Abstract
The maximum rates (V max) of some enzymatic activities related to energy consumption (ATP-ases) were evaluated in two types of synaptic plasma membranes (SPM) isolated from cerebral cortex of rats subjected to in vivo treatment with l-acetylcarnitine at two different doses (30 and 60 mg kg−1 i.p., 28 days, 5 days/week). The following enzyme activities were evaluated: acetylcholinesterase (AChE); Na+, K+, Mg2+-ATP-ase; ouabain insensitive Mg2+-ATP-ase; Na+, K+-ATP-ase; direct Mg2+-ATP-ase; Ca2+, Mg2+-ATP-ase; Low- and High-affinity Ca2+-ATP-ase. Sub-chronic treatment with l-acetylcarnitine increased Na+, K+-ATP-ase activity on SPM 2 and Ca2+, Mg2+-ATP-ase activity on both SPM fractions. These results suggest (1) that the sensitivity to drug treatment is different between the two populations of SPM, confirming the micro-heterogeneity of these sub-fractions, probably originating from different types of synapses, (2) the specificity of the molecular site of action of the drug on SPM and (3) its interference on ion homeostasis at synaptic level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
l-acetylcarnitine, an essential compound for long chain fatty acid uptake and utilization in mitochondria, is involved in brain energy metabolism [1–5].
In vivo, l-acetylcarnitine crosses the blood–brain barrier primarily via the high affinity, Na+-dependent cation/carnitine transporter and to a lesser extent via the B(0,+) amino acid transporter [6–8]; in particular, the drug has a brain uptake index of 2.4 ± 0.2, which is similar to that of GABA, indicating an affinity of l-acetylcarnitine for the GABA transport system [9].
Pharmacological studies with l-acetylcarnitine have shown that the drug: (a) increases energy production [1–4, 10–12], (b) stabilizes cellular membranes particularly in ageing, increasing cardiolipin content of the inner mitochondrial membrane [13, 14], (c) plays a role in cholinergic transmission as an acetylcholine precursor, positively affecting acetylcholine release [15–17] and shows a muscarinic agonist effect [18, 19]. These pharmacological characteristics have proposed l-acetylcarnitine for the therapy of ageing [12] and neurodegenerative diseases as Alzheimer’s Dementia [12, 15, 20]. At this case, l-acetylcarnitine administration opposes to membrane viscosity changes caused by cardiolipin decrease and lipid peroxidation, that increased in diseases such as Dementia [21].
Other studies have indicated a therapeutic role for l-acetylcarnitine in conditions of: (1) hypoxia [22–25], (2) ischaemia [26–29], (3) peripheral neuropathies with pain by increasing neurotrophic factors (NGF) [25, 30] and potentiating NGF action possibly through the stimulation of p 75NGFR synthesis and expression [31]. The drug also possesses (4) a neuromodulatory role in neuronal plasticity during early neuroembryogenesis [32] and in brain energy metabolism [1–5, 10, 12].
Therefore, the aim of the present research was to study the effect of the in vivo treatment with different doses of l-acetylcarnitine on the maximum rate of some representative cerebral enzymes linked to energy utilizing systems (ATP-ases) evaluated in two different fractions of synaptic plasma membranes, indicated as synaptic plasma membranes type I (SPM 1) and synaptic plasma membranes type II (SPM 2). These two different synaptic plasma membranes populations have been obtained from synaptosomal fraction subjected to osmotic shock and layered on discontinuous sucrose gradients [33].
Particularly, the effect of pharmacological treatment was evaluated on the enzyme activities of the so-called “ionic pumps”, formally energy consuming enzyme systems of ATP-ases, that modulate the presynaptic nerve ending homeostasis [33, 34].
Na+, K+-ATP-ase is located in synaptic plasma membranes and functions as an electrogenic Na+/K+-pump that maintains a low intracellular Na+ concentration and Na+ and K+ gradient, modulating the resting transmembrane potential, some postsynaptic activities and transmitter’s turnover [34].
Synaptic low- and high-affinity Mg2+ independent Ca2+-dependent ATP-ase and Ca2+, Mg2+-ATP-ase help to maintain the homeostasis of the intracellular Ca2+ that initiates the interaction between ATP and ATP-ases to promote both the attachment of secretory granules to plasmalemma and the extrusion of their neurotransmitters to the nerve terminal exterior [34].
The ectoenzyme Mg2+-ATP-ase has externally oriented active sites on synaptic plasma membrane and it is involved in the hydrolysis of ATP to adenosine. In contrast, the enzyme located in synaptic vesicles is involved in the turnover of different transmitters [34].
The effect of l-acetylcarnitine treatment was never studied on these enzyme systems (ATP-ases) on synaptic plasma membranes separated in two fractions: SPM 1 and SPM 2; furthermore, the present study was performed utilizing synaptic plasma membranes from the cerebral cortex and not from the whole brain because of the well-known brain heterogeneity and metabolic compartmentation [1, 4, 5].
Materials and Methods
Care of the Animals and Pharmacological Treatment
The experiments were performed on 4 month-old female Sprague–Dawley rats (Cobs-Charles River). The animals were selected according to randomized experimental procedures and kept from birth under standard cycling and housing conditions (temperature: 22 ± 1°C, relative humidity 60 ± 3%, lighting cycle: 12 h light and 12 h darkness; low noise disturbances), fed with a standard diet in pellets with water ad libitum and housed three and subsequently two per cage. The selection of the animals and time course of pharmacological treatment was established by permutation tables.
The animals were divided into three groups and treated by intraperitoneal injections of: (a) vehicle only (NaHCO3 0.8-1.0 M) (Merck Darmstadt, F.R. Germany) for 4 weeks, 5 days a week (control animals for sub-chronic treatment); (b) two different doses of l-acetylcarnitine (30 and 60 mg kg−1 i.p.) for 4 weeks, 5 days a week (treated animals for sub-chronic treatment). The drug was injected under anaesthesia by ether at 09:00 a.m. and, at the end of sub-chronic pharmacological treatment, the animals were sacrificed under anaesthesia by a lethal dose of urethane (1.4 g kg−1, i.p.) at 10:00 a.m. (to avoid any circadian changes of enzyme activities) and 48 h after the last drug administration (to avoid any acute effect due to the last drug’s administration).
Preparation of Purified Synaptic Plasma Membranes
Purified synaptic plasma membranes (S.P.M.) were obtained from rat cerebral areas according to the method of Lin and Way [33] as modified by an analytical technique adapted to single animal, as described in details by Gorini et al. [12].
