Abstract
Achyranthes bidentata Blume is a commonly prescribed Chinese medicinal herb. Our previous studies have proved the neuroprotective function of Achyranthes bidentata polypeptides (ABPP), a major constituent from aqueous extracts of the herb. Now we have separated an active fraction, referred to as ABPP-E4, from ABPP by HPLC methods. This study aimed to investigate the possible therapeutic potential of ABPP-E4. Assessments of cell viability and apoptosis indicated that ABPP-E4 pretreatment, in a concentration-dependent manner, antagonized the cell viability loss and cell apoptosis of cultured SH-SY5Y cells deprived of serum. ABPP-E4 pretreatment also resulted in increase of Bcl-2/Bax ratio and inhibition of caspase-3 activation in the cells on exposure to serum deprivation. Signaling pathway analysis indicated that ABPP-E4 treatment stimulated the activation of Akt/Gsk3β signaling in cultured SH-SY5Y cells, and anti-apoptotic effects of ABPP-E4 could be blocked by chemical inhibition of PI3K. Taken together, all the results suggest that ABPP-E4 might exert protective effects against serum deprivation-induced neuronal apoptosis through modulation of PI3K/Akt/Gsk3β pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Achyranthes bidentata Blume (Amaranthaceae family), a popular Chinese medicinal herb [1], is commonly prescribed in traditional Chinese medicine for the treatment of spasm, osteodynia of the lumbar region and knees, and flaccidity of limbs due to its medical functions of dissipating blood stasis, nourishing the liver and kidney, and strengthening the bones and muscles [2]. Our previous study demonstrates that an aqueous extract of Achyranthes bidentata Blume, composed of sterones, polysaccharides and polypeptides, could accelerate peripheral nerve regeneration after a crush injury to rabbit common peroneal nerves [3]. Our other previous studies also show that Achyranthes bidentata polypeptides (ABPP), a major constituent from aqueous extracts of Achyranthes bidentata Blume, could attenuate the NMDA-induced hippocampal neuron apoptosis in vitro [4, 5] or aid the regeneration of crushed mouse sciatic nerves in vivo [6]. Now we have separated several eluted fractions form ABPP by high-performance liquid chromatography (HPLC) methods for examining the possible therapeutic potential of these different active fractions.
Neuron death induced by various neurotoxic insults is implicated in the pathogenesis of acute and chronic diseases of the central nervous system, including trauma, epilepsy, ischemia stroke and many degenerative disorders [7–9]. The development of neuroprotective drugs or agents, especially of plant origin, has attracted great research interest. In this study, we aimed to investigate whether one of the eluted fractions from ABPP, referred to as ABPP-E4, could protect cultured SH-SY5Y cells against neuronal cell death induced by serum deprivation. Here, human neuroblastoma SH-SY5Y cells were chosen as a cell model in that this neuron-like cell line is widely used to study intracellular events linked to neurotoxic insults [10, 11].
There are different cellular signaling pathways activated in neurons to prevent them from neurotoxic insults. The phosphatidylinositol 3-kinase (PI3K)/Akt pathway is a critical anti-apoptotic pathway involved in neurodegenerative disorders [12], and phosphorylated Akt promotes cell survival by inhibiting proapoptotic proteins and stimulating anti-apoptotic proteins [13]. Gsk3β is a major target of PI3K/Akt, and inhibition of the Gsk3β activity contributes to the cell survival-promoting function of PI3K/Akt [14]. Therefore, Gsk3β has been shown to play a role in oxidative stress-induced neuronal apoptosis and the pathogenesis of neurodegenerative diseases [15, 16]. In this study, we also aimed to investigate whether the activation of PI3K/Akt/Gsk3β was involved in the influences of ABPP-E4 on cultured SH-SY5Y cells deprived of serum.
