Abstract
Multidrug resistance (MDR) is a significant problem underlying the poor prognosis associated with gliomas. Hypoxia-inducible factor-1α (HIF-1α) is thought to induce the genes expression involved in MDR. To evaluate the effect of silencing HIF-1α in human glioma T98G cells, cells were transfected with HIF-1α-small interference RNA (HIF-1α-siRNA) and cultured under hypoxic conditions. The effect of HIF-1α-siRNA on HIF-1α and multidrug resistance-associated protein 1 gene (MRP1) and protein levels was determined. Silencing rates of HIF-1α were 90%, 85%, and 88% at 24, 48, 72 h post-transfection, respectively. Corresponding rates of HIF-1α protein were 74.5%, 61.1% and 59.1%. MRP1 protein levels decreased by 7.6%, 36.8% and 45.2%. HIF-1α-siRNA transfected cells were significantly more sensitive to doxorubicin and etoposide compared to non-transfected cells. These findings suggest that the HIF-1α plays a role in mediating chemotherapeutic drug resistance in glioma cells. HIF-1α silencing may prove to be an effective therapeutic means of treating gliomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomas are the most common and lethal type of primary brain tumor [1], affecting approximately 20,000 people in the United States annually and causing over 13,000 deaths per year [2]. Due to the invasive nature of gliomas, a multimodal therapeutic treatment approach has been advocated [1]. The current regimen for treatment of primary tumors recommended by the National Comprehensive Cancer Network includes surgery combined with chemotherapy (i.e., carmustine wafer) and fractionated external beam radiotherapy with or without adjuvant chemotherapeutic agents such as etoposide [2]. Despite intervention, gliomas are associated with very poor clinical outcomes. The average survival time following diagnosis is between 12 and 18 months, with the majority (90–95%) of affected individuals surviving less than 24 months [3–5].
One reason for the poor survival rate associated with gliomas is the development of multidrug resistance (MDR) [6–9]. While the exact mechanisms involved in MDR remain elusive, induction of the MDR1 gene appears to play a role. It encodes the protein P-glycoprotein (P-gp), an ATP-dependent membrane efflux pump that transports a variety of anti-cancer drugs to maintain low subtoxic cytoplasmic levels [10]. Expression of one or more isoforms of multi-drug-associated protein (MRP), another drug efflux pump, has also been demonstrated in many cancer lines [11]. Decleves and colleagues reported that both MRP1 mRNA and protein levels were over expressed in glioma cell lines exhibiting drug resistance to vincristine (VCR) and etoposide (VP16) [12]. Findings from further studies have indicated that MRP is highly expressed in glioma cells, suggesting that MRP, rather than P-gp, may be the primary drug resistance gene underlying the MDR phenomenon [13]. As such, MRP has become an important target of research in the field of drug resistance.
Previous studies have also shown that hypoxia, which is often prevalent in the tumor microenvironment, induces the nuclear transcription factor hypoxia-inducible factor-1α (HIF-1α). HIF-1α subsequently initiates transcription of a multitude of genes including P-gp and MRP1 [6, 7, 14]. Given this, HIF-1α is widely thought to be an important target for anti-cancer therapy [6, 7, 15, 16]. Indeed, Song and colleagues have demonstrated that hypoxia-induced resistance to cisplatin and doxorubicin was reversed in human non-small cell lung cancer (NSCLC) cells in which the HIF-1α gene was silenced by RNA interference (RNAi) [6].
To date, no study has clearly reported on the synchronized, positive correlation between changes in MRP and HIF-1α in glioma research. Considering the demonstrated positive impact of HIF-1α silencing in non-small cell lung cancer and the ubiquitous consensus that novel therapies for gliomas are urgently required, the objective of this study examine the role that the HIF-1α gene plays in mediating human brain glioma drug resistance. HIF-1α gene expression was altered using RNAi and drug resistance was indicated by MRP1 expression. Studies were performed using highly malignant human glioma T98G cells, which exhibit high endogenous HIF-1α expression. The chemotherapeutics doxorubicin (ADM) and VP16 were chosen as both are highly efficient glioma chemotherapeutic agents when used alone and are popular choices for adjuvant chemotherapy. In addition, resistance to these drugs is thought to involve MRP [17].