The subsequent procedures were performed at 0–4°C. The brain was isolated (<20 s) in a refrigerated box at 0–4°C and immediately placed in an isolation medium (0.32 M sucrose, Merck Darmstadt, F.R. Germany, 1.0 mM EDTA-K+, Sigma Chemicals Company St. Louis Mo, 10 mM Tris–HCl, Merck Darmstadt, F.R. Germany, pH 7.4). The cerebral cortex (right-side) was carefully dissected, isolated and immediately placed in the isolation medium; the homogenate was obtained using a Teflon-glass homogenizer (Braun S Homogenizer) by five up and down strokes of the pestle (total clearance: 0.1 mm) rotating at 800 r.p.m., with electronic control of the pestle speed. The homogenate (usually 5 ml final volume) was then diluted to 7–10% (w/v) for differential centrifugation carried out in conditions previously determined: the “crude” nuclear fraction was removed by centrifugation at 5.5×103 g min (3.9 × 107 ω2t) in a Sorvall RC-5B Supercentrifuge, rotor SS-34.
The nuclear pellet was washed twice and the combined supernatants were centrifuged at 300×103 g min, at a total applied force of 213 × 107 ω2t, to yield the “crude” mitochondrial pellet. The “crude” mitochondrial fraction was resuspended in the isolation medium by soft homogenization in a Braun S Homogenizer (total clearance 0.1 mm) and this suspension was applied to a two steps discontinuous Ficoll-sucrose gradient, consisting of Ficoll (Pharmacia Biotech, AB Uppsala Sweden) 7.5 and 12% (w/v) in 0.32 M sucrose, 50 μM EDTA-K+, 10 mM Tris–HCl, pH 7.4. After centrifugation in a Sorvall Ultracentrifuge OTD65B, rotor AH-650, at 140×104 g min (988 × 107 ω2t) the synaptosomal fraction was obtained at the interface of the 7.5–12% Ficoll-sucrose layer. The band of synaptosomes was collected by aspiration and was then diluted with 5 volumes of isolation medium, and centrifuged at 375×103 g min (266 × 107 ω2t), rotor SS-34. The synaptosomal pellet was resuspended by soft homogenization in a Braun S Homogenizer (total clearance 0.1 mm) in a small volume of 0.32 M sucrose, pH 7.4 and osmotically lysed in 3 volumes of 6 mM Tris–HCl, pH 8.1, for 1.5 h, at 0–4°C.
After osmotic shock, the lysate was applied on a discontinuous sucrose-HEPES (Boehringer Mannheim Gmbh, Biochemica Germany) gradient consisting of successive 1 ml layers of 0.32; 0.64; 0.80; 1.0 M, pH 7.4. This gradient was centrifuged at 5×104 g min (381 × 108 ω2t) in a rotor AH-650; at the end of this centrifugation, the bands at the interfaces between 0.64 and 0.80 M sucrose-HEPES (synaptic plasma membranes, type I—SPM 1) and 0.8–1.0 M sucrose-HEPES (synaptic plasma membranes, type II—SPM 2) were collected by aspiration, diluted to the 0.32 M sucrose buffered solution and centrifuged at 342×104 g min (242 × 108 ω2t).
The pellets of synaptic plasma membranes SPM 1 and SPM 2 were resuspended by soft homogenization in a small volume of 0.32 M sucrose buffered solution (pH 7.4) for the assay of the catalytic activity of enzymes.
Enzyme Assays
The purity of the different sub-cellular fractions, was previously determined [12] evaluating the maximum rate (V max) of the following enzyme activities: acetylcholinesterase (acetylcholine hydrolase, EC 3.1.1.7) [35]; cytochrome oxidase (ferrocytochrome c: oxygen oxidoreductase, EC 1.9.3.1) [36, 37]; lactate dehydrogenase (L-lactate: NAD+ oxidoreductase, EC 1.1.1.27) [38].
Acetylcholinesterase activity was assayed by following the increase of extinction at 412 nm produced from thiocholine when it reacts with 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB). The principle of the method is the measurement of the rate of production of thiocholine as acetylthiocholine is hydrolized [35], then reacting with DTNB. The assay medium (1.02 ml final volume) consisted of 0.97 ml 0.1 M phosphate buffer (pH 8.0), 30 μl of DTNB 0.01 M, NaHCO3 0.018 M (all reagents from Merck) dissolved in phosphate buffer (pH 7.0) and 20–25 μl of synaptic plasma membranes sample. After 3 min of equilibration of the assay mixture at 20°C, the assay was initiated by the addition of 20 μl of acetylthiocholine iodide 0.075 M.
Cytochrome oxidase activity was assayed by following the decrease of extinction at 550 nm of cytochrome c (Boehringer Mannheim Gmbh, Biochemica Germany), previously reduced with 2–3 mg of ascorbic acid (Merck Darmstadt, F.R. Germany) and subsequently dialysed with 0.01 M phosphate buffer for 24 h with four changes of buffer [36, 37]. The assay medium (1.00 ml final volume) consisted of 0.9 ml 0.1 M phosphate buffer (pH 7.0), 0.1 ml of solution at 1% cytochrome c reduced and synaptic plasma membranes sample.
Lactate dehydrogenase activity was assayed by following the decrease of extinction at 340 nm produced by NADH (Merck Darmstadt, F.R. Germany) [38]. The assay medium (1.02 ml final volume) consisted of 1 ml sodium pyruvate 0.63 mM (Sigma Chemical Company, St. Louis, Mo) dissolved in 50 mM phosphate buffer (pH 7.5), 0.02 ml of NADH 11.3 mM dissolved in NaHCO3 119 mM and synaptic plasma membranes sample.
On SPM1 and SPM2 samples, ATP-ases activities were determined by measuring the Pi released from the hydrolysis of ATP in presence of different ions and synaptic plasma membranes (type I and type II); ATP-ases activities were thus expressed as μmoles of Pi released h−1 (mg of protein)−1 of each sub-cellular fraction tested [39, 40].
