Abstract
Phenelzine is a potent monoamine oxidase inhibitor that is used in patients with depression. It is also well known that nitric oxide (NO) synthase inhibitors show preclinical antidepressant-like properties, which suggests that NO is involved in the pathogenesis of depression. The purpose of this study was to determine if phenelzine affects the production of NO and tumor necrosis factor-alpha (TNF-α) in activated microglia cells. BV-2 microglia cells and primary microglia cells were cultured in DMEM and DMEM/F12 and then cells were treated with LPS or LPS plus phenelzine for 24 h. The culture medium was collected for determination of NO, TNF-α, and IL-6 and cells were harvested by lysis buffer for Western blot analysis. Phenelzine increased the lipopolysaccharide (LPS)-induced expression of inducible nitric oxide synthase (iNOS), as well as the release of TNF-α and IL-6 in BV-2 microglia cells. It is also confirmed that phenelzine increased the levels of NO, TNF-α and IL-6 in LPS-activated primary microglia cells. Phenelzine increased nuclear translocation of NF-κB by phosphorylation of IκB-α in LPS-activated microglia cells. These findings suggest that high doses of phenelzine could aggravate inflammatory responses in microglia cells that are mediated by NO and TNF-α.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenelzine is a potent monoamine oxidase (MAO) inhibiting antidepressant drug that is efficacious in the treatment of depression, social phobia and panic disorders [1, 2]. Phenelzine increases brain levels of the amine neurotransmitters noradrenaline, dopamine, and serotonin. Moreover, acute and chronic treatment with phenelzine has been found to significantly reduce the depolarization-induced outflow of excitatory neurotransmitter glutamate [3]. Phenelzine also causes a significant increase in the levels of the brain’s major inhibitory neurotransmitter, GABA [4]. In addition to these neurological effects, phenelzine inhibits adipocyte lipid storage and differentiation and attenuates the MPP+-induced loss of viability in PC12 cells by reducing the alteration of mitochondrial membrane permeability [5, 6]. However, few studies have been conducted to evaluate the effects of phenelzine on immune cells such as microglia in the brain.
Microglia, which are the resident immune cells in the central nervous system (CNS), in the healthy brain interact with neuronal and nonneuronal elements, both structurally and functionally. Forms of interactions include phagocytosis of synaptic structures during postnatal development, phagocytosis of newborn neurons during adult neurogenesis, and active remodeling of the perisynaptic environment and release of soluble factors in the mature and aging brain. These interactions can influence neuronal plasticity and function directly or indirectly [7]. Microglia also plays a critical role in inflammatory responses in the brain. It has been proposed that microglia can play either a neurotoxic role through the production of inflammatory molecules such as TNF-α, IL-1, IL-6 and NO, or a neuroprotective role by supporting neural growth and metabolism and by scavenging agents that are toxic to neurons [8].
Depression is also frequently associated with inflammatory diseases such as multiple sclerosis [9], allergies [10], and rheumatoid arthritis [11], all of which involve the overexpression of pro-inflammatory cytokines [12]. Up to 45 % of patients with hepatitis C that are treated with IFN-α develop depression [13]. However, there is evidence that treatment of inflammatory diseases reduces the depressive symptoms. Etanercept is an anti-inflammatory drug that is used to treat arthritis that acts as an antagonist of the inflammatory mediator TNF-α. In a trial of patients with psoriasis [14], etanercept reduced depressive symptoms independent of the improvement in psoriatic symptoms, such as skin involvement and joint pain. These results suggest that blocking TNF-α can relieve depressive symptoms, which is consistent with the elevations in TNF-α levels that are observed in depression. There is also evidence that effective antidepressant treatment largely attenuates changes in inflammation [15].
Microglia produce molecules such as nitric oxide (NO) and TNF-α, which are agents known to play roles in inflammatory responses. Over the past decade, NO has received increasing attention as a potent molecule against a variety of bacteria, parasites, and tumors [16]. However, inappropriate activation of microglia leads to excessive NO secretion, which has been implicated in many neurological disease states, including Alzheimer’s disease [17], Parkinson’s disease [18] and stroke [19].
