Abstract
Brain-derived neurotrophic factor (BDNF) plays an essential regulatory role in the survival and differentiation of various neural cell types during brain development and after injury. In this study, we used neural stem cells (NSCs) genetically modified to encode BDNF gene (BDNF/NSCs) and naive NSCs transplantation and found that BDNF/NSCs significantly improved neurological motor function following traumatic brain injury (TBI) on selected behavioral tests. Our data clearly demonstrate that the transplantation of BDNF/NSCs causes overexpression of BDNF in the brains of TBI rats. The number of surviving engrafted cells and the proportion of engrafted cells with a neuronal phenotype were significantly greater in BDNF/NSCs than in naive NSCs-transplanted rats. The expression of pre- and post-synaptic proteins and a regeneration-associated gene in the BDNF/NSCs-transplanted rats was significantly increased compared to that in NSCs-transplanted rats, especially at the early stage of post-transplantation. These data suggest that neurite growth and overexpression of synaptic proteins in BDNF/NSCs-transplanted rats are associated with the overexpression of BDNF, which is hypothesized to be one of the mechanisms underlying the improved functional recovery in motor behavior at the early stage of cell transplantation following TBI. Therefore, the protective effect of the BDNF-modified NSCs transplantation is greater than that of the naive NSCs transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI), a form of acquired brain injury, is a major cause of death and disability worldwide, especially in children and young adults; causes include falls, vehicle accidents, and violence [1]. Currently, effective strategies are required for treating TBI patients and supporting their recovery to minimize their functional deficits. Long-term strategies incorporating the sustained release of trophic factors and encapsulated cell therapy have demonstrated feasibility [2] but may face clinical limitations such as volume constraints and the inability of donor cells to interact with the host tissue.
The isolation and characterization of neural stem/progenitor cells (NSCs) have led to new possibilities for the direct transplantation of these cells into the injured brain because of their multipotential ability to renew themselves and give rise to neurons, astrocytes and oligodendrocytes. Moreover, their inherent ability to adapt to signals from host cells and the extracellular environment is necessary for interaction with the host tissue and regeneration of injured tissue [3]. Previous experimental studies in animal models of human neurodegenerative disease have demonstrated that recovery of lost neurological functions and partial reconstruction of neural circuitry is possible with NSCs transplantation in the damaged adult brain [4]. It has been suggested that transplanted NSCs could differentiate into various phenotypes that could promote the restoration of function after injury or disease in the central neural system (CNS), including TBI, depending on transplant location; these studies also indicated that transplanted NSCs might respond to signals present in the injured brain via cell–cell mediated repair, release specific neurotransmitters, and/or produce factors that promote neuronal growth [5, 6]. However, the environment associated with TBI in adult rats, including secondary injury, negatively affects the survival and migration of grafted NSCs. The post-traumatic inflammatory environment, which includes the entry of macrophages into the brain parenchyma, releases a myriad of cytokines that might mediate cell death and lead to reduce survival of grafted NSCs [7, 8]. Therefore, increasing the survival rate of grafted cells and subsequently improving repair to damaged tissue are key factors that may contribute to the ability of donor cells to interact with the host tissue in TBI treatment with NSCs transplantation.
It has been demonstrated that several neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), neurotrophin (NT)-3, ciliary neurotrophic factor (CNTF) and glial cell line-derived neurotrophic factor (GDNF), have neurogenesis, neurotrophic, or neuroprotective effects on the brain. Of the neurotrophic agents, BDNF has showed the most promise for the future management of brain injury. Cografted Wharton’s jelly cells-derived neurospheres and BDNF promoted functional recovery after rat spinal cord transection [9]; animals treated with BDNF following TBI showed an improved regeneration of the neural network that resembled developmental neuroplasticity. The improved regeneration was directly related to improvement in synchronized movement and spatial orientation [10, 11].
However, the delivery of BDNF via direct infusion may face clinical limitations, such as volume constraints, local inflammation and tissue injury. BDNF has been shown to affect the proliferation, survival, and differentiation of NSCs in vitro and in vivo [12–14]. The potential of BDNF gene transfer for the treatment of CNS injury has been demonstrated [15]. Following acute cervical spinal cord injury, viral-mediated gene transfer of BDNF prevented rubrospinal neuronal atrophy and enhanced the growth propensity of injured neurons by stimulating regeneration-associated gene expression [16]. However, the functional mechanisms underlying transplant-mediated recovery following TBI are not clear. Thus, the main objective of the present study was to investigate whether transplantation of NSCs genetically modified to encode BDNF gene would provide a better therapeutic result than naive NSCs by implanting these cells into a rat model of TBI. We evaluated the ability of BDNF to contribute to improving cell survival and whether BDNF overexpression through BDNF gene transfer could stimulate pre- and post-synaptic protein and regeneration-associated gene expression.
Experimental Procedure
Culturing of NSCs and Establishment of BDNF-Modified NSCs
Primary NSCs were isolated and cultured as previously described [17]. In brief, the forebrain tissue of embryonic 14-day-old Wistar rats (Experimental Animal Center of Dalian Medical University, China) was harvested under a dissecting microscope. The shredded tissue was settled down in the bottom of tube with approximately 100 μl of PBS, and then PBS was aspirate and 1 ml of Accutase (Sigma, St. Louis, MO, USA) was added to the shredded tissue and incubated 10 min at room temperature. Gentle pipetting was used to generate a single cell suspension. The suspension was centrifuged for 5 min, resuspended with culture medium, and then cells plated into T25 flasks at cell density of 0.8 × 105 cells/ml as a suspension. Cells were maintained at 37°C in a humidified atmosphere with 5% CO2. Expansion medium (DMEM and F12, 1:1; l-glutamine, 2.92 g/100 ml; HEPES, 5 mM; NaHCO3, 7.5%; glucose, 0.915 g/100 ml; and heparin, 50 mg/100 ml; all from Sigma, USA) contained N2 supplement (1%; Invitrogen, Carlsbad, CA), epidermal growth factor (EGF), 10 ng/ml and basic fibroblast growth factor (bFGF), 10 ng/ml (Invitrogen, USA). Sphere formation was observed after 4–7 days. NSCs cultured in suspension were passed every 5–7 days by dissociation to a single cell suspension with Accutase. The neurospheres were expanded for an additional 3–10 weeks in suspension. The pcDNA3.1-BDNF recombinant plasmid was constructed (Takara Biotechnology (Dalian, China). For stable cell transfection, 10 μg of the pcDNA3.1-BDNF construct or the pcDNA3.1 was transfected into the NSCs via the Effectenen transfection reagent (Invitrogen Corp., Carlsbad, CA) according to the manufacturer’s instructions. This transfection paradigm was based on previous experiments in which liposome-mediated BDNF cDNA transfer exhibited a similar profile of BDNF cDNA when it was injected following cortical impact injury [18, 19]. Twenty-four hours after transfection, cells were dissociated with Accutase and plated at clonal density into two 6-well plates and allowed to form solitary colonies. Forty-eight hours after transfection, G418 (400 mg/ml; Sigma, USA) was added for selection. Resistant NSCs subclones were then harvested, plated into 24-well plates, and expanded and tested for protein and mRNA expression. Clones containing 80–100% BDNF-positive NSCs were classified as BDNF/NSCs and propagated for later experiments.