Na+, K+-ATP-ase activity was calculated from the difference between the Na+, K+, Mg2+-ATP-ase activity and Mg2+-ATP-ase activity [39]. Na+, K+, Mg2+-ATP-ase activity was assayed in 1.0 ml of a medium containing 50 mM Imidazole-HCl buffer, pH 7.4; 120 mM NaCl; 10 mM KCl; 5 mM MgCl2 (all reagents from Merck). Ouabain insensitive Mg2+-ATP-ase activity was evaluated in 1.0 ml of a medium containing 50 mM Imidazole-HCl buffer, pH 7.4; 120 mM NaCl; 10 mM KCl; 5 mM MgCl2; 1 mM freshly prepared ouabain (Sigma Chemical Company, St. Louis, MO). The mixture with synaptic plasma membranes (24–48 μg of protein) was preincubated for 5 min at 37°C and the reaction was then started by the addition of 4 mM ATP (Sigma Chemical Company, St. Louis, Mo). Vanadium-free Na2-ATP solutions were made fresh prior to use by neutralizing ATP to pH 7.4 with Tris. At the end of the incubation period (10 min; 37°C) with the sample of the sub-fraction tested, the reaction was stopped by cooling to 4°C and by adding 0.5 ml of TCA solution 10% (w/v) (Merck Darmstadt, F.R. Germany).
“Direct” Mg2+-ATP-ase activity was evaluated according to Shallom and Katyare [39] in 1.0 ml of a medium containing 50 mM Imidazole-HCl buffer, pH 7.4; 5 mM MgCl2. The mixture with synaptic plasma membranes (24–48 μg of protein) was preincubated for 5 min at 37°C and the reaction was then started by the addition of 4 mM ATP. Vanadium-free Na2-ATP solution was made fresh prior to use by neutralizing ATP to pH 7.4 with Tris. At the end of the incubation period (10 min; 37°C) with the sample of the sub-fraction tested, the reaction was stopped by cooling to 4°C and by adding 0.5 ml of TCA solution 10% (w/v).
Ca2+, Mg2+-ATP-ase activity was evaluated according to Palayoor et al. [40] in 1.0 ml of a medium containing 50 mM Tris–HCl buffer, pH 7.5; 100 mM KCl; 2 mM MgCl2; 120 μM CaCl2 (Merck Darmstadt, F.R. Germany); 100 μM EGTA (Sigma Chemical Company, St. Louis, Mo); 100 μM freshly prepared ouabain. The mixture with synaptic plasma membranes (32–48 μg of protein) was preincubated for 5 min at 37°C and the reaction was then started by the addition of 1.23 mM ATP. Vanadium-free Na2-ATP solution was made fresh prior to use by neutralizing ATP to pH 7.4 with Tris. At the end of the incubation period (10 min; 37°C) with the sample of the sub-fraction tested, the reaction was stopped by cooling to 4°C and by adding 0.5 ml of TCA solution 10% (w/v).
Ca2+-ATP-ase, Mg2+-independent activities with Low (L) or High (H) affinity for Ca2+ were evaluated according to Palayoor et al. [40] in 1.0 ml of a medium containing 25 mM Tris–HCl buffer, pH 7.4; 100 μM EGTA; 0.2 M sucrose; for the determination of the Low affinity Ca2+-ATP-ase in the reaction mixture the concentration of CaCl2 was 2 mM; for the determination of the High affinity Ca2+-ATP-ase, CaCl2 was 200 μM. The mixture with synaptic plasma membranes (32–48 μg of protein) was preincubated for 5 min at 37°C and the reaction was then started by the addition of 1.84 mM ATP. Vanadium-free Na2-ATP solution was made fresh prior to use by neutralizing ATP to pH 7.4 with Tris. At the end of the incubation period (12 min; 37°C) with the sample of the sub-fraction tested, the reaction was stopped by cooling to 4°C and by adding 0.5 ml of TCA solution 10% (w/v).
For all the enzyme activities tested, after centrifugation of the TCA-treated sample, 1.0 ml of the clear supernatant was assayed for Pi [41] by adding and mixing: (1) 3.0 ml of a solution containing both 4.6% of CH3COONa.3H2O and 0.25% of CuSO4·5H2O in 2 N CH3COOH; (2) 0.5 ml of 5% (NH4)6Mo7O24·4 H2O (all reagents from Merck).
Subsequently, 0.5 ml of 2% MAPS [(HOC6H4NHCH3)2·H2SO4] in 5% Na2SO3 (all reagents from Merck) was added and mixed; after 10 min, the absorbance was read at 870 nm versus a blank sample containing all the reagents without cations. A titration curve for Pi was made with anhydrous KH2PO4.
The protein concentration of the tested sub-fractions was determined using crystalline bovine serum albumin (Merck Darmstadt, F.R. Germany) as standard, according to Lowry et al. [42].
Enzyme Activity Calculation and Statistical Analysis
Enzyme activities of acetylcholinesterase, cytochrome oxidase and lactate dehydrogenase were measured by graphic recordings for at least 3 min in double beam recorder Spectrophotometers (Perkin–Elmer 554 or 551 s) and each value was calculated from two blind determinations on the same sample. Catalytic activities were expressed as specific activities in (μmoles of substrate transformed) min−1 (mg of protein)−1 of the sub-fraction tested.
Enzyme activities of ATP-ases were determined by measuring the Pi released from the hydrolysis of ATP in the presence of different ions and synaptic plasma membranes (type I and type II), as previously described in details, using a 554 or 551 s Perkin–Elmer Spectrophotometer. ATP-ases activities were expressed as specific activities in (μmoles of P i released) h−1 (mg of protein)−1 of the sub-fraction tested or as enzyme catalytic activity if expressed as nanokatal (S.I. Units).
This value is an extensive quantity and the quantities derived from it include “specific catalytic activity” and “catalytic activity”; hence, the values calculated may be: (1) enzyme specific activity (S.A.) if expressed as (μmoles of substrate transformed) min−1 (mg of protein)−1 (acetylcholinesterase); (2) (μmoles of P i released) h−1 (mg of protein)−1, if assayed as “end point” methods for ATP-ases; (3) enzyme catalytic activity (S.I. Units) if expressed as nanokatal, (nmoles of substrate transformed) s−1 ml−1.
The two ways ANOVA test was used to evaluate the comparisons of enzyme activity between the different cellular sub-fractions. The ANOVA test for multiple comparisons was used to evaluate the interactions between the values of different groups of animals, of each cellular sub-fractions, of each individual enzyme activity and of each biochemical parameter tested. The homogeneity of variance was checked by Bartlett’s test and post hoc tests were used to compare the differences between individual groups, controlling statistical evaluation by the Tukey’s and Dunnett’s tests.
Results
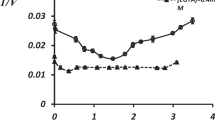
The data concerning acetylcholinesterase activity and ATP-ases enzyme activities evaluated on synaptic plasma membranes type I and type II sub-fractions of both control and treated animals with 30 and 60 mg kg−1 i.p. of l-acetylcarnitine are reported in Table 1 as Specific Activities (S.A.) and in Table 2 as S.I. Units.
