Abstract
We have determined the plasma (p) concentration of gamma-aminobutyric acid (GABA) and the dopamine metabolite homovanillic acid (HVA), and the pHVA/pGABA ratio in schizophrenic and bipolar patients. The research was undertaken in a geographic area with an ethnically homogeneous population. The HVA plasma concentrations were significantly elevated in the schizophrenic patients compared to the bipolar patients. The levels of pGABA was significantly lower in the two groups of patients compared to the control group, while the pHVA/pGABA ratio was significantly greater in the both groups of patients compared to the controls. As the levels of pHVA and pGABA are partially under genetic control it is better to compare their concentrations within an homogeneous population. The values of the ratio pHVA/pGABA are compatible with the idea of an abnormal dopamine-GABA interaction in schizophrenic and bipolar patients. The pHVA/pGABA ratio may be a good peripheral marker in psychiatric research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the past decades the psychiatric diagnosis has been standardized based on patient features of a phenomenological nature, given that the biological basis for most syndromes, although recognized in its existence, remains unknown. Therefore, the resulting categories are mere hypotheses put across with little knowledge of the brain alterations that sustain them. In these circumstances, clinical and scientific attempts to orient research towards the discovery of the mechanisms that accompany psychiatric diseases meet great difficulties. Psychotic destabilization, depending on the interaction of diverse genes with different circumstances, co-exists with diverse phenotypes. For example, here prevail, (to a greater or smaller degree): alterations in the course of thought, affective symptoms or cognitive disorders. Consequently, to facilitate the advance in neurobiological investigation in psychiatry it is absolutely necessary to identify markers that may help define endophenotypes which objectively sub-group psychiatric patients. It is evident that we lack such markers [1].
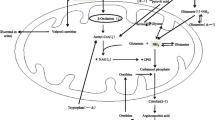
Direct investigation of the human brain is obviously difficult; and, for ethical reasons as much as for practical ones, human neurobiological research is mostly limited to indirect, noninvasive methods. One of these methods is to determine the plasma concentration levels of some neurotransmitters and their metabolites. Although plasma metabolites come from the central nervous system as well as from the periphery, in suitable experimental conditions they reflect central changes [2, 3]. Significant correlations between peripheral and central changes are possible because the central contribution is sufficient to dominate a statistical relation, or because the central and peripheral pools, under genetic control, are affected in a co-relative way [4]. An example of interest to our study is homovanillic acid (HVA), dopamine’s main metabolite. Haloperidol, which blocks central and peripheral dopamine receptors, elevates the plasma concentration of homovanillic acid (pHVA); whereas domperidone, being a powerful antagonist in the peripheral receptors of dopamine but not penetrating into the brain, does not elevate pHVA [5]. The determination of plasma levels of gamma-aminobutyric acid (pGABA) has also been suggested as an index of cerebral GABA activity [6, 7]. The concentrations of GABA in the plasma and in the brain change in a proportional and correlated way after pharmacological manipulation [7–11].
The plasma concentration of both substances has been related to psychiatric disorders. Two decades ago several groups, among them our own, demonstrated the existence of a relationship between the pHVA concentration and the response to neuroleptic treatment in schizophrenic patients, based on the dopaminergic hypothesis of the schizophrenic process. The clinical improvement correlated positively with the decrease of pHVA [12–15]. Furthermore, a greater initial increase of pHVA in response to neuroleptic treatment was a predictive factor of good clinical evolution [13]. In general these works were confirmed later, including, on occasion, the determination of other metabolites [16, 17]. The plasma concentration of homovanillic acid also proved to be a useful tool in other pathologies such as the Hungtinton disease [18].
The studies of catecholamines metabolites in plasma in patients with BPD have given contradictory results, although high values of pHVA and pMHPG (3-methoxy-4-hydroxyphenylethylenglycol) have been generally found before treatment [19, 20]. In other series of studies concentrations of pHVA or pMHPG, or their changes during treatment, have been related to clinical evolution [20–24].