Preparation of Cells for Transplantation
On the day of transplantation, BDNF/NSCs or naive neurospheres were harvested, dissociated to a single cell suspension with Accutase, counted on a hemocytometer, washed again, centrifuged (400×g) for 5 min into a loose pellet, and resuspended in sterile phosphate-buffered saline at a concentration of 2 × 107/ml. We prelabeled these cells through exposure to the non-diffusible vital fluorescent membrane dye PKH-26 (Sigma, USA) immediately before transplantation as per the manufacturer’s protocol. Cells were washed with complete medium, centrifuged and resuspended at a density of 1 × 105 cells/μl. The nondiffusibility of PKH-26 was verified for NSCs in a previous study [20]. Cells were maintained on ice during surgery, and the remaining cells were assayed for cell viability through the use of trypan blue dye exclusion.
Surgical Procedures and Transplantation
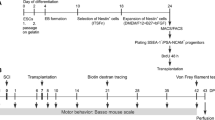
Forty-eight adult male Wister rats (weight, 200–220 g, Experimental Animal Laboratory of Dalian Medical University, China) were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee of the Dalian Medical University, Dalian, China. The controlled cortical impact (CCI) model of brain injury has been described previously [21]. Briefly, on the day of surgery the animals were deeply anesthetized with sodium pentobarbital (60 mg/kg intraperitoneally) and placed in a stereotactic frame (Stoelting Co., USA). After the skull was exposed, a 3 mm craniotomy was performed over the left parietotemporal cortex between lambda and bregma, with the dura matter left intact. CCI brain injury was induced by the use of a 2.0 mm diameter pneumatic impactor (Air-Power, Inc., High Point, NC) which indented the exposed surface of the brain by a fixed distance at a fixed velocity (tissue deformation 1 mm, 4.5 m/s tip velocity). CCI animals were randomly assigned to two groups for transplantation. One group received delivery of the BDNF/NSCs suspension that had been exposed to PKH-26 (3 μl per animal at a density of 1 × 105 cells/μl) to the cortex below the injury cavity in the ipsilateral hemisphere: anteroposterior, −3.0 mm from bregma; mediolateral, 1.0 mm; dorsoventral, 1.1 mm (n = 24). The second (control) group received an equivalent naive NSCs suspension in the same location (n = 24). Seventy-two hours after CCI injury, the animals were again anesthetized and a 10 μl Hamilton syringe was slowly advanced through the dura and cortex until the desired depth was reached. With controlled use of the Stoelting quintessential injector (Stoelting Co., USA), the cell suspension was injected over a period of 3 min (1 μl/min). The needle was left in place for an additional 5 min and then withdrawn slowly. An immunosuppressant was not used in any of the animals subjected to cell transplantation.
Assessment of Neurological Sensorimotor Dysfunction
Neurological motor function was evaluated in terms of latency to move and a gridwalk test; both tests were conducted by trained observers blinded to treatment.
Latency to move is an indicator of motor coordination and balance function after experimental TBI in rats and mice [22]. Rats were placed on a plate and the time to move one body length (30 cm) was recorded. All rats were trained prior to surgery and reached a stable baseline level of performance within 3 days. Latency to move was measured at 1 day after injury and 1, 2, 4, and 8 weeks post-transplantation (n = 6 in each group at different time points).
Gridwalk, which is sensitive in revealing sensorimotor deficits, has been used to evaluate behavioral improvements in rat ischemia, spinal cord injury, and TBI models [23]. Briefly, the grid area was 64 × 40 × 50 cm (length × width × height) with 25 × 25 mm diameter openings. Behavior was recorded using a camera in order to assess the animals’ stepping errors. Animals were given 5 min to walk atop the elevated wire surface. Footfaults and overall step number were counted for each limb. Thus, the percentage of footfaults was calculated by: (footfaults/steps + footfaults) × 100, and the asymmetry difference by: (% contralateral footfaults − % ipsilateral footfaults). A step was considered to be a stepping error if it was not providing support and the foot went through the grid hole.
Histological Analysis
At 1, 2, 4 and 8 weeks, animals (n = 3 in each group at different time points) were given a lethal dose of sodium pentobarbital (200 mg/kg intraperitoneally) and transcardially perfused with cold phosphate-buffered saline and freshly hydrolyzed 4% paraformaldehyde (pH 7.4 in 0.1 mol/l phosphate buffer). Brains were harvested, postfixed overnight, cryoprotected in increasing sucrose concentrations, and then cryosectioned at a 16 μm thickness along the coronal plane for immunohistochemistry. In order to determine the distribution and characterization of grafted cells and to assess the expression of synaptic proteins and growth-associated protein, immunohistochemistry was performed. The concomitance of PKH26 was evaluated within neurons identified by immunoreactivity to the neuron-specific protein of the cytoskeleton, βIII-tubulin, within astrocytes identified by immunoreactivity to glial fibrillary acidic protein (GFAP), with important pre- and post-synaptic proteins synaptophysin (SYP) and ProSAP1/Shank2, and with growth-associated protein (GAP-43). BDNF immunohistochemistry was also performed to determine if the transgene protein was actually being produced in BDNF/NSCs. Sections were rinsed in 0.3% Triton X-100 in phosphate-buffered saline (T-PBS) and preincubated in 10% normal goat serum in T-PBS. The sections were incubated overnight at 4°C with one of the following primary antibodies: mouse monoclonal anti-βIII-tubulin (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), GFAP (1:200; Boster Biotechnology, Wuhan, China), BDNF (1:200; Boster, China), mouse monoclonal anti-SYP (1:500; Sigma, St Louis, MO), rabbit anti-ProSAP1/Shank2 (1:300; Santa Cruz). After being washed three times, the sections were incubated with the appropriate fluorescent secondary antibody for 1 h at room temperature; these included fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (1:1,000; Sigma, USA) and FITC-conjugated goat anti-rabbit IgG (1:1,000; Sigma, USA). The sections were washed three times with PBS and then coverslipped with Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, CA) for a nuclear counterstain. Images were taken with a Nikon compound fluorescent microscope and a Nikon C1 Plus confocal microscope.