In control (vehicle-treated) animals, the biochemical machinery is differently expressed in SPM 1 respect to SPM 2, in that specific enzyme activities are higher in SPM 2. These results are confirmed also when enzyme activities are expressed in S.I. Units (Table 2), suggesting an intrinsic characterization of SPM 2 with respect to SPM 1.
Sub-chronic administration of l-acetylcarnitine (30 and 60 mg kg−1 i.p. 28 days 5 days/week) modified enzyme activities of ATP-ase systems in both fractions of synaptic plasma membranes. Particularly, pharmacological treatment at the dose of 30 and 60 mg kg−1 increased the activity of Ca2+, Mg2+-ATP-ase on synaptic plasma membrane SPM 1 and SPM 2 (Table 1), both if the results are expressed as Specific Activity (S.A.) and as S.I. Units (Table 2). On the contrary Na+, K+-ATP-ase activity is increased by the drug at both doses only on synaptic plasma membranes type II fraction (Tables 1, 2).
Thus, l-acetylcarnitine treatment mainly affected enzyme activities of ATP-ases located on synaptic plasma membranes type II, that is the most representative sub-fraction of synaptic plasma membranes, suggesting a specific molecular site of action at synaptic level for the drug.
These results are confirmed also when enzyme activities are expressed as S.I. Units, indicating that the sub-chronic treatment with the drug was ineffective on protein concentration and content, by this way avoiding a possible masking effect due to sub-chronic drug administration on protein synthesis [4].
On the whole, the present results attest that the sensitivity to pharmacological treatment is different between the two types of synaptic plasma membranes, confirming that the micro-heterogeneity of these sub-fractions may play a role when certain physiopathological conditions or the pharmacodynamic properties of drugs are evaluated at sub-cellular levels [1–4, 12, 43–46].
Discussion
Methodological Aspects
In this study, energy consuming systems of ATP-ases were assayed after sub-chronic administration of l-acetylcarnitine on synaptic plasma membranes, to take into account the in vivo sub-cellular distribution of these enzyme systems, differentiable in synaptic plasma membranes type I (SPM 1) and synaptic plasma membranes type II (SPM 2) demonstrating that these two fractions are different, as underlined by enzyme analysis [47–49].
As extensively described by Gorini [49], the catalytic properties of ATP-ases systems, as assayed in different types of synaptic plasma membranes (SPM 1, SPM 2, SPM 3) and in somatic plasma membranes (SM) from the frontal cerebral cortex of 5-month-old rats, markedly differ according to the different types of considered SPM and SM, suggesting that the metabolic role of each ATP-ase is determined by their sub-cellular in vivo localization. Moreover, in the same work, assaying ATP-ases catalytic properties at 5, 10, 22 months of age, their subsynaptic localization likely influences the age-induced specific modifications in individual ATP-ases activity [49].
The purity of SPM 1 and SPM 2 sub-fractions was determined evaluating lactate dehydrogenase activity as a marker of cytoplasmic contamination (equal to 0.3–0.6% respect to homogenate, respectively), and cytochrome oxidase activity as a marker of mitochondrial membranes contamination (equal to 1.5–1.8% respect to homogenate, respectively) [49].
The SPM 2 fraction according to Cotman’s research [47] is the richest population of SPM and also Gurd [48] described some SPM sub-fractions, called A and B, possessing the highest Na+, K+-ATP-ase activity. These results, i.e. the higher enzymatic activities detected in SPM 2 fraction, are in accordance also with other works previously published by us [45, 46] and the analogies between all these data confirm that these SPMs are concentrated between 0.6–0.8 M sucrose, and 0.8–1.0 M sucrose, being thus possible to select two distinct populations [48], to be sedimented, separated and assayed distinctively [33, 50, 51].
This opportunity seems particularly interesting, because these two fractions exhibit different metabolic machinery as indicated by the differences of specific activities of ATP-ases of these two fractions (Table 1—control animals); in particular, the higher activity of acetylcholinesterase on SPM 2 suggests that this sub-fraction may possibly derive from cholinergic terminals of cerebral cortex, as previously suggested by us [4, 45].
The working pharmacological hypothesis was therefore that these sub-fractions could respond differently to pharmacological treatment, because these are derived presumably from cerebral synapses (i.e. synaptosomes) with likely different density and physico-chemical and metabolic characteristics, even if it is not possible to specify their origin [1–4, 12, 43–46].
Selective modifications by pharmacological treatment on enzyme systems of different neuronal sub-fractions have already been described [1–4, 12, 45, 46] indicating that brain macro-heterogeneity (specific cerebral areas), together with micro-heterogeneity of sub-cellular fractions, are not only subtle or unclear problems, but also possible relevant factors respect to physiopathology as well as to pharmacological treatment.
Effect of l-Acetylcarnitine on ATP-ases Activities
Sub-chronic l-acetylcarnitine treatment at both doses on 4-month-old female Sprague–Dawley rats cerebral cortex (right-side) increased Na+, K+-ATP-ase activity only on SPM 2 and Ca2+, Mg2+-ATP-ase activity on SPM 1 and SPM 2 (Tables 1, 2).
The “sodium pump” is responsible for the maintenance of resting membrane potential and in neurotransmitter turnover, while the physiological role of Ca2+, Mg2+-ATP-ase activity is to regulate intraneuronal calcium ion concentrations within the range of 0.1–1.0 μM [51], together with other cellular mechanisms [52].
Ca2+, Mg2+-ATP-ase activity is stimulated by calmodulin association respect to the level of free calcium in the cytoplasm, so that the enzyme system regulates the cytosolic calcium levels in a feedback process [53]. Therefore, during depolarization of presynaptic nerve endings, the increase of free calcium ion involves the interaction between ATP and ATP-ase to promote exocitosis of secretory granules content [51, 54].
The Ca2+ in combination with calmodulin exerts a stimulating effect on Na+, K+-ATP-ase as well [55], but it might be misleading not to point out that, in both cases, this regulation is possible only in steady-state condition. In physiopathological conditions, an impairment in energy metabolism, and therefore in ATP-ases activities, entails a loss of ionic homeostasis, thus suggesting that energy (ATP) availability is the real necessary condition to maintain a proper regulatory control of these enzyme systems.
In any case, the results show that the drug, modifying both Na+, K+-ATP-ase and Ca2+, Mg2+-ATP-ase activities, can interfere with ionic homeostasis, suggesting that the effect of the l-acetylcarnitine may be selective to specific enzymatic ATP-ase activities related to transport of Na+ and Ca2+.