TNF-α, which is one of the most important proinflammatory cytokines, is primarily produced by activated microglia [20]. TNF-α induces various biological responses, including tissue injury, shock, and apoptosis [21, 22]. TNF-α also induces the secretion of other cytokines such as IL-1, IL-6 and IL-10, as well as the activation of T cells and other inflammatory cells [23]. Although proinflammatory cytokines and NO are necessary for normal function, the microglia response must be tightly regulated to avoid over-activation and disastrous neurotoxic consequences.
The induction of cytokine genes such as TNF-α by LPS occurs primarily at the transcriptional level and involves the action of several transcription factors, including members of the nuclear factor-κB (NF-κB)/rel, C/EBP, Ets, and AP-1 protein families [24]. Particularly, binding of NF-κB to specific consensus DNA elements present on the promoters of target genes initiates the transcription of TNF-α, iNOS, cyclo-oxygenase-2 and IL-6 [25]. Thus, the inhibition of NF-κB may be key to suppression of inflammation effectors such as NO and TNF-α.
These observations raise the possibility that monoamine oxidase inhibitors inhibit inflammatory responses and led us to examine the potential effects of several monoamine oxidase inhibitors on proinflammatory cytokines production by LPS activated microglia cells. Unexpectedly, we found that phenelzine increased NO, TNF-α and IL-6 production in LPS activated microglia cells via the NF-κB pathway.
Materials and Methods
Reagents
An OptEIA IL-6 ELISA kit and OptEIA TNF-α ELISA kit (BD Biosciences, Bedford, MA, USA), N-(1-naphtyl)-ethylenediamine dihydrochloride, LPS (Escherichia coli serotype 0111:B4) and sodium nitrite (Sigma, St. Louis, MO, USA), rabbit polyclonal antisera to iNOS and p65 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) protein extraction solution (Intron Biotechnology, Republic of Korea), FBS (Hyclone, Logan, UT, USA), 0.2 μm syringe filters, 96 and 24 well tissue culture plates and 100-mm diameter dishes (Corning Inc. NY, USA), DMEM containing l-arginine (84 mg/l) and other tissue culture reagents (Invitrogen Life Technologies, Carlsbad, CA, USA) were used.
Cell Cultures
The immortalized murine BV-2 microglial cell line, which has been generated by infecting primary microglial cell cultures with a v-raf/v-myc oncogene carrying retrovirus [26], were kindly provided by Dr. S. Go (Kyung Hee University, Seoul, Korea) and maintained in 5 % CO2 at 37 °C in DMEM supplemented with 10 % fetal bovine serum (FBS), 1 % streptomycin, and penicillin (Invitrogen Life Technologies, Rockville, USA).
Mouse primary microglial cultures were prepared by mild trypsinization as described previously, with minor modifications [27, 28]. In brief, cerebral cortices of 0–3 day-old C57BL/6 mice were chopped and dissociated by mechanical disruption using a nylon mesh, and the cells were seeded in culture dishes. After in vitro culture for 10–14 days, microglial cells were isolated from these mixed glial cultures by mild trypsinization. The mixed glial cultures were incubated with trypsin solution (0.25 % trypsin, 1 mM EDTA in HBS) diluted 1:3 in DMEM/F12 for 30–60 min. This resulted in detachment of an upper layer of astrocytes in one piece, while the microglia remained attached to the bottom of the culture flask. The detached layer of astrocytes was aspirated, and the microglia was used in our experiments.
Measurement of Nitrite Concentration
Microglia cells (2 × 105 cells/well) were treated with various concentrations of phenelzine. The microglia cells were then stimulated with LPS (0.2 μg/ml) and incubated for 24 h. NO synthesis in cell cultures was measured by the microplate assay method as previously described [29]. To measure the nitrite levels, 100 μl aliquots were removed from conditioned medium and incubated with an equal volume of Griess reagent (1 % sulfanilamide/0.1 % N-(1-naphtyl)-ethylenediamine dihydrochloride/2.5 % H3PO4) at room temperature for 10 min. The absorbance at 530 nm was then determined using a microplate reader (Molecular Devices, Sunnyvale, CA, USA) using sodium nitrite as the standard.
