Abstract
Astrocyte-rich primary cultures were used to investigate the consequences of a copper exposure on the glucose metabolism of astrocytes. After application of CuCl2 (30 μM) the specific cellular copper content increased from initial 1.5 ± 0.2 nmol/mg to a steady state level of 7.9 ± 0.9 nmol/mg within about 12 h. The copper accumulation was accompanied by a significant increase in the extracellular lactate concentration. The stimulating effect of copper on the lactate production remained after removal of extracellular copper. Copper treatment accelerated the rates of both glucose consumption and lactate production by about 60%. The copper induced acceleration of glycolytic flux was prevented by inhibition of protein synthesis, and additive to the stimulation of glycolysis observed for inhibitors of respiration or prolyl hydroxylases. A copper induced stimulation of glycolytic flux in astrocytes could have severe consequences for the glucose metabolism of the brain in conditions of copper overload.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copper is an essential trace metal that is required as catalytic co-factor or as structural component of several enzymes [1–4]. However, an excess of copper is harmful to cells, most likely by the copper-catalysed generation of hydroxyl radicals in a Fenton-like reaction [5, 6]. To avoid both copper deficiency and copper toxicity, cellular uptake, distribution and excretion of copper are tightly regulated. However, disturbances of these homeostatic mechanisms in copper metabolism have been considered to contribute to the development and progression of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and prion disorders [7–9].
Astrocytes fulfil many important functions for the brain. These cells modulate synaptic transmission, synaptic plasticity and extracellular ion homeostasis [10–12]. In addition, astrocytes are metabolically coupled to neurons and have been discussed to supply neurons with metabolites for the synthesis of the antioxidant glutathione (GSH) and with substrates for energy production [13–15]. Thus, compromised astrocytic functions will also affect neighbouring neurons in brain.
Astrocytes have been shown to efficiently accumulate copper in vitro [16–18]. One transporter that is likely to be involved in this copper accumulation by astrocytes is the copper transport receptor 1 (Ctr1), but also the divalent metal transporter 1 (DMT1) has been considered to mediate uptake of copper ions into cultured astrocytes [17]. Efficient copper uptake by astrocytes may be important for the protection of neighboring neurons in brain. At least in co-cultures, astrocytes protect cerebellar neurones against copper toxicity [16]. In addition, astrocytes prevent the copper catalysed extracellular loss of GSH [19]. However, excess of copper has been reported to damage astrocytes. Exposure of cultured astrocytes to copper increases membrane permeability and mitochondrial permeability transition, induces a mild oxidative stress that leads to lipid peroxidation and alterations in the GSH/GSSG ratio, and inactivates enzymes such as glutathione reductase [18, 20, 21].
Astrocytes are considered as rather glycolytic cell type that produces substantial amounts of ATP by glycolysis, as indicated by the high rate of lactate production compared for example to neurons [22, 23]. However, the stimulation of glycolysis by inhibitors of mitochondrial respiration in cultured astrocytes demonstrates that these cells produce ATP also by respiration and that they do not depend exclusively on glycolytic ATP production [22, 24]. In addition to inhibitors of the respiratory chain, lactate production in cultured astrocytes is stimulated by a variety of substances such as glutamate, cytokines, ammonia, noradrenalin or pioglitazone [15, 25–28]. Furthermore, compounds that stabilise the transcription factor hypoxia inducible factor (HIF)-1α increase the glycolytic rate in cultured astrocytes by an upregulation of glycolytic enzymes [29].
To study the consequences of subtoxic concentrations of cellular copper on the glucose metabolism of astrocytes, we have investigated lactate release and glucose consumption of astrocyte-rich primary cultures after application of micromolar concentrations of CuCl2. Here we show that copper treatment stimulated glycolytic flux in a time- and concentration- dependent manner, in a process that depends on protein synthesis and that is additive to the expected effects of inhibitors of mitochondrial respiration and of stabilisers of HIF-1α.