In particular, as regards Ca2+ ions, Kobayashi and co-workers have found an increment of depolarization-induced calcium ion influx into synaptosomes in brain cortices of rats given l-acetylcarnitine [17]. Therefore, it seems likely that the brain tissue undergoes to a series of adaptations that tend to establish a new dynamic equilibrium, improving its ability to buffer and extrude intracellular Ca2+ concentration increases. In fact, the drug grants a proper calcium homeostasis increasing the Ca2+, Mg2+-ATP-ase activity in both SPM 1 and SPM 2 and the Na+, K+-ATP-ase activity in SPM 2.
However, synaptic low- and high-affinity Mg2+ independent Ca2+-dependent ATP-ases are both unaffected by the sub-chronic treatment with l-acetylcarnitine: this might be related to the fact that their role is to help Ca2+, Mg2+-ATP-ase to maintain the homeostasis of the intracellular calcium, together with the Na+, Ca2+ antiport process.
In fact, the K m values suggest that is actually the Ca2+, Mg2+-ATP-ase to play a key role in calcium homeostasis respect to Ca2+-ATP-ases. As regards the low-affinity Ca2+-ATP-ase, the K m value is 82.6 μM Ca2+ [56], suggesting that it is a “capacitative” component in the calcium extrusion mechanism, while the high-affinity Ca2+-ATPase is an allosteric enzyme showing a K m = 1.33 μM Ca2+ [57]. Nevertheless, the Ca2+, Mg2+-ATP-ase has a lower K m for calcium (K m = 0.23 μM) [51], signifying that this enzyme is activated at even lower calcium concentrations and it is therefore primarily involved in calcium homeostasis regulation respect to both low- and high-affinity Mg2+ independent Ca2+-ATPase.
Interestingly, it has been found that both Ca2+, Mg2+-ATP-ase and high affinity Mg2+-independent Ca2+-ATPase share practically the same K m from ATP: respectively, 18.9 and 19.0 μM [51, 57]; this suggests that the ratio of the two enzyme activities, and therefore the contribution of the single enzyme to calcium homeostasis, likely remains constant even in conditions of partial ATP depletion, possibly because the ATP moiety of the substrate complex may bind to the same site for both enzymes.
However, also the effect of l-acetylcarnitine on Na+, K+-ATP-ase explains why the low- and high-affinity Mg2+ independent Ca2+-dependent ATP-ases activities are not affected by the drug treatment, at least as regards synaptic plasma membranes type II. The sustained Na+, K+-ATP-ase activity, creating the necessary ion gradients, allows in fact the appropriate functioning of the Na+, Ca2+ antiport process, further sustaining the control of intracellular calcium concentrations.
Effect of l-acetylcarnitine on Brain Energy Metabolism in Relation to ATP-ases Activities
As regards ATP-ases activities in relation to brain energy metabolism, in previous studies on l-acetylcarnitine in vivo administration [1–3], the inhibition of citrate synthase activity, key enzyme of the Krebs cycle (ΔG (kJ/mol) = negative; ΔG0′ (kJ/mol) = −31.5) [58, 59], was observed in mitochondrial fractions from hippocampus, striatum and cerebral cortex. This event, together with an increased carnitine level, leads to an enhanced mitochondrial formation of acetylcarnitine or activates carnitine acetyl-tranferase reactions. Nevertheless, carnitine uptake is abolished by ouabain, suggesting an involvement of the Na+, K+-ATP-ase system. Thus, an increased activity of Na+, K+-ATP-ase by l-acetylcarnitine may also be related to the increased concentration of carnitine in cerebral tissue, and carnitine could have an important role in biochemical systems that are involved in excitatory and inhibitory cerebral functions [1].
Moreover, it has been assessed that l-acetylcarnitine treatment decreases the Embden-Meyerhof pathway and therefore brain lactate production after ischaemia, reducing tissue acidosis and improving the neurologic outcome of the patient [28]: since the drug is a source of acetyl-CoA, it promotes aerobic energy metabolism by-passing the reaction catalyzed by the pyruvate dehydrogenase complex, a target of reactive oxygen species that is inhibited following cerebral ischaemia.
In a study on SPM from hippocampus after ischaemia and recovery during ageing [44], the changes observed in ATP-ase activities, related to ATP availability, are in parallel in a time-dependent manner with the abnormalities in oxidative metabolism, indicating that ATP-ases possibly may be implicated in metabolic processes of physiopathological relevance, in addition to the their classical electrophysiological significance. Therefore, energy-related synaptic enzymes catalytic activities may play an important functional role during recovery time in cerebral tissue in vivo, especially as regards the responsiveness to noxious stimuli and particularly during the recirculation period from acute (or chronic) brain injury [44].
However, it should be remembered that the drug inhibits citrate synthase activity and therefore the acetyl groups would not be solely utilized in acetyl-CoA formation, even if the aerobic metabolism is sustained by l-acetylcarnitine treatment. This would prevent an otherwise excessive increase of acetyl-CoA/CoA ratio that in turn would decrease pyruvate dehydrogenase activity, the mitochondrial fatty acid β-oxidation and citric acid cycle flux [60], observing that the acetyl groups are utilized in fatty acids and acetylcholine synthesis [17], besides l-acetylcarnitine synthesis itself. An explanation could be that the drug actually favours the aerobic metabolism, but mainly through alternative metabolic pathways, so that the drug increases energy production as a result.
In fact, α-ketoglutarate dehydrogenase activity was found to be enhanced by l-acetylcarnitine sub-chronic treatment in intrasynaptic “light” mitochondria isolated from 4 month-old female Sprague-Dawley rats cerebral cortex (left-side) [3], suggesting that the drug stimulates a preferential utilization of the metabolite in the Krebs cycle, also considering the stimulating action of l-acetylcarnitine treatment on cytochrome oxidase activity of mitochondrial fractions obtained from rat hippocampus, striatum and cerebral cortex [1–3].
These results are partially in accordance with a recent study by Long et al. [61], where l-acetylcarnitine and α-lipoic acid treatment led to the recovery of complex I and IV activities in 22-month-old rats compared to 4.7 month-old ones, highlighting the supposed synergistic effect of α-lipoic acid on aerobic metabolism [62].
