Abstract
Focal cerebral ischemia results in an increased expression of matrix metalloproteinase-9 (MMP-9), which induces vasogenic brain edema via disrupting the blood–brain barrier (BBB) integrity. Recent studies from our laboratory showed that baicalin reduces ischemic brain damage by inhibiting inflammatory reaction and neuronal apoptosis in a rat model of focal cerebral ischemia. In the present study, we first explored the effect of baicalin on the neuronal damage, brain edema and BBB permeability, then further investigated its potential mechanisms. Sprague–Dawley rats underwent permanent middle cerebral artery occlusion (MCAO). Baicalin was administrated by intraperitoneally injected twice at 2 and 12 h after the onset of MCAO. Neuronal damage, brain edema and BBB permeability were measured 24 h following MCAO. Expression of MMP-9 protein and mRNA were determined by western blot and RT–PCR, respectively. Expression of tight junction protein (TJP) occludin was detected by western blot. Neuronal damage, brain edema and BBB permeability were significantly reduced by baicalin administration following focal cerebral ischemia. Elevated expression of MMP-9 protein and mRNA were significantly down-regulated by baicalin administration. In addition, MCAO caused the decreased expression of occludin, which was significantly up-regulated by baicalin administration. Our study suggested that baicalin reduces MCAO-induced neuronal damage, brain edema and BBB permeability, which might be associated with the inhibition of MMP-9 expression and MMP-9-mediated occludin degradation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The blood–brain barrier (BBB), which is consisted of cerebral microvascular endothelial cell via connecting with tight junction complex, is critical to control the exchange of materials between the peripheral circulation and the central nervous system (CNS), and to maintain the homeostasis and proper function of CNS tissue [1, 2]. Tight junctions form a metabolic and physical barrier to restrict the paracellular permeability. Occludin, the most important tight junction protein, is considered to seal the tight junctions, and degradation of occludin alone is enough to enhance the BBB permeability [3, 4].
Abundant evidence demonstrates that cerebral ischemia-induced BBB disruption contributes to vasogenic brain edema and neutrophil infiltration, eventually aggravating the ischemic brain damage [5, 6]. Matrix metalloproteinases (MMPs), a family of proteolytic enzymes, are reported to degrade important structures in the microvascular wall, resulting in an increase of the microvascular permeability and BBB disruption [7, 8]. Among the MMPs, MMP-9 expression is up-regulated after ischemic stroke and induces the degradation of occludin, leading to BBB leakage, brain edema, brain hemorrhage and secondary brain damage [9, 10].
Recent studies from our laboratory have shown that baicalin reduces ischemic brain damage by inhibiting inflammatory reaction and neuronal apoptosis [11, 12]. In the present study, we, for the first time, explored whether baicalin reduces ischemia-induced brain edema and BBB permeability, and further investigated the effect of baicalin on the expression of MMP-9 and occludin.
Materials and Methods
Middle Cerebral Artery Occlusion Model
Adult male Sprague–Dawley rats (weighing 250–300 g, provided by Shanghai Laboratory Animal Center, Chinese Academy of Sciences, Shanghai, China) were used in this study. The surgical procedures were carried out according to the NIH Guide for the Care and Use of Laboratory Animals. Permanent middle cerebral artery occlusion (MCAO) was induced by intraluminal filament occlusion method in rats as described in our previous report [13]. Briefly, rats were anesthetized with an intraperitoneal injection of chloral hydrate (300 mg/kg), the right common carotid artery (CCA), external carotid artery (ECA) and internal carotid artery (ICA) were exposed. A monofilament suture (0.32 mm diameter at the tip) was introduced from the ECA into the ICA, and then into the circle of Willis to occlude the origin of the right MCA. The filament was inserted 18–20 mm from the carotid bifurcation, confirmed with mild resistance. The sham-operated rats underwent the same surgery except that the filament was inserted only 10 mm and withdrawn a minute later.