Experimental Procedures
Materials
Fetal calf serum and penicillin/streptomycin solution were obtained from Biochrom (Berlin, Germany). Dulbecco’s modified Eagle’s medium (DMEM, without or with 25 mM glucose) was from Gibco (Karlsruhe, Germany). Saccharose and dimethyl sulfoxide (DMSO) were purchased from Janssen Chimica (Geel, Belgium). Bovine serum albumin, NAD+, NADH and NADP+ were purchased from AppliChem (Darmstadt, Germany). Antimycine A, cycloheximide, deferoxamine mesylate (DFx), EDTA, rotenone and Tris were purchased from Sigma (Steinheim, Germany). Glucose-6-phosphate dehydrogenase (G6PDH), glutamate pyruvate transaminase (GPT), hexokinase and lactate dehydrogenase (LDH) were purchased from Roche Diagnostics (Mannheim, Germany). CoCl2, disodium hydrogen phosphate, Folin-Ciocalteus phenol reagent, HNO3, neutral red (NR; 3-amino-7-dimethylamino-2-methyl-phenazine hydrochloride), Pd(NO3)2, potassium sodium tartrate, sodium azide, sodium dihydrogen phosphate, sodium pyruvate and triethanolamine hydrochloride were obtained from Merck (Darmstadt, Germany). Acetic acid, glutamine, MgCl2 and NaOH were purchased from Fluka (Buchs, Switzerland). Argon was from Linde (Hamburg, Germany). HCl was purchased from Fisher Scientific (Schwerte, Germany). CuCl2 was from Riedel-de-Haen (Seelze, Germany) and glucose from Serva (Heidelberg, Germany). Ethanol was purchased from Roth (Karlsruhe, Germany) and dimethyloxallyl glycine (DMOG) from Cayman Chemical (Ann Arbor, USA). Microtiter plates and sterile cell culture dishes were from Sarstedt (Nümbrecht, Germany).
Cell Cultures
Astrocyte-rich primary cultures were prepared from brains of newborn Wistar rats according to the method of Hamprecht and Löffler [30]. Specific lactate release and glucose consumption rates were determined for cell cultures on 6-well plates, while all other experiments were performed on cultures in 24-well plates. Total numbers of 300,000 and 1,500,000 viable cells were seeded in 1 ml culture medium (90% DMEM containing 25 mM glucose and 1 mM pyruvate, 10% foetal calf serum, 20 U/ml penicillin G, 20 μg/ml streptomycin sulfate) per well of 24-well culture plates and in 2.5 ml culture medium per well of 6-well culture plates, respectively. The cultures were maintained at 10% CO2 in the humidified atmosphere of a Sanyo (Osaka, Japan) incubator and were used for experiments at an age between 14 and 20 days. The culture medium was renewed every 7 days and 24 h prior to the individual experiment.
Experimental Incubation with Copper
Cells were washed twice with 1 ml (24-well plates) or 2.5 ml (6-well plates) of prewarmed (37°C) incubation medium (DMEM containing 25 mM glucose, 1 mM sodium pyruvate, 20 U/ml penicillin G and 20 μg/ml streptomycin sulfate) and then incubated at 37°C for up to 24 h in 1 ml (24-well plates) or 2.5 ml (6-well plates) incubation medium with CuCl2 in the concentrations given in the legends of the figures and the table. The experiments were stopped by washing the cells once with 1 ml (24-well plates) or 2.5 ml (6-well plates) of ice-cold phosphate buffered saline (PBS; 10 mM potassium phosphate buffer, pH 7.4, containing 150 mM NaCl) and twice with 1 ml (24-well plates) or 2.5 ml (6-well plates) of ice-cold PBS containing 10 mM EDTA. Dry cells were stored at −20°C for the quantitation of copper and of protein. For determination of other cellular parameters the cells were processed as described below. For the determination of extracellular lactate and LDH activity, the incubation medium was collected and processed as described below.
Determination of Lactate Release Rates and Glucose Consumption Rates
If not stated otherwise, cells on 6-well culture plates were preincubation for 16 h at 37°C in 2.5 ml incubation medium without or with CuCl2 in the concentrations given in the legends of the figures or the table, washed twice with 2.5 ml prewarmed (37°C) DMEM (containing 5 mM glucose and 1 mM pyruvate) and then incubated at 37°C for up to 4 h in 1 ml DMEM (containing 5 mM glucose and 1 mM pyruvate). The rates of glucose consumption and lactate production were calculated from the almost linear decreases and increases of the extracellular glucose and lactate concentrations, respectively. Lactate and glucose contents of the media were determined as described previously [31–33].
Determination of Cellular Copper and Protein Contents
The cells were lysed in 1 ml 50 mM NaOH and this lysate was used to determine cellular protein contents according to the Lowry method [34], using bovine serum albumin as standard, and the cellular copper content as previously described in detail [17].