A deeper insight into the possible relationships with mitochondrial respiratory chain components would be of great interest, in particular in relation to Coenzyme Q, which actually controls the efficiency of oxidative phosphorylation, as discussed in details by Lenaz et al. [63–65]. It was shown that l-acetylcarnitine sub-chronic treatment at the dose of 30 mg kg−1 negatively affects CoQ10 levels in rat brain free mitochondria [5]. Since electron transfer is limited by the concentration of ubiquinone in the inner mitochondrial membrane phospholipids [63–65], the drug likely exerts a direct action particularly on subunits of complex IV. This hypothesis is consistent with previous studies [10, 43] showing that l-acetylcarnitine increased the amount of a 16 kDa mitochondrial inner membrane protein [43] identified as the subunit IV of cytochrome oxidase [66].
The stimulating action of l-acetylcarnitine treatment on cytochrome oxidase activity of intra-synaptic mitochondria [1–3] and Na+, K+-ATP-ase activity (present data) confirms the functional correlation of these two enzymes, the activation of Na+, K+-ATP-ase representing a critical event that links energy metabolism with electric activity [67]. This suggests the fundamental role of Na+, K+-ATP-ase (and ATP availability) in the control of neuronal transmission [58].
In the same metabolic context, after neuronal activity stimulation, there is an increase of Ca2+ ion concentration into presynaptic nerve endings and this event explains the drug-induced increase of Ca2+, Mg2+-ATP-ase activity in order to remove intracellular Ca2+ ions and to maintain the homeostatic level of these Ca2+ ions.
These results show that l-acetylcarnitine treatment increases energy metabolism and the enzyme activities involved in neurotransmission processes. Taken together these pharmacodynamic characteristics of l-acetylcarnitine at sub-cellular levels (intrasynaptic mitochondria—synaptic plasma membranes) may be of interest in the therapeutic role of the drug in several physiopathologic states such as ageing and in neurodegenerative age-related diseases such as Alzheimer’s Dementia.
In particular, as regards ageing, some studies [68, 69] pointed out that either ageing or peroxidative stress imply a decreased activity of Na+, K+-ATP-ase: such an effect might depend on the modification of the lipidic composition and on decreased membrane fluidity which occurs during ageing [70], suggesting that l-acetylcarnitine is involved in the stabilization of the cellular membrane [13, 14] and thus helps to re-create the structural microenvironment necessary to maintain the proper physiological synaptic functions.
Effect of l-acetylcarnitine on AChE Activity
Cholinergic system dysfunction is claimed to be involved in the pathogenesis of Alzheimer’s Disease, but also a defect in energy metabolism may play a role; particularly, cytochrome oxidase activity seems to be reduced in this neurodegenerative pathology leading to a reduction in energy stores and thereby contributing to neurodegenerative process [71], while l-acetylcarnitine has been proven to be able to slow the natural course of Alzheimer’s disease [72].
With regard to the catabolism of acetylcholine, l-acetylcarnitine treatment in 4 month-old rats has not modified acetylcholinesterase activity (AChE) of both SPM fractions, in agreement with previous studies evaluating acetylcholinesterase activity on SPM from frontal cerebral cortex of 4-, 8-, 12-, 20- and 24-month-old female Sprague–Dawley rats [12]. Interestingly, the catalytic properties of AChE [73], as well as Na+, K+-ATP-ase [68, 69], is significantly influenced by the membrane composition of phospholipids; therefore, it should be expected an increase in this enzyme activity after l-acetylcarnitine administration. However, since AChE enters the membrane double phospholipid layer less than Na+, K+-ATP-ase does [74], it is therefore less susceptible to changes in membrane phospholipid composition [75].
Conclusion
In summary, as we discussed in previous studies and in present research about the effects of in vivo treatments of l-acetylcarnitine on enzymes related to energy-yielding as well as energy-consuming ATP-ase systems of cerebral tissue [1–5, 12, 43], it seems likely that the drug primarily interferes with the energy metabolism and consequently with the neuronal electric activity and the neurotransmission processes.
Moreover, l-acetylcarnitine acts both on intra-synaptic mitochondria (citrate synthase, cytochrome oxidase activity) and on synaptic membranes (Na+, K+-ATP-ase and Ca2+, Mg2+-ATP-ase activities), therefore particularly on synaptic structures [4]. These results confirm that cellular sub-fractionation might be a useful method to verify the pharmacodynamic characteristics of drugs acting on cerebral energy mechanisms, in this research taking into account the macro-heterogeneity and micro-heterogeneity of ATP-ases systems of cerebral tissue, whose individual roles are determined by their in vivo sub-cellular localization [34, 76–78].
References
Villa RF, Gorini A, Zanada F et al (1986) Action of l-acetylcarnitine on different cerebral mitochondrial populations from hippocampus. Arch Int Pharmacodyn Ther 279:195–211
Villa RF, Gorini A (1991) Action of l-acetylcarnitine on different cerebral mitochondrial populations from hippocampus and striatum during aging. Neurochem Res 16:1125–1132
Gorini A, D’Angelo A, Villa RF (1998) Action of l-acetylcarnitine on different cerebral mitochondrial populations from cerebral cortex. Neurochem Res 23:1485–1491
Gorini A, D’Angelo A, Villa RF (1999) Energy metabolism of synaptosomal subpopulations from different neuronal systems of rat hippocampus: effect of l-acetylcarnitine administration in vivo. Neurochem Res 24:617–624
Battino M, Quiles JL, Huertas JR et al (2000) Cerebral cortex synaptic heavy mitochondria may represent the oldest synaptic mitochondrial population: biochemical heterogeneity and effect of l-acetylcarnitine. J Bioenerg Biomembr 32:163–173
Inano A, Sai Y, Nikaido H et al (2003) Acetyl-L-carnitine permeability across the blood-brain barrier and involvement of carnitine transporter OCTN2. Biopharm Drug Dispos 24:357–365