Drug Administration and Experimental Groups
Baicalin (Sigma, with a purity >95%), was dissolved in normal saline and intraperitoneally injected twice at 2 h and 12 h after the onset of ischemia. Rats were divided into three groups as follows: (a) sham control group, which underwent sham operation and received vehicle; (b) MCAO group, which was subjected to permanent MCAO and received vehicle; (c) Baicalin administration group, which was subjected to permanent MCAO and intraperitoneally administrated with baicalin 100 mg/kg. The dosage of baicalin was determined according to the previous report [11], in which showed that baicalin intraperitoneally administrated at 100 mg/kg could produce the best protective effect on the brain of rats subjected to permanent focal cerebral ischemia. Rats were treated with normal saline as the vehicle control at the same volume and time point as baicalin.
Histological Examination
To confirm the effect of baicalin on neuronal injury produced by focal cerebral ischemia, we used light microscope to observe the morphological characteristic of neurons in ischemic brain according to hematoxylin and eosin (HE) staining. Briefly, rats were deeply anesthetized and perfused with 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4). Then the brains were removed, fixed, and embedded in paraffin. Coronal sections (4 μm thick) were obtained from embedded paraffin and deparaffinized with xylene and rehydrated with graded alcohol for HE staining and immunohistochemical analysis. For HE staining, coronal sections were counterstained with hematoxylin and eosin. The sections were visualized with a light microscope (Olympus, Japan).
Assessment of Brain Edema
Brain edema was evaluated on the basis of cerebral water content. Briefly, rats were killed 24 h following focal cerebral ischemia, and the brains were quickly removed. The ischemic hemispheres were gently blotted with filter-paper and weighed on electronic balance, as the wet weights (WW), and then dried to constant weight to obtain the dry weights (DW). Cerebral water content was calculated according to the following formulation: H2O (%) = (WW − DW)/WW × 100%.
Measurement of Blood–Brain Barrier Permeability
Endogenous IgG immunostaining was performed to determine the BBB permeability. Briefly, the paraffin-embedded coronal sections (4 μm thick) were deparaffinized with xylene and rehydrated with graded alcohol. After washing in PBS, sections reacted, respectively, and successively with a biotinylated anti-rat IgG antibody, horseradish peroxidase-streptavidin (1:200, Boster Biological Technology, LTD, Wuhan, China) and 0.05% diaminobenzidine (Zhongshan Biotechnology CO. LTD, Beijing, China). The IgG positive areas of coronal sections were analyzed with a computer using image analysis software. The percentage of IgG immunoreactivity in the sections was determined according to the following formula: IgG positive area in ischemic hemisphere/contralateral hemisphere area × 100%.
Reverse Transcription-Polymerase Chain Reaction (RT–PCR)
Brain samples were homogenized and the total RNA was extracted by Trizol reagents (Invitrogen) and reverse-transcribed to obtain single-strand cDNA using a Reverse Transcription System (Promega) in accordance with the manufacturer’s protocol. Single-strand cDNA was amplified by PCR in a 100 μl reaction mixture containing 50 mM KCl, 10 mM Tris–HCl (pH 9.0), 2 mM MgCl2, 200 μM dNTPs, 0.5 μM sense and antisense primers and 2.5 units Taq DNA polymerase (Promega). The primer sequences used in this study were as follows: MMP-9 (primers, sense 5′-AAG GAT GGT CTA CTG GCA C-3′; antisense 5′-AGA GAT TCT CAC TGG GGC-3′, product size 279 bp), β-actin as an internal standard (primers, sense 5′-TCA TGA AGT GTG ACG TTG AC-3′; antisense 5′-CCT AGA AGC ATT TGC GGT GC-3′, product size 285 bp). The reactions were initially heated at 94°C for 4 min; then at 94°C for 40 s, 60°C for 40 s and 72°C for 50 s, totally 40 cycles; finally stopped at 72°C for 7 min. PCR products of 10 μl were separated by 2% agarose gel electrophoresis and visualized by ethidium bromide staining. The optical density of DNA band was measured by UVP gel analysis system (Quantity One, Bio-Rad).