Western Blot Analysis
Another 3 animals per group were decapitated at each time point. Brain tissue encompassing the transplant site was obtained immediately and dissected into two parts on a bed of ice along the center of the injury cavity for Western blot and reverse transcription-polymerase chain reaction (RT-PCR) detection (described below). Western blot analysis was performed as described previously [24]. Each sample was sonicated in 0.0625 M Tris–HCl (pH 6.8) and then centrifuged at 12,000×g for 15 min at 4°C. The supernatant was assayed for protein using a Bradford assay. Aliquots (50 μg) of protein were boiled in denaturing sample buffer (62.5 mmol/l Tris (pH 6.8), 2% SDS, 5 mmol/l EDTA, 10% glycerol, 0.25% 2-mercaptoethanol, and 0.01% bromophenol blue). Brain samples were loaded onto SDS–PAGE mini-gels, electrophoresed, and transferred to nitrocellulose membranes. After transferring, membranes were blocked with 5% milk in TBST (0.1% Tween 20 in 20 mM Tris–HCl, pH 7.4, and 410 mM NaCl) for 2 h at room temperature. Blots were incubated overnight at 4°C with mouse monoclonal anti-SYP (1:1,000; Sigma, USA), rabbit anti-ProSAP1/Shank2 polyclonal antibody (1:500; Santa Cruz), or rabbit anti-GAP-43 polyclonal antibody (1:500, KeyGEN, China). Following primary antibody incubations, blots were washed three times for 5 min each with TBST, incubated for 1 h with horseradish peroxidase (HRP)-linked secondary antibodies, washed four times for 10 min each with TBST, and developed with the enhanced chemiluminescence Western blotting detection system kit (ECL Plus, Amersham Biosciences, NJ, USA). In some instances, membranes were stripped by incubation in stripping buffer (62.5 mM Tris–HCl (pH 6.8), 2% SDS, and 100 mM 2-mercaptoethanol) for 30 min at 50°C and reused.
Semiquantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from the tissue using an RNA isolation reagent (TRIZOL, TaKaRa, Dalian, China) according to the manufacturer’s instructions and treated with RNase-free DNase (TaKaRa, Dalian, China). Single-stranded cDNA synthesis was performed using AMV Reverse Transcriptase (TaKaRa, Dalian, China) according to the manufacturer’s protocol. PCR was performed using Taq Dynazyme (TaKaRa, Dalian, China) under standard conditions (5× PCR Buffer 10 μl, dH2O 28.75 μl, TaKaRa Ex Taq HS 0.25 μl, 0.5 μl of each specific primer and 10 μl of cDNA) using a 4 min hot start at 94°C followed by 30 cycles at 94°C for 30 s, 55°C for 30 s, 72°C for 1 min, and a 10 min final extension at 72°C. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was co-amplified as an internal control in each reaction. PCR products were analyzed by 1.5% agarose gel electrophoresis and visualized using ethidium bromide fluorescence. The products were run on 1% agarose gels containing 10 ng/ml ethidium bromide and visualized under UV light. All primer pairs were obtained from TaKaRa Biotechnology (Dalian) Co., Ltd. and were as follows:
BDNF (rat) | Forward, 5′-AAGCTTATGACCATCCTTTTCCTTAC-3′ |
Reverse, 5′-GAATTCCTATCTTCCCCTTTTAATGG-3′ | |
SYP | Forward, 5′-GCACCAAGTGTACTTTGATG-3′ |
Reverse, 5′-GAACACGAACCATAAGTTGC-3′ | |
Shank2 | Forward, 5′-CTTTGTGGTTGACAAGCCCCCAGTAC-3′ |
Reverse, 5′-CCTGAGGTCCTGCTTTCATAGTC-3′ | |
GAP-43 | Forward, 5′-TGCTGTGCTGTATGAGAAGAACC-3′ |
Reverse, 5′-GGCAACGTGGAAAGCCGTTTCTTAAAGT-3′ | |
GAPDH | Forward, 5′-TGTGATGGGTGTGAACCACGAGA-3′ |
Reverse, 5′-GAGCCCTTCCACAATGCCAAAGTT-3′ |
Quantification and Statistical Analysis
All transplanted cells in the CCI injury core and along the lesion border were quantified at different time points post-transplantation. For each cell marker, we immunostained the 3 sections with the highest number of transplanted cells per animal (n = 3); 3 regions/section were counted for double-labeled cells (cells positive for both PKH26 and the specific antibody). The proportion of double-labeled PKH26-positive cells was calculated for each animal. To quantify the number of transplanted cells, every tenth 16 μm thick section throughout the thickness of the transplantation site and along the lesion border (1.0 to −4.0 mm from bregma) was analyzed, and all PKH26-positive cells were counted. The number of PKH26-positive cells in all of the counted sections was multiplied by 10 to compensate for the sampling frequency [25]. Differences between mean values for different time points within a group were analyzed using one-way ANOVAs, and independent sample t tests were performed to detect the difference between mean values for these groups at the same time point. Data are presented as the means ± SD, and the difference was considered significant at P < 0.05.