Experimental Procedure
Chemicals and Reagents
Hoechst 33342, 3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2-tetrazolium bromide (MTT), trypsin, monoclonal mouse anti-Bcl-2 antibody, monoclonal mouse anti-Bax antibody, monoclonal rabbit anti phospho-Akt (Ser473) antibody and monoclonal mouse anti-β-actin antibody were purchased from Sigma (St. Louis, MO). LY294002 (an inhibitor of PI3K) and monoclonal mouse anti-protein kinase Bα (PKBα/Akt) antibody were purchased from Cell Signaling (Danvers, MA). Polyclonal rabbit anti-Gsk3beta, phospho (Ser9) antibody and mouse anti-Gsk3β monoclonal antibody were purchased from Abcam (Cambridge, UK). Dulbecco’s modified eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY). IRDye 800-conjugated goat anti-mouse IgG and IRDye 800-conjugated donkey anti-rabbit IgG were purchased from Rockland (Gilbertsville, PA). The terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) assay kit, annexinV-Fluos Staining Kit, and caspase-3/CPP32 colorimetric assay kit were provided by Promega (Madison, WI), Roche (Mannheim, Germany) and Biovision (Mountain View, CA), respectively.
Preparation of ABPP Active Fractions
The root of Achyranthes bidentata Blume was obtained from a local Chinese medicine grocery, and its identity was confirmed by Porf. Haoru Zhao, an experienced pharmacologist from China Pharmaceutical University. ABPP was prepared from Achyranthes bidentata Blume as described previously [4], and characterized with a HPLC method to ensure the consistency between batches.
Then, HPLC was further applied to ABPP for separation of it into seven fractions. A Waters HPLC system consisted of Waters 600 Controller, Waters 717 plus Autosampler and Waters 2996 Photodiode Array Detector (Waters, Milford, MA). A DEAE anion exchange column (Protein-Pak™ DEAE 8HR 1000Å 8 μm 5 mm × 100 mm Anion Exchange Column, Waters) was used, and a linear gradient eluting was performed with 1.0 M NaCl in water containing 12% Tris/Hcl (pH 6.0) and 8% Tris/Base (pH 10.0) from 0% to 80% during 40 min at a flow rate of 0.5 ml/min. For characterization, the eluted 7 fractions, referred to as ABPP-E1 − ABPP-E7 (E stands for eluent) respectively, were monitored by UV spectrophotometry at 214 nm. They were lyophilized to yield powders, which could be dissolved in medium to furnish desired concentrations prior to use.
Cell culture and Treatment
The human SH-SY5Y cells, obtained from the American Type Culture Collection (Manassas, VA), were plated and cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 U/ml streptomycin in a humidified atmosphere of 5% CO2 and 95% air at 37°C. The medium was changed every other day. After they were pretreated with ABPP-E4 at different concentrations or with ABPP (0.1 μg/ml) for 24 h, cultured SH-SY5Y cells were deprived of serum by washing twice with phosphate buffer saline (PBS) and transferring them into a culture medium identical to the original one still containing ABPP-E4 or ABPP but lacking fetal bovine serum to allow incubation for the indicated time periods. The cells treated with the vehicle alone were considered as control. In some tests, LY294002 (10 μM) was added to the culture medium 30 min before ABPP-E4 treatment. At the end of cell treatments, the following assessments were conducted.
MTT Assay
The SH-SY5Y cells were seeded at a density of 5 × 104 cells/ml onto 96-well plates to undergo different treatments. The cells were incubated with MTT (0.5 mg/ml) for 4 h at 37°C. The resultant blue-colored formazan was dissolved in 20% sodium dodecyl sulfide for spectrophotometric analysis at 570 nm using ELx-800 Microplate reader (Bio-Tek Inc., Winooski, VT). The cell viability was expressed as the absorbance percentage relative to that of control cells.