Determination of Cell Viability
Compromised cell viability and functions were determined by quantifying the release of the cytosolic enzyme LDH and the accumulation of NR into lysosomes as indicators. The activity of cellular and extracellular LDH was determined as described previously [35]. Neutral red (NR) retention was investigated using a modification of a published assay [36]. Briefly, cells in wells of 24-well plates were washed twice with 1 ml of prewarmed (37°C) incubation medium and were subsequently incubated for 1 h at 37°C in 500 μl of DMEM (containing 25 mM glucose and 1 mM pyruvate) with 16 μg/ml NR. Cells were then washed twice with 1 ml PBS and lysed in 500 μl of lysis solution (50% (v/v) ethanol, 1% (v/v) acetic acid, 49% (v/v) H2O). Of these lysates, 250 μl were transferred into wells of a microtiter plate and the absorbance at 540 nm was measured.
Presentation of Data
If not stated otherwise, results are presented as means ± SD of data obtained in at least three experiments that were performed on independently prepared astrocyte-rich primary cultures. Significance of differences between two sets of data were analysed by the t test and differences between groups of data were analysed by ANOVA followed by the Bonferroni post hoc test, with *P <0.05, **P < 0.01 and ***P < 0.001. P > 0.05 was considered as not significant.
Results
Copper Accumulation and Lactate Production by Copper-Exposed Cultured Astrocytes
Treatment of astrocyte-rich primary cultures with 30 μM CuCl2 resulted in a time dependent increase in the cellular copper content from a basal value of 1.5 ± 0.2 to 7.9 ± 0.9 nmol/mg within 24 h. The initial strong increase of the cellular copper content within the first hours after copper application slowed down after 8 h and reached a plateau after about 12 h of incubation (Fig. 1a). To investigate the concentration dependency of the copper accumulation, the cells were incubated with up to 100 μM CuCl2 for 16 h and the contents of cellular copper were determined (Fig. 1c). The specific cellular copper content increased almost proportional to the concentration of copper applied. However, concentrations above 60 μM did not further increase the cellular copper content (Fig. 1c), but significantly compromised cell viability and functions as indicated by the increase in extracellular LDH activity (Fig. 1e) and by the significantly lowered NR retention after 16 and 24 h of incubation (Fig. 1f).
Consequences of a copper treatment on cultured astrocytes. The cells were incubated without (open circles) or with (filled circles) 30 μM CuCl2 for up to 24 h (a, b) or with CuCl2 in the indicated concentrations for 16 h (c, d). The contents of cellular copper (a, c) and of extracellular lactate (b, d) were determined. The panel’s e and f show the extracellular LDH activity and the NR retention, respectively, of cells that were exposed to the indicated concentrations of CuCl2 for 16 h (filled triangles) or 24 h (open triangles). The significance of differences of the data compared to those of the control condition (absence of CuCl2) is indicated
To determine whether exposure to CuCl2 alters the glycolytic flux in cultured astrocytes, the lactate content in the medium was determined during incubation of the cells with or without copper for up to 24 h (Fig. 1b). During the first 8 h of incubation in the absence or presence of 30 μM CuCl2, lactate accumulated in the medium proportional to time, reaching within 8 h a concentration of 4.0 ± 0.4 mM. However, longer incubations revealed significantly elevated concentrations of lactate in the media of cells that had been exposed to CuCl2 compared to control cells (Fig. 1b). The extracellular lactate content strongly increased with the concentration of CuCl2 applied, showing a half-maximal effect after 16 h exposure of the cells to about 30 μM CuCl2 (Fig. 1d). A maximal increase in extracellular lactate concentration to about 160% of controls (absence of copper) was observed after incubation of astrocytes for 16 h with 60 μM CuCl2 (Fig. 1d).
Lactate Production and Glucose Consumption Rates After Removal of Extracellular Copper
To investigate whether presence of extracellular copper is required to maintain the increased lactate release, lactate production and glucose consumption rates were determined for astrocytes that had been preincubated for various periods with 30 μM CuCl2. The specific lactate release and glucose consumption rates of cultured astrocytes were not significantly altered, if the cells were preincubated without copper for up to 24 h (Fig. 2a). In contrast, the lactate release rate increased almost linearly during the first 12 h of preincubation with CuCl2 from a basal rate of 19.2 ± 0.8 nmol/(mg × min) to 28.1 ± 2.5 nmol/(mg × min) (Fig. 2a). This increase was accompanied by a similar increase in the glucose consumption rate from 10.0 ± 0.5 nmol/(mg × min) to 15.7 ± 1.3 nmol/(mg × min) within 12 h (Fig. 2b). Longer preincubations than 12 h with CuCl2 did not lead to any further increase of these rates (Fig. 2a, b).