Results
Survival, Distribution and Differentiation of Engrafted Rat Cortical NSCs
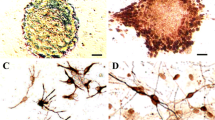
CCI injury caused a marked cell loss in the left parietal cortex and ipsilateral hippocampus of the rats. The distribution pattern of the engrafted NSCs in the naive NSCs and BDNF/NSCs groups was similar. The vast majority of the surviving cells labeled with PKH26 were located within the implantation sites and injection tracks at 1 week post-implantation (see Fig. 1a), but many transplanted cells had migrated extensively outside of the transplantation sites and were located in the granular layer of the ipsilateral or contralateral cortex in the rostral migratory stream (see Fig. 1b), the hippocampus, the pons and even the Purkinje cell layer of the cerebellum by 2 weeks post-transplantation (see Fig. 1c). Cells labeled with PKH26 were quantified. Both BDNF/NSCs-transplanted rats and NSCs-transplanted rats had a decrease in the number of surviving cells during the 8 week period, especially NSCs-transplanted rats. We found that the number of surviving engrafted cells was more marked in BDNF/NSCs-transplanted rats than in NSCs-transplanted rats at 1, 2, 4 and 8 weeks post-transplantation (P < 0.05; see Fig. 1d). Figure 1e–j showed the surviving PKH26-positive cells in the BDNF/NSCs-transplanted (see Fig. 1e, g, i) and NSCs- transplanted groups (see Fig. 1f, h, j) at 1, 4 and 8 weeks post-transplantation, respectively. Most of the engrafted cells located in the implantation sites had undifferentiated morphology and were nestin positive. Some engrafted cells were also GFAP positive and βIII-tubulin positive at 1 week post-transplantation (data not shown). Representative GFAP positive (see Fig. 2a) and βIII-tubulin positive (see Fig. 2b) staining in theses engrafted cells was demonstrated at 2 weeks post-transplantation. What’s more important, we also found that the proportion of βIII-tubulin positive cells was significantly different in BDNF/NSCs compared to NSCs-transplanted rats during the 8 week period post-transplantation (12.9 ± 2.4% vs. 6.1 ± 1.7%, 20.0 ± 1.8% vs. 9.6 ± 2.0%, 21.6 ± 1.6% vs. 16.0 ± 1.9%, and 20.7 ± 1.7% vs. 14.4 ± 0.9% at 1, 2, 4 and 8 weeks, respectively; see Fig. 2c). As shown in Fig. 2d–g, the proportion of βIII-tubulin positive cells with more extensive neurite growth was significantly different in BDNF/NSCs-transplanted rats (see Fig. 2d, f) compared to NSCs-transplanted rats (see Fig. 2e, g) at 4 and 8 weeks post-transplantation. Although, there was an increased proportion of neuronal cells at 4 compared to 2 weeks in BDNF/NSCs-transplanted rats, no significant difference was found (P > 0.05). In contrast, there was a significant difference in the proportion of neuronal cells in NSCs-transplanted rats at 4 compared to 2 weeks (P < 0.05).
Survival and distribution of fluorescently labeled cortical NSCs in the damaged rat brain. Stereotactic CCI resulted in a discrete focal neural tissue lesion (a, outlined). Low magnification image of a 16-μm-thick optical section shows the number of surviving engrafted PKH26-positive cells in the CCI injury core and along the lesion border in BDNF/NSCs and NSCs-transplanted rats at 1 week post-transplantation (a). Photomicrographs show NSCs in the granular layer of the cortex in the rostral migratory stream (b) and in the Purkinje cell layer of the cerebellum (c) at 2 weeks post-transplantation in a TBI rat. The number of surviving grafted cells was more marked in BDNF/NSCs-transplanted rats than in NSCs-transplanted rats at 1, 2, 4 and 8 weeks post-transplantation (d). The surviving PKH26-positive cells in the BDNF/NSCs (e, g, i) and NSCs (f, h, j) transplant groups at 1, 4 and 8 weeks post-transplantation, respectively
Differentiation of engrafted NSCs in the damaged rat brain. (a, b) Representative immunostains with GFAP astrocytes (a, ↑) and neuron-specific tubulin (b, ↖) in fluorescently labeled NSCs in the damaged rat brain at 2 weeks post-transplantation were shown. The proportion of βIII-tubulin positive cells with more extensive neurite growth was significantly different in BDNF/NSCs-transplanted rats compared to NSCs-transplanted rats at various time points (c). βIII-tubulin positive cells in NSCs transplant rats (d, f) and BDNF/NSCs transplant rats (e, g) at 4 and 8 weeks post-transplantation, respectively. Data are expressed as the mean ± SD (n = 3 in each group). The * and ** indicate P < 0.05 and P < 0.01 versus the NSCs group at each time point; the #indicates P < 0.05 versus the NSCs group at 2 weeks post-transplantation
Overexpression of BDNF in BDNF/NSCs In Vitro and In Vivo at Different Time Points Post-Transplantation
When NSCs were transfected by the exogene BDNF for 48 h, few cells showed immunoreactivity to BDNF. Ten days after G418 was added for selection, the neurospheres showed immunoreactivity to BDNF and many scattered cells were negative (see Fig. 3a). In addition, BDNF mRNA expression in an aliquot of cells from the transplanted cells in NSCs and BDNF/NSCs was detected by RT-PCR. The results showed overexpression of BDNF in BDNF/NSCs (see Fig. 3b). We performed RT-PCR to detect BDNF expression in the transplant site of damaged brains at different time points post-transplantation [26]. As shown in Fig. 3c and d, compared to the NSCs-transplanted group, engrafted BDNF/NSCs significantly increased BDNF mRNA expression in rats subjected to CCI injury at 1 and 2 weeks after transplantation as analyzed by RT-PCR (P < 0.05).
Overexpression of BDNF in BDNF/NSCs. a Immunostaining of neurospheres genetically modified to encode BDNF gene for 10 days after the addition of G418 for selection indicates that successful gene transfer has occurred. b RT-PCR shows strong expression of BDNF mRNA in the BDNF/NSCs compared to the pcDNA3.1 or NSCs (M, Marker 2000; 1, BDNF/NSCs at 10 days after transfer; 2, pcDNA3.1; 3, NSCs). c, d Semi-quantitative RT-PCR analysis shows a marked increase in BDNF gene expression in the brains of BDNF/NSCs-transplanted rats compared to that in NSCs-transplanted rats at 1 and 2 weeks post-transplantation. The intensity of each band was standardized by the band intensity of GAPDH. Lanes 1, 3, 5 and 7 are from BDNF/NSCs-transplanted rats, and lanes 2, 4, 6 and 8 are from NSCs-transplanted rats; lanes 1–8 are derived from rats at 1, 2, 4 and 8 weeks post-transplantation, respectively. Data are expressed as the mean ± SD (n = 3 in each group). The * indicates P < 0.05
Functional Recovery of Motor Behavior Mediated by BDNF/NSCs and NSCs Transplantation
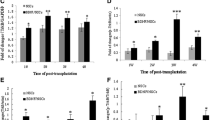
All injured groups displayed significant motor impairment 1 day following TBI. Latency to move was significantly longer in all injured rats 1 day, 1 and 2 weeks after CCI than before CCI. Latency to move was significantly longer in NSCs-transplanted rats than in BDNF/NSCs rats at 1 week post-transplantation (P < 0.05). Although, BDNF/NSCs-transplanted rats exhibited shorter latencies than did NSCs-transplanted rats, this effect did not reach statistical significance at 2 weeks post-transplantation (P > 0.05) when the BDNF/NSCs-transplanted rats’ performance returned to pre-surgery levels (see Fig. 4a). All NSCs-transplanted rats had significantly more contralateral than ipsilateral footfaults for at least 2 weeks following injury relative to baseline. ANOVAs of fore- and hind-limb performance in both BDNF/NSCs and NSCs-transplanted rats revealed significant effects of time (P < 0.05). t tests indicated that at 1 week, NSCs-transplanted rats exhibited significant footfault asymmetry compared to BDNF/NSCs-transplanted rats (P < 0.05). At 2 weeks post-transplantation, hindlimb performance in NSCs-transplanted rats was significantly different compared to baseline and BDNF/NSCs-transplanted rats; however, no significant difference in forelimb performance was found between NSCs and BDNF/NSCs-transplanted rats (see Fig. 4b, c). The performance of both NSCs and BDNF/NSCs-transplanted rats returned to pre-surgery levels at 8 weeks in selected behavioral tests (data not shown).