Western Blot
Brain samples were homogenized and the total proteins were extracted by RIPA lysis buffer (Beyotime Biotech. CO., China). Different samples with an equal amount of protein (50 μg) were separated on 10% SDS polyacrylamide gels, transferred to nitrocellulose membranes, and blocked in 5% nonfat dry milk buffer overnight at 4°C. The membranes were then incubated overnight at 4°C with a rabbit polyclonal antibody against MMP-9 (1:500, Santa Cruz, USA), or a rabbit polyclonal antibody against occludin (1:1000, Santa Cruz, USA) followed by incubation with horseradish-peroxidase conjugated secondary antibodies (1:2000, KPL Inc). Protein expression was detected with an enhanced chemiluminescence detection system (KPL Inc) and exposed on an X-ray film. GAPDH was used as a loading control. The optical densities of MMP-9, occludin and GAPDH protein bands on the X-ray film were quantitatively analyzed with Quantity One software (Bio-Rad).
Statistical Analysis
Experimental results were presented as mean ± SD. Statistical significance was assessed by one-way ANOVA followed by Bofferoni’s test. A value P < 0.05 was considered to be significant.
Results
Morphological Characteristic
HE staining showed the morphological changes of neuronal cells in the surrounding penumbra from sham control, vehicle-treated and baicalin-treated rats. There was no neuronal damage in sham control (Fig. 1a). MCAO caused an obvious increase of necrotic neuronal death in the ischemic hemisphere (Fig. 1b), which was attenuated by baicalin administration (Fig. 1c).
Morphological characteristic of neuronal cells following MCAO was determined by HE staining. Representative microphotographs of neuronal damage from sham control (a), vehicle-treated (b) and baicalin-treated (c) rats after 24 h of MCAO were present. Cerebral ischemia caused an obvious increase of necrotic neuronal death characterized with eosinophilic cytoplasm and triangulated pyknotic nuclei in ischemic brain, which was attenuated by baicalin administration. Scale bar 50 μm
Effect of Baicalin on Brain Edema
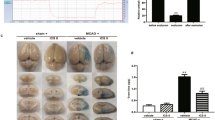
The surface areas of the ischemic hemisphere in rat brains were increased 24 h following MCAO, indicating ischemia-induced brain edema (Fig. 2a, b). Brain edema was determined by assessing the content of cerebral water. MCAO caused an increase of cerebral water content in ischemic hemisphere, which was significantly attenuated by baicalin administration (P < 0.05 vs. vehicle-treated group, Fig. 2c).
Effect of baicalin on the cerebral ischemia-induced brain edema. The surface areas of the right (ischemic) hemisphere were increased compared with the contralateral (non-ischemic) hemisphere following MCAO, indicating cerebral ischemia-induced brain edema (a, b). Moreover, the surface area of ischemic hemisphere in (b) was obviously smaller than that in (a), suggesting the inhibitory effect of baicalin on ischemic brain edema. Baicalin reduced the cerebral water content of ischemic hemisphere, but not the non-ischemic hemisphere, and the results were summarized (c). IS, ischemic hemisphere; NIS, non-ischemic hemisphere. n = 5, *P < 0.05 compared to vehicle-treated rats
Effect of Baicalin on Blood–Brain Barrier Permeability
Blood–brain barrier permeability was determined according to assessing the area of endogenous IgG immunoreactivity by the method of immunohistochemical study. The area of endogenous IgG immunoreactivity was obviously increased 24 h following MCAO, which was significantly reduced by baicalin administration (P < 0.01 vs. vehicle-treated group), indicating the inhibitory effect of baicalin on blood–brain barrier permeability (Fig. 3).