Assay of TNF-α and IL-6 Release
Microglia cells (2 × 105 cells/well) were treated with various concentrations of phenelzine, after which the cells were then stimulated with LPS (0.2 μg/ml) and incubated for 24 h. The release of TNF-α and IL-6 were detected by OptEIA calibrated according to the manufacturer’s instructions.
RNA Extraction
Total RNA was extracted from cultured cells using an EasyBlue RNA extraction kit (iNtRON Biotechnology, Inc. Seongnam, Korea). Extracts were assayed to determine the quality and concentration of the RNA using a ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). Extracts were stored at −20 °C.
Real-Time PCR
Reverse transcription of the total RNA was performed by mixing 1 μg of RNA, DW, 4 μl of transcriptor reverse transcriptase reaction buffer, 0.5 μl of protector RNase inhibitor (Roche Applied Science, Indianapolis, IN, USA) and 2 μl of deoxynucleotide mixture (10 mM each) with 2.5 μM oligo (dT) primer and 0.5 μl of transcriptor reverse transcriptase (Roche Applied Science, Indianapolis, IN). The solution was then incubated for 1 h at 50 °C, after which it was heated at 85 °C for 5 min to inactivate the transcriptor, reverse transcriptase. The samples were then stored at −20 °C until further use.
Real-time quantitative PCR was performed using a LightCycler 480 (Roche Applied Science, Indianapolis, IN, USA) employing SYBR Green I as the dsDNA-specific binding dye for continuous fluorescence monitoring. Amplification was conducted in a reaction mixture with a total volume of 20 μl that contained 0.25 μM of each gene-specific primer, 10 μl of 2× PCR Master Mix (Roche Applied Science, Indianapolis, IN, USA) and 2 μl of cDNA. The primer sequences were as follows; iNOS sense: GGC AGC CTG TGA GAC CTT TG; iNOS antisense: CAT TGG AAG TGA AGC GTT TCG; TNF-α sense: ACG GCA TGG ATC TCA AAG AC; TNF-α antisense: GTG GGT GAG GAG CAC GTA GT; IL-6 sense: GAC AAC TTT GGC ATT GTG G; IL-6 antisense: ATG CAG GGA TGA TGT TCT G; GAPDH sense: ACC CAG AAG ACT GTG GAT GG; GAPDH antisense: CAC ATT GGG GGT AGG AAC AC. PCR was conducted by subjecting the samples to 45 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 10 s and extension at 72 °C for 10 s with the fluorescence being measured at the end of each cycle. After the cycles were terminated, the signal of each temperature between 65 and 95 °C was also measured to generate a dissociation curve. All reactions were performed in duplicate to confirm reproducibility and included a negative control (without template) to verify that no primer-dimers were generated. The results of the iNOS, TNF-α, and IL-6 mRNA level were compared by calculating the CP value and normalized by the reference genes (GAPDH). Three independent experiments were performed, and the results were expressed as a percentage of control.
Western Blotting
For p-IκBα western blotting, cytoplasmic extracts were prepared as previously described [30] and whole cell lysates were prepared for iNOS western blotting. Proteins were then separated by Tris–glycine 4–12 % (KOMA Biotech, Korea) gel electrophoresis and transferred to a nitrocellulose membrane. Next, the membrane was blocked with 5 % skim milk in TBS-Tween-20 for 1 h at room temperature, after which it was incubated with anti-iNOS, anti-p-IκB-α or β-actin antibody. After washing three times in TBS-Tween-20, the blot was incubated with secondary antibody for 1 h. Antibody-specific proteins were then visualized with an enhanced chemiluminescence detection system as recommended (Amersham Corp., Newark, NJ).