Effect of copper on the glycolytic flux in cultured astrocytes. The cells were preincubated without (open circles) or with (filled circles) 30 μM CuCl2 for up to 24 h (a–c) or with CuCl2 in the concentrations indicated for 16 h (d–f). After removal of the extracellular copper, the rates of lactate release (a, d) and of glucose consumption (b, e) as well as the ratio of lactate release rate to glucose consumption rate (c, f) were determined. The significance of differences of data compared to those of controls (absence of CuCl2 during preincubation) is indicated
Both, lactate release rate (Fig. 2d) and glucose consumption rate (Fig. 2e) increased almost proportional to the copper concentration that had been present during a 16 h preincubation, at least for copper concentrations of up to 50 μM. Higher CuCl2 concentrations yielded lower rates (Fig. 2d, e), most likely due to copper-induced toxicity at high CuCl2 concentrations (Fig. 1e, f). The ratio of the lactate production rate to the glucose consumption rate was almost two for all conditions used and was not affected by the time of preincubation with CuCl2 (Fig. 2c) nor by the concentration of CuCl2 that had been present during preincubation of the cells (Fig. 2f).
Effects of Cycloheximide on Copper Accumulation and Lactate Production
To determine whether the copper-induced increase in lactate production and glucose consumption requires the synthesis of proteins, the cells were preincubated for 16 h without or with 30 μM CuCl2 in the absence or the presence of 10 μM of the protein synthesis inhibitor cycloheximide, before the main incubation was performed to determine glycolytic flux rates. As described above, preincubation with copper for 16 h increased the specific cellular copper content 5fold, did not affect cell viability, but increased the extracellular lactate concentration by 37% (Table 1). Presence of cycloheximide during preincubation without or with CuCl2 did not increase the extracellular LDH activity, but lowered significantly the cellular protein content and the NR retention by 15 and 25% of the respective controls (absence of cycloheximide), respectively (Table 1). Under these conditions, the specific cellular copper content was slightly elevated by 20%, but the copper-induced increase in extracellular lactate concentration during the preincubation was completely prevented (Table 1). In addition, both the copper-induced increases in glucose consumption and lactate production rates of copper treated cells were abolished, if the cells had been preincubated with CuCl2 plus cycloheximide (Table 1).
Effects of Respiratory Chain Inhibitors and of HIF-1α-stabilising Compounds
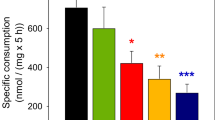
Inhibition of respiratory chain complexes has been reported to increase glucose consumption and lactate release of cultured astrocytes [37]. To test whether such a process is involved in the observed copper-induced acceleration of glycolytic flux, astrocytes were preincubated for 16 h without or with 30 μM CuCl2, before the rates of glucose consumption and lactate production were assessed in the absence or presence of rotenone, antimycine A or sodium azide. Inhibition of the oxidative phosphorylation by each of these inhibitors increased significantly (P < 0.001) both lactase release (Fig. 3a) and glucose consumption rates (Fig. 3b) 2–3 fold, whereas copper-preincubation increased lactate production and glucose consumption by only around 60% (Fig. 3). However, the effects observed for copper-preincubated cells on lactate release and glucose consumption rates were additive to those observed by each of the inhibitors (Fig. 3).
Effects of inhibitors of mitochondrial respiration on the copper induced stimulation of lactate release (a) and glucose consumption (b). Cultured astrocytes were preincubated without or with 30 μM CuCl2 for 16 h. The specific copper contents of cells that were preincubated without and with CuCl2 were 1.3 ± 0.1 and 10.9 ± 1.8 nmol/mg, respectively. The rates of lactate release and glucose consumption were determined in the absence or the presence of 10 μM rotenone, 10 μM antimycin A or 10 mM sodium azide. The difference of the data for inhibitor-treated cells compared to the respective controls (absence of respiratory chain inhibitors) was highly significant for all inhibitors (P < 0.001, not indicated in the figure). Indicated by stars is the significance of differences between the data obtained for cells that were preincubated without or with CuCl2
Copper is known to stabilise the transcription factor HIF-1α, which induces increased expression of genes that are involved in glucose uptake and glycolysis during hypoxia [38]. To test whether copper-induced stabilisation of HIF-1α is involved in the observed copper-induced stimulation of glycolytic flux, cultured astrocytes were preincubated for 16 h without or with 30 μM CuCl2 in the presence of compounds that stabilise HIF1α. No differences in the cellular copper content were observed after pretreatment of cells with 30 μM CuCl2 alone or in combination with DFx or DMOG, whereas the presence of CoCl2 resulted in a significantly higher (P < 0.001) cellular copper content (Fig. 4a). The presence of each of the three stabilisers of HIF-1α, as well as presence of CuCl2 alone, led to significantly elevated rates of lactate release and glucose consumption (P < 0.01 or P < 0.001) to about 150% of the values obtained for control cells (absence of stabilisers and copper; Fig. 4b, c). However, co-incubation of cells with both copper plus stabiliser of HIF-1α caused additive increases in lactate production and glucose consumption rates, thereby almost doubling both rates (Fig. 4b, c).