Effect of baicalin on the blood–brain barrier permeability following focal cerebral ischemia. Immunohistochemical analysis of endogenous IgG-positive area from sham control (a), vehicle-treated (b) and baicalin-treated (c) rats after 24 h of MCAO were present, and the results were summarized in (d). Representative image of endogenous IgG immunoreactivity was shown in (e, arrowhead). n = 5, **P < 0.01 compared to vehicle-treated rats. Scale bar 50 μm
Effect of Baicalin on the Expression of MMP-9
MCAO caused the elevated expression of MMP-9 protein and mRNA in ischemic hemisphere, both of which were significantly down-regulated by baicalin administration (P < 0.05 and P < 0.01 vs. vehicle-treated group, Fig. 4).
Effect of baicalin on the protein and mRNA expression of MMP-9 following focal cerebral ischemia. Representative bands of MMP-9 protein expression in ischemic brain following MCAO were detected by western blot (a), and the results were summarized in (b). Representative bands of MMP-9 mRNA expression in ischemic brain following MCAO were detected by RT–PCR (c), and the results were summarized in (d). n = 5, **P < 0.01 compared to sham control, #P < 0.05 and ##P < 0.01 compared to vehicle-treated rats
Effect of Baicalin on the Tight Junction Protein Occludin
Expression of occludin was significantly decreased 24 h following MCAO (P < 0.01 vs. sham control), which was significantly up-regulated by baicalin administration (P < 0.01 vs. vehicle-treated group, Fig. 5).
Effect of baicalin on the expression of TJP occludin following focal cerebral ischemia. Representative bands of occludin protein expression in ischemic brain following MCAO were detected by western blot (a), and the results were summarized in (b). n = 5, **P < 0.01 compared to sham control and ##P < 0.01 compared to vehicle-treated rats
Discussion
Stroke is at least the third most common cause of death and the leading cause of adult neurological disability worldwide [14, 15]. Cerebral ischemic damage produced by focal cerebral ischemia develops a series of complicated pathophysiological events including ion homeostasis imbalance, free radical injury, BBB disruption, inflammatory reaction and neuronal apoptosis [16]. In experimental ischemic stroke, a great deal of potential neuroprotective drugs targeted at different pathophysiological mechanisms of cerebral ischemic damage have been investigated [17].
The previous studies from our laboratory demonstrated that baicalin, a flavonoid compound isolated from Scutellaria baicalensis Georgi, exits a neuroprotective role in a rat model of permanent focal cerebral ischemia, and this neuroprotection might be attributed to its anti-inflammatory and anti-apoptotic properties [11]. The experimental results also showed that the neuroprotective and anti-inflammatory role of baicalin in ischemic stroke might be associated with the inhibition of toll-like receptor 2/4 (TLR2/4) signaling pathway [12]. Similarly, baicalin is also considered to protect brain from cerebral ischemia–reperfusion by down-regulating the expression of nucleotide-binding oligomerization domain protein 2 (NOD2) and TNF-α [18]. Furthermore, baicalin is reported to attenuate ischemic brain damage through inhibiting NF-κB p65 activation [19]. In addition, baicalin protects SH-SY5Y cells from oxidative injuries induced by Aβ1-42 aggregation through decreasing H2O2 production [20]. To our best knowledgement, however, it is never known that whether baicalin could improve the cerebral ischemia-induced BBB disruption.
The present study first demonstrated that baicalin attenuates neuronal damage produced by cerebral ischemia, indicating baicalin’s neuroprotection against ischemic brain damage. Next, the alleviated effect of baicalin on brain edema was examined. The results showed that, 24 h of cerebral ischemia, the cerebral water content of ischemic hemisphere in baicalin-treated group was lower compared with vehicle-treated group (P < 0.05). Similarly, our experimental results showed that the IgG-positive area of ischemic hemisphere in baicalin-treated group was smaller compared with vehicle-treated group (P < 0.01), suggesting the alleviation of BBB permeability by baicalin administration. Since cerebral ischemia-induced BBB disruption contributes to vasogenic brain edema and eventually to ischemic brain damage [21], we presumed that the baicalin’s neuroprotection in ischemic stroke was at least partly attributed to the alleviation of BBB permeability.