TUNEL Assay
The SH-SY5Y cells were seeded at a density of 2 × 104 cells/ml to undergo different treatments. For the detection of apoptotic cells, a TUNEL assay kit was used according to manufacturer’s instructions to label fragmented DNA and visualize apoptotic cells by fluorescence microscopy. In brief, the cultured SH-SY5Y cells were fixed in 4.0% methanol-free formaldehyde in PBS at room temperature for 25 min. After wash with PBS, the cells were permeabilized in 0.2% Triton X-100 in PBS for 5 min, and covered with 100 μl of equilibration buffer at room temperature for 5–10 min. DNA strand breaks were labeled with fluorescein-12-dUTP using the recombinant terminal deoxynucleotidyl transferase (rTdT) in equilibration buffer at 37°C for 1 h, avoiding exposure to light. The negative control was incubated with an incubation buffer but without rTdT enzyme. The reaction was terminated with 2 × SSC (300 mM sodium chloride and 30 mM sodium citrate, pH7.4). After wash with PBS, the cells were stained with 5 μg/ml Hoechst 33342 dye at 37°C for 10 min. Apoptotic (TUNEL-positive) cells were detected as localized bright green cells in a blue background by using scanning laser confocal microscopy (Leica, Heidelberg, Germany). Data were expressed as the percentage of apoptotic cells to total cells.
Flow cytometry with Annexin V/Propidium Iodide (PI) Double Staining
The SH-SY5Y cells were seeded at a density of 1 × 105 cells/ml onto 6-well plates to undergo different treatments. For quantitative assessment of cell apoptosis, flow cytometry (BD Bioscience, San Jose, CA) was performed with an annexinV-Fluos staining kit according to the manufacturer’s instruction. In brief, the SH-SY5Y cells were resuspended in 1 × binding buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2) at a concentration of 1 × 106 cell/ml. To a 100-μl aliquot of the cell suspension, 5 μl of FITC-conjugated annexin V and 5 μl of PI (50 μg/ml) were added. After 15 min incubation in the dark at room temperature, cells were analyzed with a flow cytometer. Viable cells are negative for both PI and annexin V; early stage apoptotic cells are positive for annexin V and negative for PI, late stage apoptotic cells are positive for both annexin V and PI.
Caspase-3 Colorimetric Assay
The SH-SY5Y cells were seeded at a density of 1 × 105 cells/ml to undergo different treatments. The caspase-3 activity was measured with a caspase-3/CPP32 colorimetric assay kit according to the manufacturer’s instructions. The SH-SY5Y cells were homogenized in a cell lysis buffer. Total protein was quantified by BCA analysis. The 50 μl of supernatant containing 50–200 μg of protein was mixed with 50 μl of 2 × reaction buffer (containing 10 mM dithiothreitol) and 200 μMDEVD-pNA substrate. The reaction was maintained in a 37°C water bath for 1–2 h and the absorbance was measured at 405 nm with an ELx-800 microplate reader. Data were expressed as the relative activity over control.
Western Blot Analysis
Cell cultures were harvested in an ice-cold lysis buffer containing protease inhibitors. The protein concentration was quantified by BCA analysis. Lysates was separated by 12% SDS–PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes using standard protocols. Membranes were blocked with 5% nonfat powdered milk in Tris–buffered saline and 0.05% Tween-20 (TBST), and then incubated overnight with the following primary antibodies diluted in TBST: mouse anti-Bcl-2 monoclonal antibody (1:400), mouse anti-Bax monoclonal antibody (1:200), mouse anti- β-actin monoclonal antibody (1:5000), rabbit anti-phospho-Akt (pAkt, Ser473) monoclonal antibody, mouse anti-protein kinase Bα (PKBα/Akt) monoclonal antibody, rabbit anti-phospho-Gsk3β (ser 9) monoclonal antibody, mouse anti-Gsk3β monoclonal antibody (1:1000). Appropriate IRDye 800 secondary antibodies (1:5000) were used and protein bands were visualized with GS800 densitometer scanner (Bio-Rad, Hercules, CA), and the absorbance data were analyzed with PDQuest 7.2.0 software (Bio-Rad).
Statistical Analysis
Data were expressed as means ± SEM for 3 separate experiments (each in triplicate). Statistical differences between groups were analyzed by one-way analysis of variance (ANOVA) followed by subsequent Turkey’s tests. Differences were considered statistically significant at P < 0.05.
Results
ABPP-E4 Protected SH-SY5Y Cells Against Serum Deprivation-Induced Neurotoxicity
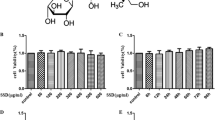
The ABPP-E4 was analyzed by HPLC with UV spectrophotometry. The chromatogram of ABPP-E4 recorded at 214 nm was shown in Fig. 1a.