Effects of stabilisers of HIF-1α on copper accumulation and on the copper-induced stimulation of lactate release and glucose consumption in cultured astrocytes. The cells were preincubated for 16 h without or with 30 μM CuCl2 in the presence or absence of 1 mM DFx, 1 mM DMOG or 200 μM CoCl2. Panel a shows the cellular specific copper content after the preincubation. The rates of lactate release (b) and glucose consumption (c) were determined as described in “Experimental Procedures”. In panels b and c, the differences of the data for cells that were treated with one of the indicated stabilisers compared to the respective controls (absence of stabilisers) was highly significant for all inhibitors used (P < 0.01 or P < 0.001), while in panel a only the specific copper content of cells treated with CuCl2 in the presence of CoCl2 was significantly elevated (P < 0.001) (not indicated in the figure). Indicated with stars is the significance of differences between the data obtained for cells that were preincubated without or with CuCl2
Discussion
The present study investigated the consequences of copper accumulation on the glycolytic flux of cultured astrocytes. Elevated cellular copper levels accelerated lactate release and glucose consumption by these cells in a process that was prevented by inhibition of protein synthesis and that was additive to the effects observed for inhibitors of the respiratory chain and for stabilisers of HIF-1α.
Cultured astrocytes accumulated copper efficiently from DMEM in a time- and concentration-dependent manner, by mechanisms that involve most likely Ctr1-dependent and Ctr1-independent transport [16, 17]. After 12 h of incubation with 30 μM CuCl2, the specific cellular copper content of astrocytes had reached a steady state level. This is likely to be a consequence of changes in the activity or the localisation of the transporters that are involved in copper uptake and efflux. For example Ctr1 has been demonstrated to be rapidly internalised in many cell types when extracellular copper levels rise [39, 40]. Also the level of DMT1 is decreased, at least in CaCo-2 cells, following exposure to copper [41]. In addition, a rising cellular copper content induces the translocation of the copper transporting ATPase ATP7A [42] into the plasma membrane of chinese hamster ovary cells, human fibroblasts and HeLa cells, thereby facilitating copper export [43, 44]. Similar alterations of the location of these proteins, which are involved in copper transport and are expressed in astrocyte cultures [17, 45], would lower copper accumulation and increase copper export, thereby generating a steady state level of cellular copper.
Exposure of cultured astrocytes to 30 μM CuCl2 in DMEM caused a 5 fold increase in cellular copper content within 12 h. This copper accumulation was much slower than that observed for astrocytes that were incubated in amino acid free incubation buffer (8 fold increase within 1 h; [18]). A similar dependency of the rates of copper accumulation from the composition of the incubation media has been reported for HEK293 cells [46]. Most likely amino acids in the DMEM such as histidine form copper complexes that are known to be taken up into cells much slower than copper ions in non-complexed form [47].
During incubation of astrocytes with copper in DMEM the cells were remarkably resistant against copper induced toxicity. Even exposure to CuCl2 in concentrations of up to 50 μM for 24 h did not lead to cell toxicity, confirming literature data for astrocytes [20, 48, 49], while higher concentrations compromised to some low but significant extent cell viability and NR retention within 16 or 24 h. This contrasts the situation of astrocytes that have been exposed to CuCl2 in amino acid free medium which leads to a very rapid accumulation of copper by the cells and to severe toxicity within 2 h [17, 18]. Thus, the speed of cellular copper accumulation appears to determine the extent and the velocity of copper-mediated cell damage in astrocytes. The toxicity observed for astrocytes that had been exposed to copper in amino acid free medium [17, 18] is most likely the consequence of the rapid accumulation of large amounts of copper which can under the conditions used not be compensated by a sufficient storage capacity for copper and/or by sufficient detoxification capacity.