In order to provide the molecular mechanisms underlying the baicalin’s neuroprotection against MCAO-induced BBB disruption, we determined the inhibitory effect of baicalin on the gene and protein expression of MMP-9. The experimental results showed that, 24 h of cerebral ischemia, both the mRNA and protein expression of MMP-9 of ischemic hemisphere in baicalin-treated group were significantly down-regulated compared with vehicle-treated group. Since that the over-expression of MMP-9 induces the disruption of BBB integrity, then enhances BBB permeability, leading to brain edema, brain hemorrhage and secondary brain damage [22], we presumed that, in acute ischemic stroke of the present study, the alleviation of BBB permeability by baicalin administration was at least partly attributed to the down-regulated expression of MMP-9.
Recent experimental data showed that the over-expression of MMP-9 could degrade the tight junction protein occludin, which disrupts the integrity of tight junction in the BBB, eventually leading to an increased BBB permeability, vasogenic brain edema and brain damage [23, 24]. In the present study, therefore, we further investigated the inhibitory effect of baicalin on the expression of occludin. The experimental results showed that, 24 h after cerebral ischemia, the expression of tight junction protein occludin of ischemic hemisphere in baicalin-treated group were significantly decreased compared with vehicle-treated group. Accordingly, we also could presume that the alleviation of BBB permeability by baicalin in ischemic stroke might be partly attributed to the up-regulated expression of TJP occludin.
Taken together, the reduction of ischemic neuronal damage and brain edema by baicalin administration might be attributed to the improvement of BBB integrity, and the attenuation of BBB permeability by baicalin administration might be associated the inhibition of MMP-9 and the enhanced tight junction in BBB indicated by the expression of TJP occludin (Fig. 6). Our experimental results further support that baicalin might be used as a neuroprotective drug for acute ischemic stroke. This study also implies that MMP-9-mediated occludin degradation may be a potential and promising therapeutic target for acute ischemic stroke in future.
The possible mechanisms underlying the baicalin’s neuroprotection against ischemic brain damage. Cerebral ischemia causes the overexpression of MMP-9 in ischemic hemisphere, which degrades the tight junction protein occludin, leading to an increased BBB permeability, vasogenic brain edema and neuronal damage, eventually aggravating ischemic brain damage. Baicalin administration provides a neuroprotection against ischemic stroke possibly through down-regulating the expression of MMP-9, inhibiting the degradation of tight junction protein occludin to enhance the tight junction, alleviating BBB permeability, attenuating brain edema and neuronal damage, thus reducing ischemic brain damage
References
Hawkins BT, Davis TP (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57(2):173–185
Correale J, Villa A (2009) Cellular elements of the blood-brain barrier. Neurochem Res 34(12):2067–2077
Tavelin S, Hashimoto K, Malkinson J, Lazorova L, Toth I, Artursson P (2003) A new principle for tight junction modulation based on occludin peptides. Mol Pharmacol 64(6):1530–1540
Lochhead JJ, McCaffrey G, Quigley CE et al (2010) Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab 30(9):1625–1636
Chi OZ, Hunter C, Liu X, Weiss HR (2009) Effects of exogenous excitatory amino acid neurotransmitters on blood-brain barrier disruption in focal cerebral ischemia. Neurochem Res 34(7):1249–1254