Effects of ABPP-E4 on serum deprivation-induced insults in cultured SH-SY5Y cells. a HPLC chromatogram of ABPP-E4, in which the column effluents were monitored at 214 nm. b Changes in the cell viability of cultured SH-SY5Y cells after exposure to different concentrations of ABPP-E4 or ABPP alone (with serum) for 24 h. c Time-dependent decreases in the cell viability of cultured SH-SY5Y cells after exposure to serum deprivation. d Protection of ABPP-E4 pretreatment for 24 h against serum deprivation (24 h)-induced neurotoxicity in cultured SH-SY5Y cells. The cell viability, as measured by MTT assay, was expressed as the percentage over control value that was considered as 100%. The data are presented as means ± SEM of three separate experiments (each in triplicate). ##P < 0.01 versus control (vehicle treated), and *P < 0.05, **P < 0.01 versus exposure to serum deprivation alone
After the SH-SY5Y cells were cultured in the serum-containing medium added with different concentrations of ABPP-E4 or ABPP, MTT assay showed no significant changes in the cell viability of SH-SY5Y cells as compared to control (Fig. 1b), indicating that ABPP-E4 of ABPP itself exerted no cytotoxicity on cultured SH-SY5Y cells. MTT assay also showed that after cultured SH-SY5Y cells were exposed to serum deprivation for 12, 24, 36, 48 h, respectively, significant decreases in the cell viability were observed as compared to control value, and they showed a time-dependent pattern (Fig. 1c). Exposure to serum deprivation for 24 h, which resulted in 52.3 ± 5.1% of relative cell viability, was chosen to induce the neurotoxic insult in subsequent experiments unless otherwise stated. The pretreatment of cultured SH-SY5Y cells with different concentrations of ABPP-E4 attenuated the cell viability loss induced by exposure to serum deprivation alone, indicating that ABPP-E4 could protect cultured SH-SY5Y cells against serum deprivation-induced cell insults in a concentration-dependent manner, with the optimum protection at 0.05 μg/ml ABPP-E4, which was similar to protection by ABPP (0.1 μg/ml) pretreatment (Fig. 1d).
ABPP-E4 Protected SH-SY5Y Cells Against Serum Deprivation-Induced Cell Apoptosis
TUNEL analysis indicated that ABPP-E4 pretreatment prevented cultured SH-SY5Y cells from serum deprivation-induced apoptosis. The TUNEL-positive cells were significantly increased from 2.02 ± 0.72% (control) to 20.33 ± 2.86% by exposure to serum deprivation alone. Pretreatment with 0.05 μg/ml ABPP-E4, as well as pretreatment with 0.1 μg/ml of ABPP significantly decreased the number of TUNEL-positive cells to 7.60 ± 2.50 and 8.90 ± 2.34%, respectively (Fig. 2a, b). Flow cytometry with annexin V/PI staining provided further evidence that ABPP-E4 pretreatment prevented cultured SH-SY5Y cells from serum deprivation-induced apoptosis. The quantitative comparison indicated that the percentage of early apoptotic SH-SY5Y cells induced by exposure to serum deprivation alone was larger than that pretreatment with ABPP-E4 or ABPP followed by serum deprivation (Fig. 2c, d). In addition, pretreatment with ABPP-E4 or ABPP alone (without serum deprivation) caused no apparent apoptosis on cultured SH-SY5Y cells (Fig. 2a–d).