Cultured astrocytes are known for their high rate of lactate production, although the reported specific rates for lactate release and glucose consumption vary strongly [50]. With basal rates of 19.2 ± 0.8 nmol/(mg × min) and 10.0 ± 0.5 nmol/(mg × min) for lactate release and glucose consumption, respectively, the values reported in the present study are similar to literature data [51, 52]. The high ratio of lactate production to glucose consumption of almost two demonstrates that cultured astrocytes are highly glycolytic under the conditions used. This predominant glycolytic ATP production is most likely a direct consequence of the strong inactivation of the pyruvate dehydrogenase complex by phosphorylation of the pyruvate dehydrogenase α-subunit in cultured astrocytes [53].
The time- and concentration-dependent increases in cellular specific copper content correlated well with the increases in extracellular lactate concentration as well as with the stimulation of the specific rates of cellular glucose consumption and lactate release, suggesting that an elevated cellular copper content stimulates glycolytic flux in astrocytes. Detectable differences in extracellular lactate concentrations of copper-treated cells compared to control cells were only observed after exposure to copper for at least 8 h. This delay in the cellular response suggests that the intracellular copper level has to reach a given threshold value to accelerate glycolytic flux and/or that protein synthesis is involved in this process. Both explanations would be supported by the observation that the copper-effect is maintained after removal of extracellular copper. The copper-induced stimulation of glycolytic flux was prevented by the presence of the protein synthesis inhibitor cycloheximide, demonstrating that indeed the time required for activation of transcription and protein synthesis contributes to the delay of the upregulation of glycolytic flux in copper-treated astrocytes. The co-incubation of astrocytes with CuCl2 plus cycloheximide did not lower the specific copper accumulation and was not accompanied by an increased toxicity, suggesting that the increased glycolytic flux is not required to maintain cell survival after exposure to copper. In addition, the synthesis of copper sequestering proteins such as metallothioneins [54, 55] appears not to be essential for cell survival under the conditions investigated. However, copper can also be detoxified by binding to the tripeptide GSH [56] which is present in cultured astrocytes in a specific cellular content (about 35 nmol/mg [18]) that is 4 fold higher than the specific cellular copper content found after exposure to 30 μM CuCl2.
Inhibitors of the respiratory chain are known to stimulate glycolysis in cultured astrocytes [22, 37]. In addition, stabilisation of HIF-1α under normoxic conditions induces the expression of genes involved in glucose uptake and glycolysis in astrocytes [57–60] and accelerates glycolytic flux [29]. However, although inhibitors of the different respiratory chain complexes as well as HIF-1α stabilisers stimulated glucose consumption and lactate production in astrocytes, these compounds showed effects that were additive to those observed for copper-treatment. Thus, the mechanism that is responsible for stimulated glycolytic flux by elevated cellular copper levels appears not to involve inhibition of respiration and stabilisation of HIF-1α.
In conclusion, we show for the first time that copper exposure modulates the cellular glucose metabolism of astrocytes by accelerating the glycolytic flux. Already a 5 fold increased cellular copper content accelerated glycolytic flux by about 60%. Protein synthesis is required for this effect, while contributions of copper-induced inhibition of mitochondrial respiration or stabilisation of HIF-1α are unlikely to be involved. So far we were unable to identify the mechanism involved. Thus, further studies are required to analyse the copper-induced stimulation of glycolytic flux in astrocytes in more detail. Assuming that astrocytes in brain react in a similar way to an excess of copper as cultured astrocytes do, the consequence of a copper accumulation in astrocytes, for example in copper overload conditions such in Wilsons disease [61, 62], could be a lactic acidosis in brain. However, since lactate has been considered as energy rich fuel that is delivered from astrocytes to neurons [13, 15], an elevated lactate release during copper-exposure could also deliver additional substrate for the energy metabolism to neurons.