Behavioral function tests after TBI. a Latency to move. b, c Fore- and hindlimb asymmetry differences (% contralateral/% ipsilateral footfaults) on the gridwalk task. Data are expressed as the mean ± SD (n = 6 in each group at different time points). The * indicates P < 0.05 versus the NSCs group at different time points. The #indicates P < 0.05 versus baseline
Effect of NSCs Transplantation on the Expression of Pre- and Post-synaptic Proteins
We detected immunofluorescent staining for pre- and post-synaptic proteins (SYP and Shank2) in brain sections from NSCs to BDNF/NSCs-transplanted rats at different time points post-transplantation. Representative SYP (see Fig. 5a, b) and Shank2 (see Fig. 5c, d) staining was found in the cytoplasm juxtaposed to the nucleus at 1 week post-transplantation. Our study shows that SYP and Shank2 staining was significantly greater in BDNF/NSCs-transplanted rats compared to NSCs-transplanted rats. It was not possible to perform a precise semiquantitative analysis due to the high number and density of cell bodies and fibers in the sections that were immunoassayed with the cytoplasm antibody.
The immunoreactivity of SYP and Shank2 in the core of the lesion graft at 1 week post-transplantation. a, b SYP-positive staining in 16 μm sections from the brains of NSCs- and BDNF/NSCs-transplanted rats. c, d Shank2-positive staining in brains from NSCs- to BDNF/NSCs-transplanted rats. b, d show improved expression of SYP and Shank2 in BDNF/NSCs-transplanted rats (right arrow) compared to that of NSCs-transplanted rats a, c. DNA was stained with DAPI. The scale bar represents 30 μm
We also performed Western blots and RT-PCR to detect protein and gene expression of SYP and Shank2 in the transplant core and boundary of the CCI injury at various time points post-transplantation. Figure 5 shows the expression of SYP and Shank2 at both the gene (see Fig. 6a–c) and protein level (see Fig. 6d–f) in the BDNF/NSCs- and NSCs-transplanted groups at various time points post-transplantation. As shown in Fig. 5, the gene expression of SYP was 0.44, 0.40, 0.68, and 0.67-fold at 1, 2, 4, and 8 weeks, respectively, after transplantation in NSCs-transplanted rats, and 0.61, 0.59, 0.77, and 0.73-fold, respectively, in BDNF/NSCs-transplanted rats (see Fig. 6a, b). Gene expression levels of Shank2 were 0.033, 0.034, 0.086, and 0.037-fold at 1, 2, 4, and 8 weeks, respectively, after transplantation in NSCs-transplanted rats, and 0.063, 0.064, 0.11 and 0.047-fold, respectively, in BDNF/NSCs-transplanted rats (see Fig. 6a, c). Significantly higher gene expression of SYP and Shank2 was found in BDNF/NSCs compared to NSCs-transplanted rats at 1 and 2 weeks after transplantation (P < 0.01). Moreover, there was a significant difference in Shank2 expression in BDNF/NSCs compared to NSCs-transplanted rats at 4 and 8 weeks after transplantation (P < 0.05). However, no significant difference was found in SYP expression in BDNF/NSCs compared to NSCs-transplanted rats at this late stage post-transplantation (P > 0.05; see Fig. 6a–c). In addition, the expression of SYP and Shank2 mRNA was highest at 4 weeks post-transplantation in both BDNF/NSCs and NSCs-transplanted rats, followed by a decrease at 8 weeks post-transplantation. The protein expression of SYP and Shank2 as detected by Western blot confirmed these results (see Fig. 6d–f). We also found that Shank2 migrated as a 165/180 kDa doublet protein in the brains from BDNF/NSCs-transplanted rats; however, one lane (180 kDa) of Shank2 product was scarce in NSCs-transplanted rats (see Fig. 6d).
The expression of SYP and Shank2 in the transplant site and the boundary zone of the lesion. In (a) and (d), lanes 1, 3, 5 and 7 are from BDNF/NSCs-transplanted rats, and lanes 2, 4, 6 and 8 are from NSCs-transplanted rats. Lanes 1–8 are derived from rats at 1, 2, 4 and 8 weeks post-transplantation, respectively. Representative results of semi-quantitative RT-PCR (a) and Western blot analysis (d) show significantly increased SYP gene and protein expression in BDNF/NSCs-transplanted rats compared to NSCs-transplanted rats at 1 and 2 weeks post-transplantation, and a clear increase in Shank2 gene and protein expression in BDNF/NSCs-transplanted rats compared to NSCs-transplanted rats during the 8 weeks after transplantation. Densitometric analysis of SYP with semi-quantitative RT-PCR (b) and Western blot bands (e). Densitometric analysis of Shank2 with semi-quantitative RT-PCR (c) and Western blot bands (f). The intensity of each band was standardized by the band intensity of GAPDH or actin. Data are expressed as the mean ± SD (n = 3 in each group). The * and ** indicate P < 0.05 and P < 0.01 versus the NSCs group at different time points
NSCs Genetically Modified to Encode BDNF Gene Promote GAP-43 Expression in the Injured Brain
GAP-43 is a growth-associated protein that has been linked to synaptic regeneration in response to injury-associated behavioral deficits [27]. We therefore examined GAP-43 expression in injured brains from BDNF/NSCs to NSCs-transplanted rats. GAP-43 mRNA levels were measured by semiquantitative RT-PCR, and the amounts of GAP-43 protein were quantified by Western blot. There was a continuous increase in GAP-43 mRNA and protein expression in both BDNF/NSCs and NSCs-transplanted rats during the 8 weeks post-transplantation. However, at 1 week post-transplantation, GAP-43 mRNA was 1.85-fold more abundant in BDNF/NSCs-transplanted rats than in NSCs-transplanted rats; this difference decreased to 1.65-fold at 2 weeks post-transplantation (Fig. 7a, b) and further decreased at 4 and 8 weeks post-transplantation. Western blot analysis indicated higher amounts of GAP-43 protein in the brains of rats treated with BDNF/NSCs than in those treated with NSCs at 1 (1.81-fold) and 2 weeks (1.77-fold) post-transplantation (see Fig. 7c, d).