Effects of ABPP-E4 on serum deprivation-induced cell apoptosis. SH-SY5Y cells were pretreated with ABPP-E4 or ABPP for 24 h, and then deprived of serum for additional 24 h. TUNEL staining (a) and flow cytometry (c) was performed after SH-SY5Y cells were subjected to vehicle (a); serum deprivation alone (b); ABPP-E4 (0.05 μg/ml) pretreatment alone (c); ABPP-E4 (0.05 μg/ml) pretreatment followed by serum deprivation (d); ABPP (0.1 μg/ml) pretreatment alone (e); ABPP (0.1 μg/ml) pretreatment followed by serum deprivation (f). The pictures (a) are the merge of green-stained (TUNEL) apoptotic cells and blue-stained (Hoechst) all the nuclei. The pictures (c) are representative flow cytograms of annexin V binding (abscissa) versus propidium iodide (PI) uptake (ordinate) in cultured SH-SY5Y cells. The percentage of TUNEL-positive cells and the rate of early apoptosis (annexin V-positive, PI-negative cells) are shown by two histograms (b and d), respectively. ##P < 0.01 versus control (vehicle treated), and **P < 0.01 versus exposure to serum deprivation alone
Effects of ABPP-E4 on Bcl-2 and Bax Expressions and Caspase3 Activity in SH-SY5Y Cells Deprived of Serum
Bcl-2 family members include both anti-apoptotic (e.g. Bcl-2, Bcl-xl) and pro-apoptotic (e.g. Bax, Bad, Bak, Bid) proteins. The ratio between the two subsets determines the susceptibility of cells to a death signal [17, 18]. Western blot analysis revealed that exposure to serum deprivation alone for 24 h reduced the Bcl-2/Bax expression ratio and pretreatment with ABPP-E4 at different concentrations as well as with 0.1 μg/ml ABPP antagonized the serum deprivation-induced decrease in Bcl-2/Bax ratio of protein expression within cultured SH-SY5Y cells (Fig. 3a, b). Caspase 3 is a key effector protease in the execution of apoptosis. We also observed that caspase 3 activity increased about two fold on exposure of cultured SH-SY5Y cells to serum deprivation alone for 6 h; however, pretreatment with different concentrations of ABPP-E4 protected the cells against serum deprivation-induced increase in caspase3 activity in a dose-dependent manner (Fig. 3c).
Effects of ABPP-E4 on Bcl-2 and Bax expression and caspase3 activity. (a) Representative Western blot images showing the expression of Bcl-2, Bax and β-actin (an internal reference) in cultured SH-SY5Y cells after indicated treatments, in which either pretreatment or serum deprivation took 24 h. (b) The change of Bcl-2/Bax protein ratio in cultured SH-SY5Y cells after the above cell treatments. (c) The caspase-3 activity in cultured SH-SY5Y cells after indicated treatments, in which pretreatment and serum deprivation took 24 and 6 h, respectively. The data are presented as means ± SEM of three separate experiments (each in triplicate). ##P < 0.01 versus control (vehicle treated).*P < 0.05 and **P < 0.01 versus serum deprivation alone
ABPP-E4 Activated Akt and Gsk3β in SH-SY5Y Cells
To explore possible mechanisms involved in the protective effects of ABPP-E4, we investigated whether ABPP-E4 resulted in the activation of Akt signaling pathways. Since Akt is activated by phosphorylation of Ser 473 and Thr 308, phosphorylation at Ser 473 is commonly used as an indirect measure of Akt activation [12]. From Western blot analysis, we found that the phosphorylated Akt (p-Akt) level, expressed as the % value relative to the total Akt (t-Akt) level that was arbitrarily adjusted to identical throughout all time points examined, in cultured SH-SY5Y cells was increased by ABPP-E4 (0.05 μg/ml) treatment for 5 min-24 h, reaching the peak level at 30 min of ABPP-E4 (0.05 μg/ml) treatment without altering the total Akt level (Fig. 4a, b).