Confocal Microscopy
BV-2 microglia cells were cultured on 4-well Lab-Tek™ II Chambered Coverglasses (Nalge Nunc International, Naperville, Illinois). The cells were then treated with phenelzine for 30 min and subsequently stimulated with LPS (0.2 μg/ml) for 10 min. Next, the cells were fixed in 4 % paraformaldehyde/PBS for 20 min and then washed in PBS with Tween-20 (0.1 %). After permeabilization with 0.1 % Triton X-100/PBS for 15 min, the cells were washed with PBST (0.1 %) and blocked with blocking buffer (PBS with 1 % BSA) at room temperature for 1 h and then incubated with p65 antibody (Santa Cruz Biotechnology, CA) at 4 °C overnight. After washing with PBST (0.1 %), the cells were incubated with FITC-conjugated secondary antibodies (Sigma) diluted 500-fold in blocking buffer for 1 h at room temperature. Finally, the cells were washed with PBST (0.1 %), mounted in Vectashield mounting medium with DAPI (Vector Laboratories Inc., Burlingame, CA, USA) and analyzed by confocal microscopy (Zeiss LSM Pascal 5).
Statistical Analysis
All results were expressed as the means ± SEM of at least three independent experiments performed in duplicate. One-way analysis of variance (ANOVA) and Tukey’s test were used to compare the groups. Differences were considered to be significant at p < 0.05.
Results
Effect of Phenelzine on NO Production in LPS-Activated Microglia Cells
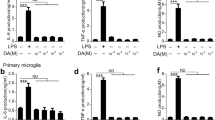
The levels of NO in primary microglia cells and BV-2 microglia cell line that were treated with various concentrations of phenelzine were then examined. As shown in Fig. 1, production of NO increased in response to phenelzine treatment in LPS-activated primary microglia cells (Fig. 1b) and BV-2 microglia cell line (Fig. 1a). But non-activated microglia did not increase NO production by phenelzine treatment alone (data not shown).
Effect of phenelzine on NO production in LPS-activated microglia. Microglia cells (2 × 105 cells/well) cultured with LPS (0.2 μg/ml) were treated with various concentrations of phenelzine. After 24 h of culture, NO release was measured by the Griess method (to detect nitrite). a BV-2 microglia cells, b primary microglia cells. NO released into the medium is presented as the mean ± SEM of at least three independent experiments performed in duplicate. *p < 0.05 compared to LPS-treated cells
Effect of Phenelzine on LPS-Activated iNOS mRNA and Protein Expression
To assess the expression of iNOS mRNA and protein in microglia, we conducted real-time PCR and western blotting of LPS-activated BV-2 microglia cells. Real-time PCR showed that iNOS mRNA was upregulated in response to exposure to phenelzine, with the highest levels being observed in LPS-activated BV-2 microglia cells that were treated with 50 μM phenelzine (Fig. 2a). Treatment with phenelzine also increased iNOS protein expression in LPS-activated BV-2 microglia cells (Fig. 2b).
Effect of phenelzine on iNOS mRNA and protein expression in LPS-activated microglia. a BV-2 microglia cells treated with various concentrations of phenelzine were incubated for 6 h with LPS (0.2 μg/ml). The amounts of iNOS mRNA were quantified by real-time RT-PCR. The expression levels of iNOS mRNA were normalized by dividing the values by the GAPDH intensity. b BV-2 microglia cells treated with various concentrations of phenelzine were incubated for 9 h with LPS (0.2 μg/ml). Protein extracts were prepared and the samples were then analyzed for iNOS expression by Western blot as described in the “Materials and Methods” section
Effects of Phenelzine on TNF-α and IL-6 Production and mRNA Expression in LPS-Activated Microglia Cells
Activation of microglia results in increased secretion of a number of proinflammatory products; therefore, we measured the cytokine production and mRNA expression by activated microglia in the presence of phenelzine. The results indicated that phenelzine led to a significant increase in TNF-α and IL-6 production by LPS-activated microglia cells (Fig. 3). Although the TNF-α and IL-6 mRNA expressions were increased by phenelzine, only TNF-α expression was significantly increased at 50 μM of phenelzine (Fig. 3e, f).
