by the MWM test (Fig. 5b–d). On the contrary, TMF treatment (8 mg/kg/day) significantly increased the BDNF and p-CREB levels compared with the model group (P < 0.05). These results indicated that TMF exerted its neuroprotective effect through increasing the BDNF and p-CREB activities in the hippocampus of d-gal treated mice.
Conclusion
We firstly investigated the protective effect of TMF with in vitro model of dopamine-induced PC12 cells, and found TMF offset the oxidative stress caused by dopamine through restoring the intracellular normal redox balance. Further, our study in vivo demonstrated that TMF ameliorated the redox imbalance status and attenuated the cognitive impairment by inhibiting the activation of AP-1 and upregulating the expression of BDNF and p-CREB in the hippocampus of d-gal treated mice. Therefore, TMF is a potential agent for the treatment of neurodegenerative diseases.
References
Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Bio Med 23:134–147
Jones DC, Gunasekar PG, Borowitz JL, Isom GE (2000) Dopamine-induced apoptosis is mediated by oxidative stress and is enhanced by cyanide in differentiated PC12 cells. J Neurochem 74:2296–2304
Ziv I, Barzilai A, Offen D, Stein R, Achiron A, Melamed E (1996) Dopamine-induced, genotoxic activation of programmed cell death. A role in nigrostriatal neuronal degeneration in Parkinson’s disease? Adv Neurol 69:229–233
Jenner P (2003) Oxidative stress in Parkinson’s disease. Ann Neurol 53(Suppl 3):26–38
Kim KJ, Jang YY, Han ES, Lee CS (1999) Modulation of brain mitochondrial membrane permeability and synaptosomal Ca2+ transport bydopamine oxidation. Mol Cell Biochem 201:89–98
Lee CS, Han ES, Jang YY, Han JH, Ha HW, Kim DE (2000) Protective effect of harmalol and harmaline on MPTP neurotoxicity in the mouse and dopamine-induced damage of brain mitochondria and PC12 Cells. J Neurochem 75:521–531
Lee CS, Han JH, Jang YY, Song JH, Han ES (2002) Differential effect of catecholamines and MPP + on membrane permeability in brain mitochondria and cell viability in PC12 cells. Neurochem Int 40:361–369
Olanow CW (1993) A radical hypothesis for neurodegeneration. Trends Neurosci 16:439–444
Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze RB, Markesbery WR, Butterfield DA (2002) Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Bio Med 33:562–571
De Iuliis A, Grigoletto J, Recchia A, Giusti P, Arslan P (2005) A proteomic approach in the study of an animal model of Parkinson’s disease. Clin Chim Acta 357:202–209
Havsteen BH (2002) The biochemistry and medical significance of the flavonoids. Pharmacol Therapeut 96:67–202
Li Q, Zhao HF, Zhang ZF, Liu ZG, Pei XR, Wang JB, Cai MY, Li Y (2009) Long-term administration of green tea catechins prevents age-related spatiallearning and memory decline in C57BL/6 mice by regulating hippocampal cyclic AMP-response element binding protein signaling cascade. Neuroscience 159:1208–1215
Spencer JPE, Vauzour D, Rendeiro C (2009) Flavonoids and cognition: the molecular mechanisms underlying their behavioural effects. Arch Biochem Biophys 492:1–9
The Editorial Committee of Chinese Materia Medica (1999) Chinese materia medica. Shanghai Scientific and Technical Publishers, Shanghai
Wei H, Wu GH, Lei YF, Xiong CM, Ruan JL (2011) Neuropective constituents from the rhizomes of Abacopteris penangiana. J Asian Nat Prod Res 13:707–713