Time-dependent effects of ABPP-E4 (0.05 μg/ml) on the phosphorylation of Akt and Gsk3β in cultured SH-SY5Y cells. Representative Western blot images showing protein expressions of phosphorylated Akt (p-Akt), total-Akt (t-Akt), phosphorylated-Gsk3β (p-Gsk3β) and total-Gsk3β (t-Gsk3β) in SH-SY5Y cells after indicated treatments (a, d). Histograms showing the ratio of p-Akt/t-Akt (b, e) or p-Gsk3β/t-Gsk3β (c, f) in SH-SY5Y cells after ABPP-E4 treatment alone for different times (b, c), or after serum deprivation alone and serum deprivation plus ABPP-E4 treatment for different times, respectively (e, f). The data are presented as means ± SEM of three separate experiments (each in triplicate). #P < 0.05 and ##P < 0.01 versus control (vehicle treated), and **P < 0.01
Since phosphorylation of Gsk3β at Ser9 by Akt can inhibit the activity of Gsk3β kinase [19, 20], we investigated whether ABPP-E4 treatment increased the level of phosphorylated Gsk3β (p-Gsk3β) at Ser9. Western blot analysis indicated that the p-Gsk3β level in cultured SH-SY5Y cells was increased by ABPP-E4 (0.05 μg/ml) treatment for 5 min-24 h, reaching a maximum value also at 30 min of ABPP-E4 treatment (Fig. 4a, c).
It was also noted that serum deprivation led to a dramatically decreased phosphorylation of Akt and GSK-3β, which was reversed by ABPP-E4 treatment. This reversing effect also peaked at 30-min treatment, and then declined (Fig. 4d–f).
Involvement of PI3K/Akt/Gsk3β signaling pathways in neuroprotective effects of ABPP-E4
We further investigated which upstream signaling pathways were involved in ABPP-E4-evoked phosphorylation of Akt and Gsk3β. If cultured SH-SY5Y cells were pretreated with LY294002 (10 μM),for 30 min before ABPP-E4 (0.05 μg/ml) treatment, Akt and Gsk3β phosphorylation would be reversed (Fig. 5a–d). In addition, if cells were pretreated with LY294002 before ABPP-E4 treatment, the anti-apoptotic effect of ABPP-E4 would be blocked as evidenced by flow cytometric data (Fig. 5e).
Effects of ABPP-E4 (0.05 μg/ml) and PI3K inhibitor, LY294002 (LY, 10 μM), on the phosphorylation of Akt and Gsk3β in cultured SH-SY5Y cells, and on ABPP-E4 neuroprotection. Representative Western blot images for the protein expression of p-Akt and t-Akt (a) or of p-Gsk3β and t-Gsk3β (c). Histograms showing that the ratio of p-Akt/t-AKt (b) or of p-Gsk3β/t- Gsk3β (d) was changed by treatments with LY alone, ABPP-E4 alone, and LY plus ABPP-E4, respectively. Histogram (e) showing the rate of early apoptosis (annexin V-positive, PI-negative cells) after indicated cell treatments. The data are presented as means ± SEM of three separate experiments (each in triplicate). ##P < 0.01 versus control (vehicle treated). **P < 0.01 versus serum deprivation, ††P < 0.01 versus ABPP-E4 pretreatment followed by serum deprivation alone
Discussion
In this study, an active fraction ABPP-E4 was isolated from ABPP. The HPLC analysis offered a quality control for ABPP-E4 although its chemical identity has not yet been determined. We found that ABPP-E4 could increase the cell survival of cultured SH-SY5Y cells deprived of serum, and the optimum concentration (showing the best result) of ABPP-E4 was lower than that of ABPP, suggesting that ABPP-E4 was likely a major active fraction responsible for the neuroprotection afforded by ABPP. The apoptosis detection further indicated that serum deprivation-induced cell apoptosis in cultured SH-SY5Y cells was sharply attenuated by ABPP-E4 pretreatment. These results confirmed that ABPP-E4 supported the cell survival and antagonized the cell apoptosis in SH-SY5Y cells deprived of serum.
The proteins of Bcl-2 family are involved in intracellular apoptotic signal transduction. The protein expression ratio of anti-apoptotic members to pro-apoptotic members is an important factor for the ultimate vulnerability of cells to diverse apoptotic stimuli [21]. We noted that a decrease in the Bcl-2/Bax ratio appeared following exposure to serum deprivation, but ABPP-E4 pretreatment significantly attenuated serum deprivation-induced decrease in the Bcl-2/Bax ratio. The data suggested that the protection of ABPP-E4 against serum deprivation-induced cell apoptosis in SH-SY5Y cells might be, at least partly, attributed to ABPP-E4 inhibition of apoptosis-related caspase-3 activity.