Effect of phenelzine on the production and mRNA expression of TNF-α and IL-6 in LPS-activated microglia. Microglia cells (2 × 105 cells/well) cultured with LPS (0.2 μg/ml) were treated with various concentrations of phenelzine. The amounts of TNF-α and IL-6 were measured by ELISA after 24 h of culture and mRNA expressions of TNF-α and IL-6 were measured by real-time RT-PCR after 6 h of culture a, b BV-2 microglia cells, c, d primary microglia cells. e, f Primary microglia cells. The values shown are the means ± SEM of at least three independent experiments performed in duplicate in each run. *p < 0.05, **p < 0.01, ***p < 0.001 compared to LPS-treated cells
Effect of Phenelzine on Translocation of NF-κB
NF-κB is usually kept inactive in the cytoplasm by association with an endogenous inhibitor protein of the IκB (inhibitor of the NF-κB) family. After being activated, the IκB proteins become phosphorylated and degraded by the proteasome subsequently. The degradation of IκB allows nuclear localization of NF-κB. We analyzed the subcellular distribution of p65, which is one of component of NF-κB. Immunocytochemical analysis revealed that treatment of cells with phenelzine and LPS resulted in greater nuclear accumulation of p65 than treatment with LPS alone (Fig. 4a). We confirmed these results using cytoplasmic cell fractions. The level of cytoplasmic phosphorylated IκB-α was increased in samples that were treated with phenelzine plus LPS when compared to samples that were treated with LPS alone (Fig. 4b). Taken together, these results show that NF-κB translocation to the nucleus is stimulated by an increase of IκB-α phosphorylation upon phenelzine treatment in LPS activated microglia.
Effect of phenelzine on the nuclear localization of NF-κB and IκB-α phosphorylation in LPS-activated microglia. a Confocal microscopy images of fluorescence immunocytochemical analysis of p65 (green) in BV-2 cells treated with or without 50 μM of phenelzine for 10 min. Nuclei were counterstained with DAPI (blue). The bar graph shows the intensity of fluorescence in the nucleus. **p < 0.0001. b BV-2 cells (8 × 106 cells) treated with 50 μM of phenelzine were incubated for 10 min with LPS (0.2 μg/ml). Cytosol extracts were prepared and analyzed for the expression of p-IκB-α and β-actin by western blotting as described in the “Materials and Methods” section (Color figure online)
Discussion
We demonstrated that phenelzine increases the production of NO and the expression of iNOS protein in LPS-treated microglia cells. In addition, phenelzine increased TNF-α and IL-6 production in LPS-activated microglia cells. To study the mechanism by which of the production of NO, TNF-α and IL-6 was induced, the effects of phenelzine on the activation of NF-κB were examined. Our findings suggest that phenelzine increases the LPS-induced translocation of NF-κB, thereby increasing the LPS-induced production of NO, TNF-α and IL-6.
One of the major glial cell types in the brain, microglia, become activated or reactive in response to diverse insults of the CNS and produce a myriad of proinflammatory cytokines, chemokines and trophic factors to render neural immunity [31]. Microglia function as the microsensors of the brain and play an important role in detection of subtle changes in the neuronal microenvironment [32]. Microglia also express and release inflammatory mediators such as NO, TNF-α and IL-6 following brain injury, as seen in neurological and neurodegenerative disorders [33] and following experimental brain injury [34].
Over-production of NO has been correlated with oxidative stress and is regulated by NF-κB, which performs pivotal roles in the immediate early stages of immune, acute phase, and inflammatory responses, as well as in cell survival [35–37]. NF-κB also plays a critical role in the activation of immune cells by up-regulating the expression of many cytokines, including TNF-α and IL-6 [38, 39].
Under physiological conditions, TNF-α orchestrates a diverse array of functions involved in immune surveillance and defense, cellular homeostasis, and protection against certain neurological insults. However, paradoxical effects of TNF-α have been observed. TNF-α is elicited in the brain following injury (ischemia, trauma), infection (HIV, meningitis), neurodegeneration (Alzheimer’s, Parkinson’s), and chemically induced neurotoxicity [34].
Phenelzine toxicity is normally characterized by agitation, seizures, sweating, tachycardia and hypertension [40, 41], although hypotension has also been described [42]. In addition, massive phenelzine overdose has been attributed to myocarditis and an inflammatory infiltrate within the myocardium [43]. Although 50 μM of phenelzine, which was the highest effective dose used in this experiment, was higher than therapeutic doses (45–90 mg/day), the results of this study do indicate that overdose of phenelzine treatment of inflammatory conditions could aggravate inflammatory responses.