Nalecz KA, Miecz D, Berezowski V et al (2004) Carnitine: transport and physiological functions in the brain. Mol Aspects Med 25:551–567
Miecz D, Januszewicz E, Czeredys M et al (2008) Localization of organic cation/carnitine transporter (OCTN2) in cells forming the blood-brain barrier. J Neurochem 104:113–123
Burlina AP, Sershen H, Debler EA et al (1989) Uptake of acetyl-L-carnitine in the brain. Neurochem Res 14:489–493
Turpeenoja L, Villa RF, Magri MG et al (1988) Changes of mitochondrial membrane proteins in rat cerebellum during aging. Neurochem Res 13:859–865
Aureli T, Miccheli A, Ricciolini R et al (1990) Aging brain: effect of acetyl-L-carnitine treatment on rat brain energy and phospholipid metabolism. A study by 31P and 1H NMR spectroscopy. Brain Res 526:108–112
Gorini A, Ghigini B, Villa RF (1996) Acetylcholinesterase activity of synaptic plasma membranes during ageing: effect of l-acetylcarnitine. Dementia 7:147–154
Paradies G, Ruggero FM, Gadaleta MN et al (1992) The effect of aging and acetyl-L-carnitine on the activity of the phosphate carrier and on the phospholipid composition in rat heart mitochondria. Biochim Biophys Acta 1103:324–326
Hagen TM, Ingersoll RT, Wehr CM et al (1998) Acetyl-l-carnitine fed to old rats partially restores mitochondrial function and ambulatory activity. Proc Natl Acad Sci USA 95:9562–9566
Carta A, Calvani M, Bravi D et al (1993) Acetyl-L-carnitine and Alzheimer’s disease: pharmacological considerations beyond the cholinergic sphere. Ann NY Acad Sci 695:324–326
Jones LL, McDonald DA, Borum PR (2010) Acylcarnitines: role in brain. Prog Lipid Res 49:61–75
Kobayashi S, Iwamoto M, Kon K et al (2010) Acetyl-L-carnitine improves aged brain function. Geriatr Gerontol Int 10:S99–S106
Toth E, Harsing LG Jr, Sershen H et al (1993) Effect of acetyl-L-carnitine on extracellular amino acid levels in vivo in rat brain regions. Neurochem Res 18:573–578
Gherlandini C, Galeotti N, Calvani M et al (2002) Acetyl-l-carnitine induces muscarinic antinocieption in mice and rats. Neuropharmacology 43:1180–1187
Pettegrew JW, Levine J, McClure RJ (2000) Acetyl-L-carnitine physical-chemical, metabolic, and therapeutic properties: relevance for its mode of action in Alzheimer’s disease and geriatric depression. Mol Psychiatry 5:616–632
Arienti G, Ramacci MT, Maccari F et al (1992) Acetyl-L-carnitine influences the fluidity of brain microsomes and of liposomes made of rat brain microsomal lipid extracts. Neurochem Res 17:671–675
Corbucci GG, Melis A, Piga M et al (1992) Influence of acetyl-carnitine on some mitochondrial enzymic activities in the human cerebral tissue in conditions of acute hypoxia. Int J Tiss Reac 14:183–194
Blokland A, Bothmer J, Honig W et al (1993) Behavioural and biochemical effects of acute central metabolic inhibition: effects of acetyl-L-carnitine. Eur J Pharmacol 235:275–281
Barhwal K, Singh SB, Hota SK et al (2007) Acetyl-L-carnitine ameliorates hypobaric hypoxic impairment and spatial memory deficits in rats. Eur J Pharmacol 570:97–107
Barhwal K, Hota SK, Prasad D et al (2008) Hypoxia-induced deactivation of NGF-mediated ERK1/2 signaling in hippocampal cells: neuroprotection by acetyl-L-carnitine. J Neurosci Res 86:2705–2721
Rosadini G, Marenco S, Nobili F et al (1990) Acute effects of acetyl-L-carnitine on regional cerebral blood flow in patients with brain ischaemia. Int J Clin Pharmacol Res 10:123–128
Shuaib A, Waqaar T, Wishart T et al (1995) Acetyl-L-carnitine attenuates neuronal damage in gerbils with transient forebrain ischemia only when given before the insult. Neurochem Res 9:1021–1025
Zanelli SA, Solenski NJ, Rosenthal RE et al (2005) Mechanisms of ischemic neuroprotection by acetyl-L-carnitine. Ann NY Acad Sci 1053:153–161
Jalal FY, Böhlke M, Maher TJ (2010) Acetyl-L-carnitine reduces the infarct size and striatal glutamate outflow following focal cerebral ischemia in rats. Ann NY Acad Sci 1199:95–104
Onofrj M, Fulgente T, Melchionda D et al (1995) l-acetylcarnitine as a new therapeutic approach for peripheral neuropathies with pain. Int J Clin Pharmacol Res 15:9–15
Taglialatela G, Navarra D, Cruciani R et al (1994) Acetyl-L-carnitine treatment increases nerve growth factor levels and choline acetyltransferase activity in the central nervous system of aged rats. Exp Gerontol 29:55–66
Kentroti S, Ramacci MT, Vernadakis A (1992) Acetyl-L-carnitine has a neuromodulatory influence on neuronal phenotypes during early embryogenesis in the chick embryo. Dev Brain Res 70:259–266
Lin SC, Way EL (1982) Calcium-activated ATPases in presynaptic nerve endings. J Neurochem 39:1641–1651
Benzi G, Gorini A, Ghigini B et al (1994) Modifications by hypoxia and drug treatment of cerebral ATPase plasticity. Neurochem Res 19:517–524
Ellman GL, Courtney KD, Andres V et al (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–96
Smith L (1955) Spectrophotometric assay of cytochrome c oxidase. In: Glick D (ed) Methods of biochemical analysis. Wiley Interscience, New York, pp 427–434
Wharton DC, Tzagoloff A (1977) Cytochrome oxidase from beef heart mitochondria. In: Estabrook RW, Pulman ME (eds) Methods in enzymology. Academic Press, New York, pp 245–250
Bergmeyer HU, Bernt E (1974) Lactate dehydrogenase: UV-assay with pyruvate and NADH. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 574–579
Shallom JM, Katyare SS (1985) Altered synaptosomal ATPase activity in rat brain following prolonged in vivo treatment with nicotine. Biochem Pharmacol 34:3445–3449
Palayoor ST, Seyfried TN, Bernard DJ (1986) Calcium ATPase activities in synaptic plasma membranes of seizure-prone mice. J Neurochem 46:1370–1375
Le Bel D, Poirier GG, Beaudolin AR (1978) A convenient method for the ATPase assay. Anal Biochem 85:86–89
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Villa RF, Turpeenoja L, Benzi G et al (1988) Action of l-acetylcarnitine on age-dependent modifications of mitochondrial membrane protein from rat cerebellum. Neurochem Res 13:909–916
Villa RF, Gorini A, Hoyer S (2002) ATPases of synaptic plasma membranes from hippocampus after ischemia and recovery during ageing. Neurochem Res 27:861–870