GABA’s cerebral activity has also been related to several neuropsychiatric disorders. Depression, panic attacks, alcohol dependency or post-traumatic stress have been associated to a reduction of GABA concentration in the cortex, demonstrated in some cases through magnetic resonance [25–28]. Other authors have suggested a certain GABA-ergic hypofunction in BPD. The first to suggest an alteration of GABA in mood disorders was Emrich et al. [29], based on the effectiveness of valproate that was found after administration to patients with BPD. Different authors have also described objective deficits in gabaergic transmission in the prefrontal cortex, the limbic system, and the cerebellum in individuals with schizophrenia [30–32]. It has been suggested that an imbalance in the GABA/dopamine interaction would contribute to the pathophysiology of schizophrenia [33–35]. In this sense some studies have demonstrated a modulatory capacity of the GABA on the dopaminergic system that causes changes in the HVA [36, 37]. Diverse studies have used pGABA concentration as a reflection of brain activity. Of particular interest were the studies in BPD done by Petty et al. [7, 25] that suggested the pGABA determination as a blood test of this disorder. Subsequently it was suggested that the decrease of pGABA values was associated only to a sub-group of bipolar patients [38] while other authors suggest a simultaneous alteration of the gabaergic function together with other monoaminergic systems [39].
In this context we have started an investigation of biological markers in schizophrenia and bipolar disorder, beginning with the analysis of the differences in pHVA and pGABA concentrations among normal controls, schizophrenics and bipolar I patients, in a geographic zone of relative ethnic homogeneity. We have also verified the influence of gender and age on the mentioned levels.
Technically we were fortunate with the wide spread of high-resolution liquid chromatography (HPLC) which enables many laboratories to determine neurotransmitters and their metabolites. In the method section we will provide a more detailed description of the one developed to determine pGABA, since we had not previously published the details of this technique, as was done with pHVA-determination techniques [40].
Experimental Procedure
Subjects
The sample consisted of: 104 healthy controls that were not undergoing any pharmacological treatment and that had no record of psychiatric disease; 74 bipolar patients of type I; and 50 patients with schizophrenia. Patients were selected among those admitted consecutively in two hospitals of the Basque Health Service (Zamudio and Basurto). Patients were of both sexes, between 18 and 64 years of age, and free from serious organic disease, as well as drug addiction, pregnancy or breastfeeding. The patients were diagnosed according to the diagnostic an statistical manual of mental disorders revised (DSM-IV-TR) [41] based on the data obtained by two experienced psychiatrists through structured interview [42]. The patients had a need for treatment, and they fulfilled the following requirements: for at least the previous 2 weeks none of them had received any neuroleptic medication, and for at least the past week they had taken no other psychoactive medication.
Patients were informed of the details of the study signing a written consent to take part in it. This study fulfills the requirements of the Helsinki declaration, and was approved by the ethics committee of the Basque Health Service. Controls for both sexes were chosen among members of the staff.
Blood Samples
Blood samples were taken from all participants between 8 and 8.30 a.m., after 30 min of rest and 12 h of fast. The samples were obtained in glass tubes with heparin, centrifuged for 10 min at 4,000g. After plasma separation, sodium metabisulfite was added in sufficient quantity to obtain a final concentration of 0.5 g/l plasma. The samples were stored in liquid nitrogen until their analysis.
Biochemical Assays
The pHVA concentration was assessed with HPLC and coulombimetric detection, as previously described [40].
The determination of pGABA concentration was carried out by HPLC with fluorescence detection, with excitation and emission wavelengths set at 330 and 360 nm, respectively, after pre-column derivatization by means of o-phthalaldehyde. The column was an ACE 5 C18HL, which is 250 × 4.6 mm with 5 μm particle size set at 28°C. The precolumn was an 5 μm C18. The mobile phase consisted of 0.1 M sodium dihydrogen phosphate (pH = 4.5) with 8% methanol and 4% acetonitrile (v/v) at a flow rate of 1 ml/min.
A GABA stock solution (1 mg/ml) was prepared in water: methanol (1/1; v/v) and stored for up to 5 days at 4°C. The working solution of the derivatization agent consisted of a mixture (2/2/1; v/v/v) of 50 mM sodium tetraborate containing 0.1 mM EDTA (pH 9.4), o-phthalaldehyde (5.4 mg/ml in methanol) and sodium sulfite (5 mg/ml in water). For the derivatization reaction, we used 11 μl of the derivatization agent per 40 μl of standard or plasma samples.