The BDNF exogene promotes regeneration-associated gene expression in the injured brain. In a and c, lanes 1, 3, 5 and 7 are from BDNF/NSCs-transplanted rats and lanes 2, 4, 6 and 8 are from NSCs-transplanted rats. Lanes 1–8 are derived from rats at 1, 2, 4 and 8 weeks post-transplantation, respectively. Representative results from semi-quantitative RT-PCR for GAP-43 (a) and densitometric analysis of these bands (b). The intensity of each band was standardized to the band intensity of GAPDH. Representative Western blots for GAP-43 (c) and densitometric analysis of protein bands (d). The intensity of each band was standardized to the band intensity of actin. Data are expressed as the mean ± SD (n = 3 in each group). The * and ** indicate P < 0.05 and P < 0.01 versus the NSCs group at different time points
Discussion
Our data show that BDNF/NSCs transplantation significantly improves neurological motor function in selected behavioral tests compared to naive NSCs transplantation after experimental TBI. The number of surviving engrafted cells was significantly greater in the brains of rats subjected to BDNF/NSCs transplantation than in those subjected to naive NSCs transplantation following TBI. Additionally, the proportion of tubulin-positive cell in engrafted cells was increased in BDNF/NSCs-transplanted rats, especially at the early stage of post-transplantation. We found that the expression of pre- and post-synaptic proteins and a regeneration-associated gene was significantly increased in the BDNF/NSCs compared to the NSCs-transplanted rats. These findings indicate that transplantation of NSCs genetically modified to encode BDNF gene may provide a better therapeutic effect than naive NSCs in a rat model of TBI and help provide an understanding of the mechanism underlying the improvement of neurological functional in TBI animals treated with cells transplantation.
Previous studies indicated that transplanted NSCs ameliorated neurological deficits and promoted repair and restoration of lost function after TBI [21, 28]. The mechanisms underlying the improvement in motor function are unclear. The beneficial effects of transplanted NSCs on motor behavior might involve the release of neurotrophic factors by the transplanted cells [13]. Shear et al. [28] showed that NSCs transplantation were capable of enhancing motor recovery as early as 1 week after transplantation. This recovery was not a consequence of the direct replacement of injured neurons, but rather suggested a mechanism of repair involving trophic support. It was also reported that NSCs have the ability to produce neurotrophic factors such as BDNF and GDNF, which might lead to neuroprotection and restoration of function [29]. Therefore, we assumed that NSCs genetically modified to encode BDNF gene might provide further beneficial therapeutic effects compared to naive NSCs in a model of TBI.
In the current study, the CCI injury model produced marked neurological motor dysfunction. BDNF/NSCs demonstrated BDNF overexpression when NSCs were genetically modified to encode BDNF gene. The engrafted BDNF/NSCs significantly increased BDNF mRNA expression in the transplant site of damaged brains at 1 and 2 weeks after transplantation. Previous studies have shown that NSCs can naively and constitutively secrete significant quantities of several neurotrophic factors in vitro, including nerve growth factor (NGF), BDNF, and GDNF. Overexpression of one growth factor has a reciprocal effect on the expression of another factor. When NSCs were genetically modified to produce BDNF, it was possible to significantly expand the effects of NSCs on the neural repair of host tissue by implanting these modified cells [30]. In the current study, BDNF/NSCs transplant into the injury cavity led to a significant reduction in latencies and improved performance on the gridwalk compared to naive NSCs transplantation at an earlier stage post-transplantation. No differences were observed in motor function between BDNF/NSCs and NSCs-transplanted rats at 4 weeks post-transplantation. Increased BDNF mRNA expression in BDNF/NSCs-transplanted rats during the initial few weeks after transplantation may partially contribute to the greater beneficial effects of these engrafted cells on functional motor outcome than those in NSCs-transplanted rats [21].
It has long been known that BDNF, a member of the NGF neurotrophin family, plays an essential regulatory role in the survival and differentiation of various neuronal populations during development and after injury. Induction of BDNF and activation of its intracellular receptors can produce neural regeneration, reconnection, and dendritic sprouting, and can improve synaptic efficacy in conditions of TBI [11]. In vitro, single EGF-generated spheres showed a twofold increase in neuron number and a marked enhancement in neurite outgrowth 10 days after a one-time exposure to BDNF; this showed that BDNF markedly enhanced the morphological differentiation of EGF-generated neuronal precursors. However, BDNF alone did not appear to act as a survival factor for neuronal precursors, nor was it sufficient for preventing their death over time [12]. In addition, it has previously been reported that BDNF could act in mediating cortical progenitor cell survival and neurogenesis through the binding of BDNF to trkB, which induces trkB phosphorylation and downstream phosphorylation of MEK and Akt in vitro [31]. BDNF gene transfer with adeno-associated virus might prevent rubrospinal neuronal atrophy after acute cervical spinal cord injury [16].
In this study, the surviving engrafted cells were significantly increased in BDNF/NSCs rats compared to NSCs rats during the experiment. The other underlying mechanism that could account for the greater survival of grafted cells in BDNF/NSCs rats might be due to the fact that BDNF and NGF have similar immunosuppressive and neuroprotective properties that contribute to the maintenance of brain immunity privileges through increased production of anti-inflammatory chemical mediators; this is crucial for the survival of engrafted cells after TBI [32]. Moreover, we have shown that the number of neurons in engrafted cells was significantly increased in BDNF/NSCs-transplanted rats compared to NSCs-transplanted rats at different time points post-transplantation, especially at 1 week post-transplantation. One-way ANOVA tests showed that there was a significant increase in the number of neurons in engrafted cells at 4 weeks compared to 2 weeks post-transplantation in NSCs-transplanted rats, but no statistically significant difference in BDNF/NSCs-transplanted rats. These results suggest that BDNF caused NSCs to promote differentiation and increased the proportion of grafted cells with a neuronal phenotype in vivo. However, the naive NSCs may acquire a mature phenotype over a longer period of time. The increase in the survival and differentiation of engrafted cells is crucial for improving the therapeutic effect of NSCs transplantation for CNS injury.
The ability of NSCs to establish and maintain synaptic contacts is one of the basic requirements for intercellular communication and functional integration into existing neuronal networks. Oxidative stress following TBI most likely contributed to the loss of synaptic proteins, which are known to affect neuronal survival and function [33]. In this study, we investigated the expression of the important pre- and post-synaptic proteins SYP and ProSAP1/Shank2. SYP, which is often used as a general marker protein for presynaptic nerve endings, interacts with other proteins involved in exocytosis and has a wide range of neuronal functions including neurotransmitter release, controlled vesicle fusion, synaptogenesis, cytoskeletal structural dynamics, energy metabolism, ion homeostasis, and protein folding [34]. It has also been suggested that SYP plays a role in activity-dependent competitive synapse formation [35]. Because the loss of synapses correlates with the degree of brain dysfunction, the level of SYP also serves as a marker of brain function [36]. ProSAP1/Shank2, a member of the ProSAP/Shank family, is a post-synaptic multidomain protein that interacts directly and indirectly with a large number of synaptic proteins [37]. In addition, ProSAPs/Shanks are very early components of post-synaptic specializations and interact with the actin-based cytoskeleton. Thus, ProSAPs/Shanks have been considered to constitute “master scaffold” post-synaptic density molecules during the maturation of synaptic contacts [38].