Gidday JM, Gasche YG, Copin JC et al (2005) Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol 289(2):H558–H568
Piao MS, Lee JK, Park CS, Ryu HS, Kim SH, Kim HS (2009) Early activation of matrix metalloproteinase-9 is associated with blood-brain barrier disruption after photothrombotic cerebral ischemia in rats. Acta Neurochir (Wien) 151(12):1649–1653
Dong H, Fan YH, Zhang W, Wang Q, Yang QZ, Xiong LZ (2009) Repeated electroacupuncture preconditioning attenuates matrix metalloproteinase-9 expression and activity after focal cerebral ischemia in rats. Neurol Res 31(8):853–858
Liu W, Hendren J, Qin XJ, Shen J, Liu KJ (2009) Normobaric hyperoxia attenuates early blood-brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia. J Neurochem 108(3):811–820
Adibhatla RM, Hatcher JF (2008) Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets 7(3):243–253
Tu XK, Yang WZ, Shi SS, Wang CH, Chen CM (2009) Neuroprotective effect of baicalin in a rat model of permanent focal cerebral ischemia. Neurochem Res 34(9):1626–1634
Tu XK, Yang WZ, Shi SS et al (2010) Baicalin inhibits TLR2/4 signaling pathway in rat brain following permanent cerebral ischemia. Inflammation [Epub ahead of print]. doi:10.1007/s10753-010-9254-8
Tu XK, Yang WZ, Shi SS et al (2010) Spatio-temporal distribution of inflammatory reaction and expression of TLR2/4 signaling pathway in rat brain following permanent focal cerebral ischemia. Neurochem Res 35(8):1147–1155
Jemal A, Ward E, Hao Y, Thun M (2005) Trends in the leading causes of death in the United States, 1970–2002. JAMA 294(10):1255–1259
Huang J, Upadhyay UM, Tamargo RJ (2006) Inflammation in stroke and focal cerebral ischemia. Surg Neurol 66(3):232–245
Durukan A, Tatlisumak T (2007) Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav 87(1):179–197
Mehta SL, Manhas N, Raghubir R (2007) Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev 54(1):34–66
Li H, Hu J, Ma L et al (2010) Comprehensive study of baicalin down-regulating NOD2 receptor expression of neurons with oxygen-glucose deprivation in vitro and cerebral ischemia-reperfusion in vivo. Eur J Pharmacol 649(1–3):92–99
Xue X, Qu XJ, Yang Y et al (2010) Baicalin attenuates focal cerebral ischemic reperfusion injury through inhibition of nuclear factor kappaB p65 activation. Biochem Biophys Res Commun 403(3–4):398–404
Yin F, Liu J, Ji X, Wang Y, Zidichouski J, Zhang J (2011) Baicalin prevents the production of hydrogen peroxide and oxidative stress induced by Abeta aggregation in SH-SY5Y cells. Neurosci Lett 492(2):76–79
Meng F, Liu R, Gao M et al (2011) Pinocembrin attenuates blood-brain barrier injury induced by global cerebral ischemia-reperfusion in rats. Brain Res 1391:93–101
Mao X, Yin W, Liu M et al (2011) Osthole, a natural coumarin, improves neurobehavioral functions and reduces infarct volume and matrix metalloproteinase-9 activity after transient focal cerebral ischemia in rats. Brain Res 1385:275–280
Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM (2011) Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab 31(1):52–57
Lischper M, Beuck S, Thanabalasundaram G, Pieper C, Galla HJ (2010) Metalloproteinase mediated occludin cleavage in the cerebral microcapillary endothelium under pathological conditions. Brain Res 1326:114–127
Acknowledgments
The Key Laboratory (Neurosurgical Department) Funding from the Affiliated Union Hospital of Fujian Medical University, supported this work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tu, Xk., Yang, Wz., Liang, Rs. et al. Effect of Baicalin on Matrix Metalloproteinase-9 Expression and Blood–Brain Barrier Permeability Following Focal Cerebral Ischemia in Rats. Neurochem Res 36, 2022–2028 (2011). https://doi.org/10.1007/s11064-011-0526-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0526-y