Conclusions
The results of this study showed that phenelzine increased the LPS-induced production of NO and TNF-α in microglia through enhancement of NF-κB activation. Because NF-κB is a critical transcription factor that regulates the production of various proinflammatory proteins and cytokines in activated microglia during the process of inflammation, the increase of this transcription factor by phenelzine treatment may aggravate inflammatory responses during inflammatory diseases.
References
Johnson MR, Lydiard RB, Ballenger JC (1995) Panic disorder. Pathophysiology and drug treatment. Drugs 49:328–344
Casacalenda N, Perry JC, Looper K (2002) Remission in major depressive disorder: a comparison of pharmacotherapy, psychotherapy, and control conditions. Am J Psychiatry 159:1354–1360
Michael-Titus AT, Bains S, Jeetle J, Whelpton R (2000) Imipramine and phenelzine decrease glutamate overflow in the prefrontal cortex—a possible mechanism of neuroprotection in major depression? Neuroscience 100:681–684
Parent MB, Master S, Kashlub S, Baker GB (2002) Effects of the antidepressant/antipanic drug phenelzine and its putative metabolite phenylethylidenehydrazine on extracellular gamma-aminobutyric acid levels in the striatum. Biochem Pharmacol 63:57–64
Chiche F, Le Guillou M, Chetrite G, Lasnier F, Dugail I, Carpene C, Moldes M, Feve B (2009) Antidepressant phenelzine alters differentiation of cultured human and mouse preadipocytes. Mol Pharmacol 75:1052–1061
Lee CS, Han ES, Lee WB (2003) Antioxidant effect of phenelzine on mpp+-induced cell viability loss in differentiated pc12 cells. Neurochem Res 28:1833–1841
Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A (2011) The role of microglia in the healthy brain. J Neurosci 31:16064–16069
Wyss-Coray T, Mucke L (2002) Inflammation in neurodegenerative disease—a double-edged sword. Neuron 35:419–432
Ehde DM, Bombardier CH (2005) Depression in persons with multiple sclerosis. Phys Med Rehabil Clin N Am 16:437–448, ix
Marshall PS (1993) Allergy and depression: a neurochemical threshold model of the relation between the illnesses. Psychol Bull 113:23–43
Katon W, Sullivan MD (1990) Depression and chronic medical illness. J Clin Psychiatry 51Suppl:3–11 (discussion 12–14)
Schrott LM, Crnic LS (1996) Anxiety behavior, exploratory behavior, and activity in nzb x nzw f1 hybrid mice: role of genotype and autoimmune disease progression. Brain Behav Immun 10:260–274
Asnis GM, De La Garza R II (2006) Interferon-induced depression in chronic hepatitis c: a review of its prevalence, risk factors, biology, and treatment approaches. J Clin Gastroenterol 40:322–335
Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R et al (2006) Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase iii trial. Lancet 367:29–35
Sluzewska A, Rybakowski J, Bosmans E, Sobieska M, Berghmans R, Maes M, Wiktorowicz K (1996) Indicators of immune activation in major depression. Psychiatry Res 64:161–167
Gantt KR, Goldman TL, McCormick ML, Miller MA, Jeronimo SM, Nascimento ET, Britigan BE, Wilson ME (2001) Oxidative responses of human and murine macrophages during phagocytosis of leishmania chagasi. J Immunol 167:893–901
Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL et al (2000) Inflammation and alzheimer’s disease. Neurobiol Aging 21:383–421
McGeer PL, McGeer EG (2004) Inflammation and neurodegeneration in parkinson’s disease. Parkinsonism Relat Disord 10(Suppl 1):S3–S7
Danton GH, Dietrich WD (2003) Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol 62:127–136
Chao CC, Hu S, Peterson PK (1995) Glia, cytokines, and neurotoxicity. Crit Rev Neurobiol 9:189–205
Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A (1987) Anti-cachectin/tnf monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330:662–664
Baker SJ, Reddy EP (1998) Modulation of life and death by the tnf receptor superfamily. Oncogene 17:3261–3270