The data of this study showed that ABPP-E4 treatment not only resulted in neuroprotection but also induced a prompt and sustained activation of PI3K/Akt/Gsk3β. Phosphorylation and activation of PI3K/Akt signaling is associated with cell survival after toxic insults in various cellular systems [22]. As is known, Akt inhibits cell apoptosis and cytochrome c release induced by several Bcl-2 family members [23]. Especially, Bax is regulated by phosphorylation of Ser184 in an Akt-dependent manner, whereas Akt-dependent phosphorylation inhibits Bax effects on the mitochondria by maintaining the protein in the cytoplasm, heterodimerized with anti-apoptotic Bcl-2 family members [24]. This study showed that p-Akt was up-regulated by ABPP-E4 treatment for 5 min-24 h. Activated Akt promotes cell survival by phosphorylating and thereby inactivating proapoptotic target proteins. In particular, Akt inactivates Gsk3β by phosphorylating it at Ser9. Recent evidences have suggested that Gsk3β is a key determinant in apoptotic processes leading to neuronal cell death following Aβ, MPTP and 6-OHDA and rotenone treatments [25–29]. Furthermore, accumulating evidence has suggested that Gsk3β contributes to the cell death of dopaminergic cells, including SH-SY5Y and PC12 cells, in response to various toxic cell insults [25, 26, 29, 30]. In this study, therefore, ABPP-E4 treatment was also found to result in a sustained phosphorylation of Gsk3β in SH-SY5Y cells.
Moreover, we observed that pretreatment of SH-SY5Y cells with LY294002, a specific PI3K inhibitor, markedly reversed ABPP-E4-activated phosphorylation of Akt and Gsk3β. On the other hand, we noted that neuroprotection afforded by ABPP-E4 against serum deprivation was antagonized by LY294002. This observation suggested that neuroprotection of ABPP-E4 was mediated by PI3K signaling. Collectively, our data indicated that PI3K/Akt/Gsk3β signaling pathways might be associated with neuroprotective effects of ABPP-E4 against serum deprivation-induced apoptosis in SH-SY5Y cells.
To conclude, this study indicates that ABPP-E4, an active fraction separated from ABPP, could prevent cultured SH-SY5Y cells from serum deprivation-induced apoptosis, and PI3K/Akt/Gsk3β pathway might be involved in neuroprotective effects of ABPP-E4.
References
Committee of National Pharmacopoeia (2005) Pharmacopoeia of the People’s Republic of China. Chemical Industry Press, Beijing, p 49
Li J, Qi H, Qi LW et al (2007) Simultaneous determination of main phytoecdysones and triterpenoids in Radix Achyranthis Bidentatae by high-performance liquid chromatography with diode array-evaporative light scattering detectors and mass spectrometry. Anal Chim Acta 596:264–272
Ding F, Cheng Q, Gu X (2008) The repair effects of Achyranthes bidentata extract on the crushed common peroneal nerve of rabbits. Fitoterapia 79:161–167
Shen H, Yuan Y, Ding F et al (2008) The protective effects of Achyranthes bidentata polypeptides against NMDA-induced cell apoptosis in cultured hippocampal neurons through differential modulation of NR2A-and NR2B-containing NMDA receptors. Brain Res Bull 77:274–281
Shen H, Yuan Y, Ding F et al (2010) Achyranthes bidentata polypeptides confer neuroprotection through inhibition of reactive oxygen species production, Bax expression, and mitochondrial dysfunction induced by overstimulation of N-methyl-D-aspartate receptors. J Neurosci Res 88:669–676
Yuan Y, Shen H, Yao J et al (2010) The protective effects of Achyranthes bidentata polypeptides in an experimental model of mouse sciatic nerve crush injury. Brain Res Bull 81:25–32
Macdonald NJ, Delderfield SM, Zhang W et al (2003) Tumour necrosis factor-alpha-versus growth factor deprivation-promoted cell death: distinct converging pathways. Aging Cell 2:245–256