References
Turski ML, Thiele DJ (2009) New roles for copper metabolism in cell proliferation, signaling, and disease. J Biol Chem 284:717–721
Balamurugan K, Schaffner W (2006) Copper homeostasis in eukaryotes: teetering on a tightrope. Biochim Biophys Acta 1763:737–746
Stern BR (2010) Essentiality and toxicity in copper health risk assessment: overview, update and regulatory considerations. J Toxicol Environ Health A 73:114–127
Tapiero H, Townsend DM, Tew KD (2003) Trace elements in human physiology and pathology. Copper Biomed Pharmacother 57:386–398
Halliwell B, Gutteridge JM (1990) Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 186:1–85
Gunther MR, Hanna PM, Mason RP et al (1995) Hydroxyl radical formation from cuprous ion and hydrogen peroxide: a spin-trapping study. Arch Biochem Biophys 316:515–522
Hung YH, Bush AI, Cherny RA (2010) Copper in the brain and Alzheimer’s disease. J Biol Inorg Chem 15:61–76
Rivera-Mancia S, Perez-Neri I, Rios C et al (2010) The transition metals copper and iron in neurodegenerative diseases. Chem Biol Interact 186:184–199
Molina-Holgado F, Hider RC, Gaeta A et al (2007) Metals ions and neurodegeneration. Biometals 20:639–654
Perea G, Araque A (2010) GLIA modulates synaptic transmission. Brain Res Rev 63:93–102
Barker AJ, Ullian EM (2010) Astrocytes and synaptic plasticity. Neuroscientist 16:40–50
Deitmer JW, Rose CR (2010) Ion changes and signalling in perisynaptic glia. Brain Res Rev 63:113–129
Hirrlinger J, Dringen R (2010) The cytosolic redox state of astrocytes: maintenance, regulation and functional implications for metabolite trafficking. Brain Res Rev 63:177–188
Barros LF, Deitmer JW (2010) Glucose and lactate supply to the synapse. Brain Res Rev 63:149–159
Pellerin L, Bouzier-Sore AK, Aubert A et al (2007) Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55:1251–1262
Brown DR (2004) Role of the prion protein in copper turnover in astrocytes. Neurobiol Dis 15:534–543
Scheiber IF, Mercer JF, Dringen R (2010) Copper accumulation by cultured astrocytes. Neurochem Int 56:451–460
Scheiber IF, Schmidt MM, Dringen R (2010) Zinc prevents the copper-induced damage of cultured astrocytes. Neurochem Int 57:314–322
Pope SA, Milton R, Heales SJ (2008) Astrocytes protect against copper-catalysed loss of extracellular glutathione. Neurochem Res 33:1410–1418
Reddy PV, Rao KV, Norenberg MD (2008) The mitochondrial permeability transition, and oxidative and nitrosative stress in the mechanism of copper toxicity in cultured neurons and astrocytes. Lab Invest 88:816–830
Krumschnabel G, Manzl C, Berger C et al (2005) Oxidative stress, mitochondrial permeability transition, and cell death in Cu-exposed trout hepatocytes. Toxicol Appl Pharmacol 209:62–73
Walz W, Mukerji S (1988) Lactate release from cultured astrocytes and neurons: a comparison. Glia 1:366–370
Itoh Y, Esaki T, Shimoji K et al (2003) Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc Natl Acad Sci USA 100:4879–4884
Kahlert S, Reiser G (2000) Requirement of glycolytic and mitochondrial energy supply for loading of Ca2+ stores and InsP(3)-mediated Ca2+ signaling in rat hippocampus astrocytes. J Neurosci Res 61:409–420
Subbarao KV, Hertz L (1991) Stimulation of energy metabolism by alpha-adrenergic agonists in primary cultures of astrocytes. J Neurosci Res 28:399–405
Kala G, Hertz L (2005) Ammonia effects on pyruvate/lactate production in astrocytes—interaction with glutamate. Neurochem Int 47:4–12
Izawa Y, Takahashi S, Suzuki N (2009) Pioglitazone enhances pyruvate and lactate oxidation in cultured neurons but not in cultured astroglia. Brain Res 1305:64–73
Garg SK, Kipnis J, Banerjee R (2009) IFN-gamma and IL-4 differentially shape metabolic responses and neuroprotective phenotype of astrocytes. J Neurochem 108:1155–1166
Schubert D, Soucek T, Blouw B (2009) The induction of HIF-1 reduces astrocyte activation by amyloid beta peptide. Eur J Neurosci 29:1323–1334
Hamprecht B, Loffler F (1985) Primary glial cultures as a model for studying hormone action. Methods Enzymol 109:341–345
Schmidt MM, Dringen R (2009) Differential effects of iodoacetamide and iodoacetate on glycolysis and glutathione metabolism of cultured astrocytes. Front Neuroenergetics 1:1–10
Liddell JR, Zwingmann C, Schmidt MM et al (2009) Sustained hydrogen peroxide stress decreases lactate production by cultured astrocytes. J Neurosci Res 87:2696–2708
Dringen R, Gebhardt R, Hamprecht B (1993) Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res 623:208–214