When required for analysis, the plasma samples were defrosted and centrifuged for 5 min at 4,000g in a refrigerated centrifuge at 5°C. Then 150 μl of plasma were de-proteinized using Vivaspin 500 filters with 30,000 NMW by centrifugation at 4,000g during 30 min at 5°C. Next, 90 μl of filtered plasma were reacted with 25 μl of the derivatization agent. Samples were placed in the automatic injector; and after 5 min of reaction time at 5°C in the dark, 50 μl [of reacted sample] were injected into the HPLC system. To calculate pGABA concentration in samples, the GABA peak heights in each sample were compared with the height of a standard GABA peak.
This method was found to be adequate, with samples presenting high recovery (90.6–99.8%); the intra and inter-day precisions were good, with the variation coefficient ranging from 4.83 to 6.48%, (intra-day) and 4.45, to 6.90% (inter-day).
Statistical Analysis
The statistical analysis of the results was carried out using Statgraphics Plus software. The data distribution analysis was carried out by means of Chi-square test; and the presence of atypical values was established by the Grubbs test. When lack of normality was observed, with a presence of atypical values, a logarithmic transformation was made to solve this problem.
Plasma concentrations of HVA and GABA, as well as pHVA/pGABA quotients through the different groups, were analyzed by means of analysis of variance (ANOVA) followed by post hoc analysis with the Student’s Newman Keuls test (SNK). We studied the possible influence of age and sex on the plasma concentrations of HVA and GABA in the different diagnostic groups by means of analysis of covariance. Correlations between plasma concentrations of HVA, GABA and age were made by means of a Pearson test.
Results
The sample data is shown in Table 1. In this sample there were: 104 controls (64 women and 40 men). The age range was between 18–64 years old, with an average age of 44.7 ± 10.2 years. There were 74 Type I bipolar patients, (46 women and 28 men), aged between 19 and 52 years old, with an average age of 35.0 ± 9.6; and 50 schizophrenic patients, (24 women and 26 men), their age ranging between 18 and 63, with an average age of 35.1 ± 11.3.
The pGABA concentrations among controls, and in bipolar and schizophrenic patients, presented a normal distribution. This was not so in the case of pHVA levels, where logarithmic transformation was required to treat the data. We also observed that the dispersion of the data was greater among schizophrenics than it was among controls and bipolar patients. Table 2 lists pHVA and pGABA and the pHVA/pGABA quotient concentrations in each experimental group. We observed a significantly greater concentration of pHVA in schizophrenic rather than in the bipolar patients, (SNK P < 0.001). When age was included in the model as a covariate, differences in pHVA concentrations remained between schizophrenics and bipolar patients (SNK P < 0.05). Influence of gender is not significant and did not alter these results.
We found significantly lower levels of pGABA in the schizophrenic and bipolar groups than in the controls (SNK P < 0.001) Effects for the diagnostic groups remained significant after controlling for gender and age.
The pHVA/pGABA quotient is significantly higher in the schizophrenic patients compared to the group of bipolar patients and controls (SNK P < 0.001). When we analyzed the data considering the influence of age, the above mentioned differences remained, and differences emerged between bipolars and controls, being the pHVA/pGABA quotient in bipolar patients greater than in controls (SNK P < 0.05). The influence of gender was non significant and did not alter those results.
The pHVA concentration correlated significantly and positively with age only among controls (r = 0.364; P < 0.001). The age-pHVA correlation for controls was not significantly influenced by the presence of a greater number of older people in the group. The correlation remained significant (P = 0.009) when including in the control group only people aged between 18 and 53.
Gender did not significantly influence pHVA and pGABA concentrations in any of the groups of our sample. There was no correlation between the values of pHVA and pGABA, either among controls or among patients.
Discussion
There are more women than men, both among controls and among patients with bipolar disorder, (61 and 62%, respectively). In the schizophrenic group the number of individuals of both genders is balanced. This fact does not seem to affect results, since in our sample gender does not influence the pGABA and pHVA concentrations. The age is higher among controls than it was among schizophrenic and bipolar patients, which influenced the results slightly. Within each group there is no age difference between men and women. It seems that the absence of a significant relation between pHVA concentration and the patient’s age has to do with either the previous medication or the pathological process, or both factors simultaneously.