Shi C, Guo K, Yew DT et al (2008) Effects of ageing and Alzheimer’s disease on mitochondrial function of human platelets. Exp Gerontol 43:589–594
Shi C, Yao Z, Xu J et al (2009) Effects of Gingko Extract (EGb761) on oxidative damage under different conditions of serum supply. J Bioenerg Biomembr 41:61–69
Macleod MR, Allsopp TE, McLuckie J et al (2001) Serum withdrawal causes apoptosis in SHSY 5Y cells. Brain Res 889:308–315
Andoh T, Chock PB, Chiueh CC (2002) The roles of thioredoxin in protection against oxidative stress-induced apoptosis in SH-SY5Y cells. J Biol Chem 277:9655–9660
Rickle A, Bogdanovic N, Volkman I et al (2004) Akt activity in Alzheimer’s disease and other neurodegenerative disorders. Neuroreport 15:955–959
Yoshimoto T, Uchino H, He QP et al (2001) Cyclosporin A, but not FK506, prevents the downregulation of phosphorylated Akt after transient focal ischemia in the rat. Brain Res 899:148–158
Hetman M, Cavanaugh JE, Kimelman D et al (2000) Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J Neurosci 20:2567–2574
Muyllaert D, Kremer A, Jaworski T et al (2008) Glycogen synthase kinase-3β, or a link between amyloid and tau pathology? Genes Brain Behav 7:57–66
Choi BS, Sapkota K, Kim S et al (2010) Antioxidant activity and protective effects of Tripterygium regelii extract on hydrogen peroxide-induced injury in human dopaminergic cells, SH-SY5Y. Neurochem Res 35:1269–1280
Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Gene Dev 13:1899–1911
Petit PX, Susin SA, Zamzami N et al (1996) Mitochondria and programmed cell death: back to the future. FEBS Lett 396:7–13
Matsui T, Rosenzweig A (2005) Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol 38:63–71
Gosens R, Dueck G, Rector E et al (2007) Cooperative regulation of GSK-3 by muscarinic and PDGF receptors is associated with airway myocyte proliferation. Am J Physiol- Lung C 293:L1348–L1358
Korsmeyer SJ (1995) Regulators of cell death. Trends Genet 11:101–105
Brazil DP, Hemmings BA (2001) Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci 26:657–664
Gardai SJ, Hildeman DA, Frankel SK et al (2004) Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem 279:21085–21095
Kennedy SG, Kandel ES, Cross TK, Hay N (1999) Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol 19:5800–5810
Chen G, Bower KA, Ma C et al (2004) Glycogen synthase kinase 3β (GSK3β) mediates 6-hydroxydopamine-induced neuronal death. The FASEB J 18:1162–1164
King TD, Jope RS (2005) Inhibition of glycogen synthase kinase-3 protects cells from intrinsic but not extrinsic oxidative stress. Neuroreport 16:597–601
Wang W, Yang Y, Ying C et al (2007) Inhibition of glycogen synthase kinase-3 [beta] protects dopaminergic neurons from MPTP toxicity. Neuropharmacology 52:1678–1684
Koh SH, Noh MY, Kim SH (2008) Amyloid-beta-induced neurotoxicity is reduced by inhibition of glycogen synthase kinase-3. Brain Res 1188:254–262
Lee JE, Kang JS, Ki YW et al (2011) Akt/GSK3β signaling is involved in fipronil-induced apoptotic cell death of human neuroblastoma SH-SY5Y cells. Toxicol Lett 202:133–141
Lee CS, Park WJ, Ko HH et al (2006) Differential involvement of mitochondrial permeability transition in cytotoxicity of 1-methyl-4-phenylpyridinium and 6-hydroxydopamine. Mol Cell Biochem 289:193–200
Acknowledgments
This study was supported by a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and a project for industrialization promotion of RD achievements in universities and colleges (Grant No. JH09-33). We thank Professor Jie Liu for assistance in manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, Y., Zhang, Q., Gao, X. et al. An Active Fraction of Achyranthes bidentata Polypeptides Prevents Apoptosis Induced by Serum Deprivation in SH-SY5Y Cells Through Activation of PI3K/Akt/Gsk3β Pathways. Neurochem Res 36, 2186–2194 (2011). https://doi.org/10.1007/s11064-011-0543-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0543-x