Vilcek J, Lee TH (1991) Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J Biol Chem 266:7313–7316
Sweet MJ, Hume DA (1996) Endotoxin signal transduction in macrophages. J Leukoc Biol 60:8–26
Kuprash DV, Udalova IA, Turetskaya RL, Rice NR, Nedospasov SA (1995) Conserved kappa b element located downstream of the tumor necrosis factor alpha gene: distinct nf-kappa b binding pattern and enhancer activity in lps activated murine macrophages. Oncogene 11:97–106
Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F (1990) Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol 27:229–237
Chung HS, Lee JH, Kim H, Lee HJ, Kim SH, Kwon HK, Im SH, Bae H (2010) Foxp3 is a novel repressor of microglia activation. Glia 58:1247–1256
Saura J, Tusell JM, Serratosa J (2003) High-yield isolation of murine microglia by mild trypsinization. Glia 44:183–189
Xie QW, Cho HJ, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Ding A, Troso T, Nathan C (1992) Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 256:225–228
Chung HS, Kang M, Cho C, Parvez S, Park CH, Kim D, Oh J, Kim H, Shin M, Hong M et al (2007) Inhibition of nitric oxide and tumor necrosis factor-alpha by moutan cortex in activated mouse peritoneal macrophages. Biol Pharm Bull 30:912–916
Stoll G, Jander S (1999) The role of microglia and macrophages in the pathophysiology of the cns. Prog Neurobiol 58:233–247
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the cns. Trends Neurosci 19:312–318
Vila M, Jackson-Lewis V, Guegan C, Wu DC, Teismann P, Choi DK, Tieu K, Przedborski S (2001) The role of glial cells in parkinson’s disease. Curr Opin Neurol 14:483–489
Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O’Callaghan JP (2006) Deficiency of tnf receptors suppresses microglial activation and alters the susceptibility of brain regions to mptp-induced neurotoxicity: role of tnf-alpha. FASEB J 20:670–682
Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA (2001) Possible new role for nf-kappab in the resolution of inflammation. Nat Med 7:1291–1297
Makarov SS (2001) Nf-kappa b in rheumatoid arthritis: a pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res 3:200–206
Boje KM, Arora PK (1992) Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res 587:250–256
Majumder S, Zhou LZ, Chaturvedi P, Babcock G, Aras S, Ransohoff RM (1998) P48/stat-1alpha-containing complexes play a predominant role in induction of ifn-gamma-inducible protein, 10 kDa (ip-10) by ifn-gamma alone or in synergy with tnf-alpha. J Immunol 161:4736–4744
Piao HZ, Choi IY, Park JS, Kim HS, Cheong JH, Son KH, Jeon SJ, Ko KH, Kim WK (2008) Wogonin inhibits microglial cell migration via suppression of nuclear factor-kappa b activity. Int Immunopharmacol 12:1658–1662
Henry JA, Antao CA (1992) Suicide and fatal antidepressant poisoning. Eur J Med 1:343–348
Ciocatto E, Fagiano G, Bava GL (1972) Clinical features and treatment of overdosage of monoamine oxidase inhibitors and their interaction with other psychotropic drugs. Resuscitation 1:69–72
Linden CH, Rumack BH, Strehlke C (1984) Monoamine oxidase inhibitor overdose. Ann Emerg Med 13:1137–1144
Waring WS, Wallace WA (2007) Acute myocarditis after massive phenelzine overdose. Eur J Clin Pharmacol 63:1007–1009
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government [MEST] (No. 2011-0006220).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hwan-Suck Chung and Hyunseong Kim contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chung, HS., Kim, H. & Bae, H. Phenelzine (Monoamine Oxidase Inhibitor) Increases Production of Nitric Oxide and Proinflammatory Cytokines via the NF-κB Pathway in Lipopolysaccharide-Activated Microglia Cells. Neurochem Res 37, 2117–2124 (2012). https://doi.org/10.1007/s11064-012-0833-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0833-y