The frequency of abnormal distributions found in the pHVA concentrations in patients, and the high standard deviations of values in schizophrenic patients, (compared with the ones found in normal controls and in patients with bipolar disorder), must be noted (see Table 2); this could be due to both previous treatments and the heterogeneity of the sample, alluded to in our introduction,. This heterogeneity can also be related to the so-called comorbidity, although this may not serve as a clarifying criterion. Using the current criteria for diagnoses (i.e. DSM-IV), it is perfectly possible that comorbidity simply responds to the fact that sometimes inevitably many patients receive several diagnoses, though their different symptoms share the same etiology [43]. Indeed, a rational use of the parameters measured would facilitate an objective subdivision of patients. Consequently, we have recently found a basis on which to build a division between bipolar patients with congruent or incongruent psychotic symptoms [23].
On the other hand, the results regarding pGABA concentrations in different groups are coherent with the observation of patients with bipolar disorder by Berrettini et al. [44] and Petty et al. [10] and our findings differ from those found by the same authors among schizophrenics: observing no differences when compared with controls [25, 38]. Nevertheless, most post-mortem studies of schizophrenic patients have revealed GABA reductions in different brain areas [45–48]. The fact that variations can be found in the pGABA concentration in both patient types supports the idea of the gabaergic system being involved in the schizophrenic and bipolar disorder, perhaps through an abnormal interaction between dopamine and GABA [10, 29, 30, 32, 34, 39, 49, 50].
Regarding the interaction between dopamine and GABA, we consider the pHVA/pGABA quotient to be more informative of the balance between neurotransmission systems than our isolated values. It is possible that in future longitudinal studies, the variations in pHVA/pGABA quotient could reflect the adaptation to pharmacological treatment and also demonstrate whether the quotient contributes to lessen the influence of uncontrolled individual deviations. The significant elevation of pHVA/pGABA quotients in the patients as opposed to the controls, supports the idea of a global inhibiting modulation of GABA in dopaminergic activity [34]. It would be interesting, given the abnormal distributions and high standard deviations of pHVA, to study a greater number of patients to see if there exist sub-groups which concentrate increased or decrease values for pHVA/pGABA quotients. For example, with patients as with non ill relatives, it would be of interest to trace the influence of family history or the role of inheritance in clinical subgroups. We considered this high quotient to have a potential value for diagnosis, or as a marker, such as in studies on how mutations of some genes (i.e., neuroligine and neurexine) [1], can alter the balance between exciting and inhibiting transmission, or in studies on the relation of certain genetic polymorphisms with certain personality features [51].
It is possible that some genetic variants which are common to bipolar and schizophrenic disorders, although they may not always coincide, could contribute to increasing the ratio of dopaminergic/gabaergic activity throughout the whole spectrum: bipolar, schizo-affective, and schizophrenia. Recent findings contribute to the idea of shared genetic factors and support the existence of an overlap in the gene of susceptibility for schizophrenia and bipolar disorder [43]. The largest family study ever published on schizophrenia and bipolar disorder [52] reported that first degree relatives of patients with schizophrenia or bipolar disorder have an increased risk of suffering from both disorders; and that this effect is mainly due to genetic factors, concluding that both disorders share a common genetic cause.
From this point of view it is difficult to explain the difference we have found in the pHVA concentration between bipolar and schizophrenic patients. It may be that our study is underpowered to explore the question of diagnostic overlap, or that it is limited by a different time of washout or by different treatments derived from diagnosis. But, ultimately, the greatest limitation is our ignorance of the ethiopathogeny of both disorders (or if they are one, two or one hundred different disorders). Due to the intervention of multiple genes and to the different intensity of participation of each gene in each individual, together with the phenomenological approach to categorial diagnosis, it is not surprising that the results of a large study [52] show an appearance of homogeneity in what is really a mixture of many illness. It is conceivable that there is no unitary basis for both disorders and that each entity is a heterogeneous one resulting from the numerous interactions between multiple genes and between the environment and of variable intensity.
Future research is necessary to include the variation of the parameters studied here during treatment, and their relation with the presence of specific polymorphisms. This would link their relation with a proneness to suffer from certain symptoms independently of categorial diagnosis.