Gorini A, Rancati A, D’Angelo A et al (2000) Effect of in vivo administration of Naloxone on ATP-ase’s enzyme systems of synaptic plasma membranes from rat cerebral cortex. Neurochem Res 25:867–873
Gorini A, Villa RF (2001) Effect of in vivo treatment of Clonidine on ATP-ase’s enzyme systems of synaptic plasma membranes from rat cerebral cortex. Neurochem Res 26:819–825
Cotman CW, Matthews DA (1971) Synaptic plasma membranes from rat brain synaptosomes: isolation and partial characterization. Biochim Biophys Acta 249:380–394
Gurd JW, Jones LR, Mahler HR et al (1974) Isolation and partial characterization of rat brain synaptic plasma membranes. J Neurochem 22:281–290
Gorini A, Canosi U, Devecchi E et al (2002) ATPases enzyme activities during aging in different types of somatic and synaptic plasma membranes from rat frontal cerebral cortex. Prog Neuropsychopharmacol Biol Psychiatry 26:81–90
Lin SC, Way EL (1982) A high affinity Ca2+-ATPase in enriched nerve-ending plasma membranes. Brain Res 235:387–392
Michaelis EK, Michaelis ML, Chang HH et al (1983) High affinity Ca2+-stimulated Mg2+-dependent ATPase in rat brain synaptosomes, synaptic membranes, and microsomes. J Biol Chem 258:6101–6108
Berridge MJ (2004) Calcium signal transduction and cellular control mechanisms. Biochim Biophys Acta 1742:3–7
Ross DH, Cardenas HL (1983) Calmodulin stimulation of Ca2+-dependent ATP hydrolysis and ATP-dependent transport in synaptic membranes. J Neurochem 41:161–171
Douglas WW (1968) Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol 34:451–474
Powis DA, Wattus GD (1981) The stimulatory effect of calcium on Na, K-ATPase of nervous tissue. FEBS Lett 126:285–288
Lin SC, Way EL (1984) Characterization of calcium-activated and magnesium-activated ATPases of brain nerve endings. J Neurochem 42:1697–1706
Gandhi CR, Ross DH (1988) Characterization of a high-affinity Mg2+-independent Ca2+-ATPase from rat brain synaptosomal membranes. J Neurochem 50:248–256
Villa RF, Gorini A, Hoyer S (2006) Differentiated effect of aging of Krebs’ cycle, electron transfer complexes and glutamate metabolism of non-synaptic and intra-synaptic mitochondria from cerebral cortex. J Neural Transm 113:1659–1670
Villa RF, Gorini A, Hoyer S (2009) Effect of ageing and ischemia on enzymatic activities linked to Krebs’ cycle, electron transfer chain, glutamate and amino acids metabolism of free and intra-synaptic mitochondria of cerebral cortex. Neurochem Res 34:2102–2116
Rosca MG, Lemieux H, Hoppel CL (2009) Mitochondria in the elderly: is acetylcarnitine a rejuvenator? Adv Drug Deliv Rev 61:1332–1342
Long J, Gao F, Tong L et al (2009) Mitochondrial decay in the brains of old rats: ameliorating effect of alpha-lipoic acid and acetyl-L-carnitine. Neurochem Res 34:755–763
Packer L, Witt EH, Tritschler HJ (1995) Alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med 19:227–250
Battino M, Fato R, Parenti-Castelli G et al (1990) Coenzyme Q can control the efficiency of oxidative phosphorylation. Int J Tissue React 12:137–144
Lenaz G, Battino M, Castelluccio C et al (1990) Studies on the role of ubiquinone in the control of the mitochondrial respiratory chain. Free Rad Res Commun 8:317–327
Rauchová H, Battino M, Fato R et al (1992) Coenzyme Q-pool function in glycerol-3-phosphate oxidation in hamster brown adipose tissue mitochondria. J Bioenerg Biomembr 24:235–241
Ragusa N, Turpeenoja L, Magri G et al (1989) Age-dependent modifications of mitochondrial proteins in cerebral cortex and striatum of rat brain. Neurochem Res 14:415–418
Mata M, Fink DJ, Gairner H et al (1980) Activity-dependent energy metabolism in rat posterior pituitary primarily reflects sodium pump activity. J Neurochem 34:213–215
Mishra OP, Delivoria-Papadopoulos M, Cahillane G et al (1990) Lipid peroxidation as the mechanism of modification of the affinity of the Na+, K+-ATPase active sites for ATP, K+, Na+, and strophanthidin in vitro. Neurochem Res 14:845–851
Chakraborty H, Sen P, Sur A et al (2003) Age-related oxidative inactivation of Na+, K+-ATPase in rat brain crude synaptosomes. Exp Gerontol 38:705–710
Viani P, Cervato G, Fiorilli A et al (1991) Age-related differences in synaptosomal peroxidative damage and membrane properties. J Neurochem 56:253–258
Mutisya EM, Bowling AC, Beal MF (1994) Cortical cytochrome oxidase activity is reduced in Alzheimer’s disease. J Neurochem 63:2179–2184
Carta A, Calvani M (1991) Acetyl-L-carnitine: a drug able to slow the progress of Alzheimer’s disease? Ann NY Acad Sci 640:228–232
Beauregard G, Roufogalis BD (1977) The role of tightly bound phospholipid in the activity of erythrocyte acetylcholinesterase. Biochem Biophys Res Commun 77:211–219
Rieger F, Vigny M (1976) Solubilization and physicochemical characterization of rat brain acetylcholinesterase: development and maturation of its molecular forms. J Neurochem 27:121–129
Nemat-Gorgani M, Meisami E (1979) Use of Arrhenius plots of Na-K ATPase and acetylcholinesterase as a tool for studying changes in lipid-protein interactions in neuronal membranes during brain development. J Neurochem 32:1027–1032
Toll L, Howard BD (1978) Role of Mg2+-ATPase and a pH gradient in the storage of catecholamines in synaptic vesicles. Biochemistry 17:2517–2523
Nagy A, Shuster TA, Rosemberg MD (1983) Adenosine triphosphatase activity at the external surface of chicken brain synaptosomes. J Neurochem 40:226–234
Fyske EM, Fonnum F (1991) Transport of gamma-aminobutyrate and L-glutamate into synaptic vesicles. Effect of different inhibitors on the vesicular uptake of neurotransmitters and on the Mg2+-ATPase. Biochem J 276:363–367
Acknowledgments
This work was supported by grants from Italian Ministry of University and Technological and Scientific Research (M.U.R.S.T.) and partially by Sigma-Tau, Rome.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Villa, R.F., Ferrari, F. & Gorini, A. Effect of In Vivo l-Acetylcarnitine Administration on ATP-ases Enzyme Systems of Synaptic Plasma Membranes from Rat Cerebral Cortex. Neurochem Res 36, 1372–1382 (2011). https://doi.org/10.1007/s11064-011-0462-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0462-x