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Dringen R, Kussmaul L, Hamprecht B (1998) Detoxification of exogenous hydrogen peroxide and organic hydroperoxides by cultured astroglial cells assessed by microtiter plate assay. Brain Res Brain Res Protoc 2:223–228
Borenfreund E, Puerner JA (1985) Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett 24:119–124
Pauwels PJ, Opperdoes FR, Trouet A (1985) Effects of antimycin, glucose deprivation, and serum on cultures of neurons, astrocytes, and neuroblastoma cells. J Neurochem 44:143–148
Martin F, Linden T, Katschinski DM et al (2005) Copper-dependent activation of hypoxia-inducible factor (HIF)-1: implications for ceruloplasmin regulation. Blood 105:4613–4619
Molloy SA, Kaplan JH (2009) Copper-dependent recycling of hCTR1, the human high affinity copper transporter. J Biol Chem 284:29704–29713
Petris MJ, Smith K, Lee J et al (2003) Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem 278:9639–9646
Tennant J, Stansfield M, Yamaji S et al (2002) Effects of copper on the expression of metal transporters in human intestinal Caco-2 cells. FEBS Lett 527:239–244
Qian Y, Tiffany-Castiglioni E, Harris ED (1997) A Menkes P-type ATPase involved in copper homeostasis in the central nervous system of the rat. Brain Res Mol Brain Res 48:60–66
La Fontaine S, Mercer JF (2007) Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch Biochem Biophys 463:149–167
van den Berghe PV, Klomp LW (2010) Posttranslational regulation of copper transporters. J Biol Inorg Chem 15:37–46
Niciu MJ, Ma XM, El Meskini R et al (2006) Developmental changes in the expression of ATP7A during a critical period in postnatal neurodevelopment. Neuroscience 139:947–964
Lee J, Pena MM, Nose Y et al (2002) Biochemical characterization of the human copper transporter Ctr1. J Biol Chem 277:4380–4387
Waldrop GL, Ettinger MJ (1990) Effects of albumin and histidine on kinetics of copper transport by fibroblasts. Am J Physiol 259:G212–G218
Chen SH, Lin JK, Liu SH et al (2008) Apoptosis of cultured astrocytes induced by the copper and neocuproine complex through oxidative stress and JNK activation. Toxicol Sci 102:138–149
Brown DR, Schmidt B, Kretzschmar HA (1998) Effects of copper on survival of prion protein knockout neurons and glia. J Neurochem 70:1686–1693
Zwingmann C, Leibfritz D, Hazell AS (2003) Energy metabolism in astrocytes and neurons treated with manganese: relation among cell-specific energy failure, glucose metabolism, and intercellular trafficking using multinuclear NMR- spectroscopic analysis. J Cereb Blood Flow Metab 23:756–771
Schmidt MM, Rohwedder A, Dringen R (2010) Effects of chlorinated acetates on the glutathione metabolism and on glycolysis of cultured astrocytes. Neurotox Res (in press)
Fonseca LL, Monteiro MA, Alves PM et al (2005) Cultures of rat astrocytes challenged with a steady supply of glutamate: new model to study flux distribution in the glutamate-glutamine cycle. Glia 51:286–296
Halim ND, McFate T, Mohyeldin A et al (2010) Phosphorylation status of pyruvate dehydrogenase distinguishes metabolic phenotypes of cultured rat brain astrocytes and neurons. Glia 58:1168–1176
Schilsky ML, Blank RR, Czaja MJ et al (1989) Hepatocellular copper toxicity and its attenuation by zinc. J Clin Invest 84:1562–1568
Singh RP, Kumar S, Nada R et al (2006) Evaluation of copper toxicity in isolated human peripheral blood mononuclear cells and it’s attenuation by zinc ex vivo. Mol Cell Biochem 282:13–21
Freedman JH, Ciriolo MR, Peisach J (1989) The role of glutathione in copper metabolism and toxicity. J Biol Chem 264:5598–5605
Maxwell PH, Wiesener MS, Chang GW et al (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271–275
Semenza GL, Roth PH, Fang HM et al (1994) Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem 269:23757–23763
Bergeron M, Yu AY, Solway KE et al (1999) Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci 11:4159–4170
Mense SM, Sengupta A, Zhou M et al (2006) Gene expression profiling reveals the profound upregulation of hypoxia-responsive genes in primary human astrocytes. Physiol Genomics 25:435–449
Lorincz MT (2010) Neurologic Wilson’s disease. Ann NY Acad Sci 1184:173–187
Gouider-Khouja N (2009) Wilson’s disease. Parkinsonism Relat Disord 15(Suppl 3):S126–S129
Acknowledgments
The authors like to thank Dr. Maike M. Schmidt (University of Bremen, Germany) for critically reading the manuscript and for many valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scheiber, I.F., Dringen, R. Copper Accelerates Glycolytic Flux in Cultured Astrocytes. Neurochem Res 36, 894–903 (2011). https://doi.org/10.1007/s11064-011-0419-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0419-0