References
Hyman SE (2008) A glimmer of light for neuropsychiatric disorders. Nature 455:890–893
Davidson M, Losonczy MF, Mohs RC et al (1987) Effects of debrisoquin and haloperidol on plasma homovanillic acid concentration in schizophrenic patients. Neuropsychopharmacology 1:17–23
Konicki PE, Owen RR, Litman RE et al (1991) The acute effects of central- and peripheral-acting dopamine antagonists on plasma HVA in schizophrenic patients. Life Sci 48:1411–1416
Kaminski R, Powchick P, Warne PA et al (1990) Measurement of plasma homovanillic acid concentrations in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry 14:271–287
Laduron PM, Leysen JE (1979) Domperidone, a specific in vitro dopamine antagonist, devoid of in vivo central dopaminergic activity. Biochem Pharmacol 28:2161–2165
Petty F, Kramer G, Feldman M (1987) Is plasma GABA of peripheral origin? Biol Psychiatry 22:725–732
Petty F (1994) Plasma concentrations of γ-aminobutyric acid (GABA) and mood disorders: a blood test for manic depressive disease? Biol Psychiatry 39:278–284
Löscher W (1979) GABA in plasma and cerebrospinal fluid of different species: effects of γ-acetylenic GABA, γ-vinyl GABA and sodium valproate. J Neurochem 38:293–295
Uhlhaas VS, Lanage H, Wappenschmidt J et al (1986) Free and conjugated CSF and plasma GABA in Huntington′s chorea. Acta Neurol Scand 74:261–265
Petty F, Kramer GL, Fulton M et al (1993) Low plasma GABA is a trait-like marker for bipolar illness. Neuropsychopharmacology 9:125–132
Adinoff B, Kramer GL, Petty F (1995) Levels of gamma-aminobutyric acid in cerebrospinal fluid and plasma during alcohol withdrawal. Psychiatry Res 59:137–144
Pickar D, Labarca R, Linnoila M et al (1984) Neuroleptic-induced decrease in plasma homovanillic acid and antipsychotic activity in schizophrenic patients. Science 225:954–957
Davila R, Manero E, Zumarraga M et al (1988) Plasma homovanillic acid as a predictor of response to neuroleptics. Arch Gen Psychiatry 45:564–567
Bowers MB Jr, Swigar ME, Jatlow PI et al (1989) Plasma catecholamine metabolites and treatment response at neuroleptic steady state. Biol Psychiatry 25:734–738
Davidson M, Kahn RS, Powchik P et al (1991) Changes in plasma homovanillic acid concentrations in schizophrenic patients following neuroleptic discontinuation. Arch Gen Psychiatry 48:73–76
Green AI, Alam MY, Boshes RA et al (1993) Haloperidol response and plasma catecholamines and their metabolites. Schizophr Res 10:33–37
Kakihara S, Yoshimura R, Shinkai K et al (2005) Prediction of response to risperidone treatment with respect to plasma concencentrations of risperidone, catecholamine metabolites, and polymorphism of cytochrome P450 2D6. Int Clin Psychopharmacol 20:71–78
Markianos M, Panas M, Kalfakis N et al (2009) Plasma homovanillic acid and prolactin in Huntington’s disease. Neurochem Res 34:917–922
Fortunati F, Mazure C, Preda A et al (2002) Plasma catecholamine metabolites in antidepressant-exacerbated mania and psychosis. J Affect Disord 68:331–334
Yoshimura R, Nakano Y, Hori H et al (2006) Effect of risperidone on plasma catecholamine metabolites and brain-derived neurotrophic factor in patients with bipolar disorders. Hum Psychopharmacol 21:433–438
Mazure CM, Bowers MB (1998) Pretreatment plasma HVA predicts neuroleptic response in manic psychosis. J Affect Disord 48:83–86
Chou JC, Czobor P, Tuma I et al (2000) Pretreatment plasma HVA and haloperidol response in acute mania. J Affect Disord 59:55–59
Basterreche N, Dávila R, Zumárraga M et al (2008) Biological correlates of the congruence and incongruence of psychotic symptoms in patients with Type 1 bipolar disorder. Neuropsychobiology 58:111–117
Zumárraga M, Dávila R, Basterreche N et al (2009) Plasma homovanillic acid and family history of psychotic disorders in bipolar I patients. Pharmacol Res 59:269–272
Petty F, Sherman AD (1984) Plasma GABA levels in psychiatric illness. J Affect Disord 6:131–138
Goddard AW, Narayan M, Woods SW et al (1996) Plasma levels of gamma-aminobutyric acid and panic disorder. Psychiatry Res 63:223–225
Behar KL, Rothman DL, Petersen KF et al (1999) Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. Am J Psychiatry 156:952–954