Zhao JH, Xu SB (1997) Effect of blumea flavanones on lipid peroxidantion and active oxygen redicals. Chin Pharm Bull 13:438–441
Xu SB, Zhao JH (1998) Protective actions of blumea flavanones on experimental liver injury in rats. Chin Pharm Bull 14:191–192
Pu HL, Zhao JH, Xu SB, Hu Q (2000) Protective actions of blumea flavanones on primary cultured hepatocytes against lipid peroxidantion. Chin Tradit Herbal Drugs 31:113–115
Chan WL, Lin YC, Zhang WH, Tang PL, Szeto YS (1996) One step synthesis of polyhydroxyflavanones from hydroxyacetophenones and hydroxybenzaldehydes. Heterocycles 43:551–554
Lu SH, Yang Y, Liu SJ (2005) An investigation on the division of neuronal PC12 cells induced by nerve growth factor. Acta Physiol Sin 57:552–556
Kaeidi A, Esmaeili-Mahani S, Sheibani V, Abbasnejad M, Rasoulian B, Hajializadeh Z, Afrazi S (2011) Olive (Olea europaea L.) leaf extract attenuates early diabetic neuropathic pain through prevention of high glucose-induced apoptosis: in vitro and in vivo studies. J Ethnopharmcol 136:188–196
Zhang GM, Yao GH (1997) Investigation on genetic background information of Chinese Kunming Mice. Chin J Lab Anim Sci 7:246–251
Lu J, Wu DM, Hu B, Cheng W, Zheng YL, Zhang ZF, Ye Q, Fan SH, Shan Q, Wang YJ (2010) Chronic administration of troxerutin protects mouse brain against d-galactose-induced impairment of cholinergic system. Neurobio Learn Mem 93:157–164
Fu W, Lei YF, Chen JL, Xiong CM, Zhou DN, Wu GH, Chen J, Cai YL, Ruan JL (2010) Parathelypteriside attenuates cognition deficits in d-galactose treated mice by increasing antioxidant capacity and improving long-term potentiation. Neurobio Learn Mem 94:414–421
Ye M, Chen WX, Qiu T, Yuan RY, Ye YW, Cai JM (2012) Structural characterisation and anti-aging activity of extracellular polysaccharide from a strain of Lachnum. sp. Food Chem 132:338–343
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Meth 11:47–60
Filomeni G, Rotilio G, Ciriolo MR (2002) Cell signalling and the glutathione redox system. Biochem Pharmacol 64:1057–1064
Tormos C, Chaves FJ, Garcia MJ, Garrido F, Jover R, O’Connor JE, Iradi A, Oltra A, Oliva MR, Sa′ez GT (2004) Role of glutathione in the induction of apoptosis and c-fos and c-jun mRNAs by oxidative stress in tumor cells. Cancer Lett 208:103–113
Hess J, Angel P, Schorpp-Kistner M (2004) AP-1 subunits: quarrel and harmony among siblings. J Cell Sci 117:5965–5973
Calter D, Mihm S, Droge W (1994) Distinct effects of glutathione disulphide on the nuclear transcription factor kB and the activator protein-1. Eur J Biochem 221:639–648
Sapolsky RM (2003) Stress and plasticity in the limbic system. Neurochem Res 28:1735–1742
Jeon SJ, Rhee SY, Seo JE, Bak HR, Lee SH, Ryu JH, Cheong JH, Shin CY, Kim GH, Lee YS, Ko KH (2011) Oroxylin A increases BDNF production by activation of MAPK-CREB pathway in rat primary cortical neuronal culture. Neurosci Res 69:214–222
Acknowledgments
This research was founded by National Natural Science Foundation of China (No. 81173065). Han Wei is a recipient of Innovation Fund for Doctoral Dissertation from Huazhong University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, H., Wu, G., Chen, J. et al. (2S)-5, 2′, 5′-Trihydroxy-7-Methoxyflavanone, a Natural Product from Abacopteris penangiana, Presents Neuroprotective Effects In Vitro and In Vivo. Neurochem Res 38, 1686–1694 (2013). https://doi.org/10.1007/s11064-013-1070-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1070-8