In our study, there were significant increases in synaptic proteins including SYP and Shank2 in the BDNF/NSCs-transplanted rats compared to the NSCs-transplanted rats at 1 and 2 weeks post-transplantation. On one hand, improved differentiation of BDNF-modified NSCs may contribute to an increase in synaptic proteins at an earlier stage; once the development of NSCs is committed toward a neuronal phenotype, the “program” of synaptogenesis is initiated, leading to the expression and localization of synaptic proteins at membranous attachment sites, which maturate into synapses [39]. Therefore, increased synaptic protein expression in BDNF/NSCs-transplanted rats could partly result from the neurons that specifically arose from differentiated NSCs. On the other hand, BDNF has been shown to enhance synaptic efficacy and promote the formation of new synapses both in vitro and in vivo [40, 41]. It was reported that BDNF induced the morphological rearrangement of individual pre-synaptic compartments and promoted the formation of new synapses; this was most likely caused by BDNF disrupting cadherin–β-catenin complexes and enhancing the motility of synaptic vesicles clusters. This might help to form new synapses as the clusters of mobile vesicles populate new territories [42]. Thus, it is possible that increased synaptic protein expression in BDNF/NSCs-transplanted rats was caused by resident host neurons that responded to exogenous BDNF/NSCs. Therefore, enhanced expression of synaptic proteins matching the observed increase in BDNF might suggest possible reconstruction of neural circuitry and enhanced functional recovery after BDNF/NSCs engrafting.
Synaptic and axonal regeneration after brain injury is essential for predicting the neural compensation that can be achieved after various types of injury. GAP-43 is a growth-associated protein that has been linked to synaptic regeneration in response to injury-associated behavioral deficits [27]. GAP-43 expression is high in the normal developing brain and decreases in adult mice, which retain the capacity to undergo synaptic remodeling [43]. Any process that involves axonal membrane remodeling, such as neurite growth, is expected to be associated with increased expression of GAP-43 [44]. We therefore assumed that increased GAP-43 mRNA and protein expression in the brains of rats subjected to BDNF/NSCs transplantation could account for a better therapeutic effect than that caused by naive NSCs transplantation following TBI. In this study, we observed a significant difference in the expression of GAP-43 between BDNF/NSCs and NSCs-transplanted rats following TBI, especially in the early stage post-transplantation. Previous studies also showed that BDNF specifically enhanced GAP-43 mRNA level in injured retinal ganglion cells (RGCs). The intravitreal administration of a single dose of BDNF to axotomized RGCs on the day of injury caused a moderate and short-lasting enhancement in GAP-43 mRNA levels in most RGCs during the first week after axotomy. It was suggested that the changes in GAP-43 expression correlated with the branching of injured neurons [45]. Effects of BDNF on GAP-43 mRNA expression might be mediated by a Nitric Oxide (NO)-dependent mechanism [46]. The injured axons remain responsive to the neurotrophins, which means that the neurotrophins might stimulate both regenerative and sprouting responses and that the grafted cells might continue to secrete the neurotrophins [47].
In conclusion, these results suggest that transplantation of BDNF/NSCs, NSCs genetically modified to encode BDNF gene, facilitates the survival of grafted cells and increases the proportion of grafted cells with a neuronal phenotype. Compared to NSCs-transplanted rats, neurite growth and overexpression of synaptic proteins in BDNF/NSCs-transplanted rats are associated with overexpression of BDNF, which is hypothesized to be one of the mechanisms underlying the improved functional recovery of motor behavior at early stages of cell transplantation following TBI. BDNF/NSCs transplantation may potentially be a more effective therapy for improving neurological outcome compared to naive NSCs transplantation.
References
Shames J, Treger I, Ring H et al (2007) Return to work following traumatic brain injury: trends and challenges. Disabil Rehabil 29:1387–1395
Aebischer P, Goddard M, Signore AP et al (1994) Functional recovery in hemiparkinsonian primates transplanted with polymerencapsulated PC12 cells. Exp Neurol 126:151–158
Gage FH (2000) Mammalian neural stem cells. Science 287:1433–1438
Lindvall O, Kokaia Z, Martinez-Serrano A (2004) Stem cell therapy for human neurodegenerative disorders: how to make it work? Nat Med 10:42–50
Benninger Y, Marino S, Hardegger R et al (2000) Differentiation and histological analysis of embryonic stem cell-derived neural transplants in mice. Brain Pathol 10:330–341
Harting MT, Sloan LE, Jimenez F et al (2009) Subacute neural stem cell therapy for traumatic brain injury. J Surg Res 153:188–194
Lenzlinger PM, Morganti-Kossmann MC, Laurer HL et al (2001) The duality of the inflammatory response to traumatic brain injury. Mol Neurobiol 24:169–181
Modo M, Stroemer P, Tang E et al (2002) Effects of implantation site of stem cell grafts on behavioral recovery from stroke damage. Stroke 33:2270–2278
Zhang L, Zhang HT, Hong SQ et al (2009) Cografted Wharton’s jelly cells-derived neurospheres and BDNF promote functional recovery after rat spinal cord transection. Neurochem Res 34:2030–2039
Willson ML, McElnea C, Mariani J et al (2008) BDNF increases homotypic olivocerebellar reinnervation and associated fine motor and cognitive skill. Brain 131:1099–1112
Kaplan GB, Vasterling JJ, Vedak PC (2010) Brain-derived neurotrophic factor in traumatic brain injury, post-traumatic stress disorder, and their comorbid conditions: role in pathogenesis and treatment. Behav Pharmacol 21:427–437
Ahmed S, Reynolds BA, Weiss S (1995) BDNF enhances the differentiation but not the survival of CNS stem cell- derived neuronal precursors. J Neurosci 15:5765–5778
Mahmood A, Lu D, Wang L et al (2002) Intracerebral transplantation of marrow stromal cells cultured with neurotrophic factors promotes functional recovery in adult rats subjected to traumatic brain injury. J Neurotrauma 19:1609–1617
Islam O, Loo TX, Heese K (2009) Brain-derived neurotrophic factor (BDNF) has proliferative effects on neural stem cells through the truncated TRK-B receptor, MAP kinase, AKT, and STAT-3 signaling pathways. Curr Neurovasc Res 6:42–53