Sanacora G, Mason GF, Rothman DL et al (1999) Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 56:1043–1047
Emrich HM, von Zerssen D, Kissling W et al (1980) Effect of sodium valproate on mania. The GABA-hypothesis of affective disorders. Arch Psychiatr Nervenkr 229:1–16
Volk DW, Austin MC, Pierri JN et al (2000) Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry 57:237–245
Guidotti A, Auta J, Davis JM et al (2005) GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology 180:191–205
Bullock WM, Cardon K, Bustillo J et al (2008) Altered expression of genes involved in GABAergic transmission and neuromodulation of granule cell activity in the cerebellum of schizophrenia patients. Am J Psychiatry 165:1594–1603
van Kammen DP, Petty F, Kelley ME et al (1998) GABA and brain abnormalities in schizophrenia. Psychiatry Res 82:25–35
Wassef A, Baker J, Kochan LD (2003) GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol 23:601–640
Winterer G (2006) Cortical microcircuits in schizophrenia—the dopamine hypothesis revisited. Pharmacopsychiatry 39:68–71
Lautin A, Angrist B, Stanley M et al (1980) Sodium valproate in schizophrenia: some biochemical correlates. Br J Psychiatry 137:240–244
Reid MS, O’Connor WT, Herrera-Marschitz M et al (1990) The effects of intranigral GABA and dynorphin a injections on striatal dopamine and GABA release: evidence that dopamine provides inhibitory regulation of striatal GABA neurons via D2 receptors. Brain Res 519:255–260
Petty F, Fulton M, Kramer GL et al (1999) Evidence for the segregations of a major gene for human plasma GABA levels. Mol Psychiatry 4:587–589
Brambilla P, Perez J, Barale F et al (2003) GABAergic dysfunction in mood disorders. Mol Psychiatry 8:712–737
Zumárraga M, Andía I, Dávila R et al (1994) Homovanillic acid in plasma determined by HPLC with direct injection of plasma filtrates. Clin Chem 40:2119–2120
American Psychiatric Association Washington (2000) Diagnostic and statistical manual of mental disorders (DSM-IV-TR). Washington, American Psychiatric Association
First MB, JBW W, Spitzer RL (eds) (1997) Structured clinical interview for the diagnostic and statistical manual, 4th edn. American Psychiatric Press, Washington DC
Thaker GK (2008) Neuropsychiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull 34:760–773
Berrettini WH, Nurnberger JI Jr, Hare TA et al (1983) Reduced plasma and CSF gamma-aminobutyric acid in affective illness: effect of lithium carbonate. Biol Psychiatry 18:185–194
Perry TL, Kish SJ, Buchanan J et al (1979) Gamma-aminobutyric-acid deficiency in brain of schizophrenic patients. Lancet 3:237–239
Spokes EG, Garrett NJ, Rossor MN et al (1980) Distribution of GABA in post-mortem brain tissue from control, psychotic and Huntington’s chorea subjects. J Neurol Sci 48:303–313
Korpi ER, Kleinman JE, Goodman SI, Wyatt RJ (1987) Neurotransmitter amino acids in post-mortem brains of chronic schizophrenic patients. Psychiatry Res 22:291–301
Toru M, Watanabe S, Shibuya H et al (1988) Neurotransmitters, receptors and neuropeptides in post-mortem brains of chronic schizophrenic patients. Acta Psychiatr Scand 78:121–137
Benes FM, Vincent SL, Molloy R et al (1996) Increased interaction of dopamine-immunoreactive varicosities with GABA neurons of rat medial prefrontal cortex occurs during the postweanling period. Synapse 23:237–245
Akil M, Pierri JN, Whitehead RE et al (1999) Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry 10:1580–1589
Noblett KL, Coccaro EF (2005) Molecular genetics of personality. Curr Psychiatry Rep 7:73–80
Lichtenstein P, Yip BH, Björk C et al (2009) Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 373:234–239
Acknowledgments
Financed by Grant 2006111050 and 2008111051 from the Basque Department of Health, and BIO 08/LF/001 from the Bioef Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arrúe, A., Dávila, R., Zumárraga, M. et al. GABA and Homovanillic Acid in the Plasma of Schizophrenic and Bipolar I Patients. Neurochem Res 35, 247–253 (2010). https://doi.org/10.1007/s11064-009-0048-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-009-0048-z