Moro K, Shiotani A, Watabe K et al (2006) Adenoviral gene transfer of BDNF and GDNF synergistically prevent motoneuron loss in the nucleus ambiguus. Brain Res 1076:1–8
Kwon BK, Liu J, Lam C et al (2007) Brain-derived neurotrophic factor gene transfer with adeno-associated viral and lentiviral vectors prevents rubrospinal neuronal atrophy and stimulates regeneration-associated gene expression after acute cervical spinal cord injury. Spine 32:1164–1173
Ma XH, Shi Y, Hou Y et al (2010) Slow-freezing cryopreservation of neural stem cell spheres with different diameters. Cryobiology 60:184–191
Iwamoto Y, Yang K, Clifton GL et al (1996) Liposome-mediated BDNF cDNA transfer in intact and injured rat brain. Neuroreport 7:609–612
Boockvar JA, Schouten J, Royo N et al (2005) Experimental traumatic brain injury modulates the survival, migration, and terminal phenotype of transplanted epidermal growth factor receptor-activated neural stem cells. Neurosurgery 56:163–171
Fauza DO, Jennings RW, Teng YD et al (2008) Neural stem cell delivery to the spinal cord in an ovine model of fetal surgery for spina bifida. Surgery 144:367–373
Riess P, Zhang C, Saatman KE et al (2002) Transplanted neural stem cells survive, differentiate, and improve neurological motor function after experimental traumatic brain injury. Neurosurgery 51:1043–1054
Lubjuhn J, Gastens A, von Wilpert G et al (2009) Functional testing in a mouse stroke model induced by occlusion of the distal middle cerebral artery. J Neurosci Methods 184:95–103
Baskin YK, Dietrich WD, Green EJ (2003) Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J Neurosci Methods 129:87–93
Bermpohl D, You Z, Korsmeyer SJ et al (2006) Traumatic brain injury in mice deficient in bid: effects on histopathology and functional outcome. J Cereb Blood Flow Metab 26:625–633
Mothe AJ, Tator CH (2008) Transplanted neural stem/progenitor cells generate myelinating oligodendrocytes and schwann cells in spinal cord demyelination and dysmyelination. Exp Neurol 213:176–190
Zaheer A, Zhong W, Lim R (1995) Expression of mRNAs of multiple growth factors and receptors by neuronal cell lines: detection with RT-PCR. Neurochem Res 20:1457–1463
Benowitz LI, Routtenberg A (1997) GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci 20:84–91
Shear DA, Tate MC, Archer DR et al (2004) Neural progenitor cell transplants promote long-term functional recovery after traumatic brain injury. Brain Res 1026:11–22
Lennard PN, Kristen JA, Lyda MR et al (2004) Neural stem cells express melatonin receptors and neurotrophic factors: colocalization of the MT1 receptor with neuronal and glial markers. BMC Neurosci 5:41
Lu P, Jones LL, Snyder EY et al (2003) Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol 181:115–129
Barnabé-Heider F, Miller FD (2003) Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways. J Neurosci 23:5149–5160
Stanisz AM, Stanisz JA (2000) Nerve growth factor and neuroimmune interactions in inflammatory diseases. Ann NY Acad Sci 917:268–272
Ansari MA, Roberts KN, Scheff SW (2008) Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med 45:443–452
Valtorta F, Pennuto M, Bonanomi D et al (2004) Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis? Bioessays 26:445–453
Tarsa L, Goda Y (2002) Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci USA 99:1012–1016
Shojo H, Kibayashi K (2006) Changes in localization of synaptophysin following fluid percussion injury in the rat brain. Brain Res 1078:198–211
Boeckers TM, Bockmann J, Kreutz MR et al (2002) ProSAP/Shank proteins: a family of higher order organizing molecules of the post-synaptic density with an emerging role in human neurological disease. J Neurochem 81:903–910
Böckers TM, Mameza MG, Kreutz MR et al (2001) Synaptic scaffolding proteins in rat brain. Ankyrin repeats of the multidomain shank protein family interact with the cytoskeletal protein alpha-fodrin. J Biol Chem 276:40104–40112
Liebau S, Vaida B, Storch A (2007) Maturation of synaptic contacts in differentiating neural stem cells. Stem Cells 25:1720–1729
Bramham CR, Messaoudi E (2005) BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol 76:99–125
Ying Z, Roy RR, Zhong H et al (2008) BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience 155:1070–1078
Bamji SX, Rico B, Kimes N et al (2006) BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin–β-catenin interactions. J Cell Biol 174:289–299
Jacobs KM, Neve RL, Donoghue JP (1993) Neocortex and hippocampus contain distinct distributions of calcium-calmodulin protein kinase II and GAP43 mRNA. J Comp Neurol 336:151–160
Hulsebosch CE, DeWitt DS, Jenkins LW et al (1998) Traumatic brain injury in rats results in increased expression of Gap-43 that correlates with behavioral recovery. Neurosci Lett 255:83–86
Fournier AE, Beer J, Arregui CO et al (1997) Brain-derived neurotrophic factor modulates GAP-43 but not T alpha1 expression in injured retinal ganglion cells of adult rats. J Neurosci Res 47:561–572
Klöcker N, Jung M, Stuermer CA et al (2001) BDNF Increases the number of axotomized rat retinal ganglion cells expressing GAP-43, L1, and TAG-1 mRNA: a supportive role for nitric oxide? Neurobiol Dis 8:103–113
Tobias CA, Shumsky JS, Shibata M et al (2003) Delayed grafting of BDNF and NT-3 producing fibroblasts into the injured spinal cord stimulates sprouting, partially rescues axotomized red nucleus neurons from loss and atrophy, and provides limited regeneration. Exp Neurol 184:97–113
Acknowledgments
We thank Professor Xuehu Ma from the Stem Cell and Tissue Engineering Laboratory of the Dalian University of Technology for his support in culturing the NSCs. This work was supported by grants No. 30850001 from the National Nature Science Foundation of China and Nos. 20072167, 2008779 and 2008851 from the S&T Research Project of Education Bureau, Liaoning Province, China.
Conflict of interest
The authors indicate no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, H., Yu, B., Kong, L. et al. Neural Stem Cells Over-Expressing Brain-Derived Neurotrophic Factor (BDNF) Stimulate Synaptic Protein Expression and Promote Functional Recovery Following Transplantation in Rat Model of Traumatic Brain Injury. Neurochem Res 37, 69–83 (2012). https://doi.org/10.1007/s11064-011-0584-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0584-1