Materials and Methods
Synthesis of the siRNA/RNAi-Mate Complexes
The 3 pairs of HIF-1α gene interference oligonucleotide sequences (siRNA) and the FAM labeled negative control sequence (FAM-siRNA) were synthesized by Yingjun Inc. (Shanghai, China: Table 1). Serum-free DMEM was used to dilute 15 μg of RNAi-Mate cationic liposomes (Shanghai Genepharma Co., Ltd., Shanghai, China) and 5 μg of the siRNAs. The mixtures was incubated at room temperature for 30 min to generate the siRNA/RNAi-Mate complexes that were subsequently used to transfect T98G cells as described below.
HIF-1α siRNA Transfection and Hypoxic Culture
T98G cells (obtained from the West China Hospital Transplant and Immunology Laboratory of Sichuan University) were plated onto 6 well plates (1.5 × 105 cells/well) in DMEM (Sigma Chemical Company, St. Louis, MO) supplemented with 5% fetal calf serum and maintained at 37°C, 19% O2 and 5% CO2 for 24 h before transfection with siRNA. When 50–60% confluence was attained, the medium was changed to serum-free DMEM and the cells were transfected with the siRNA/RNAi-Mate complexes (80 nM), and cultured for a further 6 h. The medium was subsequently switched to DMEM supplemented with 5% fetal calf serum and the transfected cultures were maintained in an incubator under hypoxic conditions (37°C, 1% O2, 5% CO2 and 94% N2). The blank controls(defined as non-transfected cells) consisted of non-transfected cells, while negative controls cells were transfected with FAM-siRNA.
The Effect of HIF-1α siRNA on HIF-1α and MRP1 mRNA Expression
T98G cells were collected 24 h, 48 h and 72 h post-transfection. TRIZOL (Invitrogen, Carlsbad, CA) was used to extract total RNA from the cells. Reverse transcription and real time PCR (RT-PCR) using SYBR green was then performed to evaluate the expression of HIF-1α and MRP1. Primers were designed according to the complete sequences of HIF-1α and MRP1 provided by Embank using Primer Premier 5.0 software. The primer sequences (including that for the internal control, β-actin) are given in Table 2. Reaction conditions for the RT-PCR were: 3 min at 94°C for initial denaturation, 30 s at 94°C for denaturation, 30 s at 53°C for annealing, and 30 s at 72°C for elongation for a total of 40 cycles, and lastly 1 min at 95°C and 1 min at 55°C. The comparative CT method was subsequently employed to calculate relative mRNA expression [18]. Interference Rate = 1 – 2 − ΔΔCT, CT is the number of cycles where the amplification curve and the threshold value intersects and ∆CT is the CT of the target gene minus the CT of β-actin and ∆∆CT is the ∆CT in the experimental group −∆CT in the control group. Therefore, 2(−∆∆CT) represents the difference in the experimental group’s gene expression relative to that of the control group, and 1 − 2(−∆∆CT) is the silencing efficiency.
The Effect of HIF-1α siRNA on HIF-1α and MRP1 Protein Levels
Western blotting was utilized to analyze HIF-1α and MRP1 protein levels in the transfected cells. Transfected cells were lysed with lysis buffer (Triton-100 0.5%, Tris-Cl 50 mM, NaCl 150 mM, PMSF 50 μg/ml, Leupeptin 5 μg/ml, Aprotinin 2/ml, Soybean Trypsin inhibitor 50 μg/ml) on ice for 30 min and centrifuged. The supernatant was collected and equal amount of protein were loaded onto a 10% SDS-PAGE gel then transferred to a polyvinylidene difluoride membrane for immunohybridization. The membrane was blocked with phosphate buffered saline with Tween 20 (PBST) containing 10% skim milk for 2 h at 37°C and then washed 3 times (for 5–10 min each) with PBST. HIF-1α antibody (1/100) and β-actin antibody (1/400) obtained from Boster (Wuhan, China) were added and incubated overnight at 4°C. The membrane was washed again with PBST and the secondary antibodies, goat-anti-rabbit (1/20000) and goat-anti-mouse (1/20000), were added before incubation at 37°C for 1 h. The target bands were detected by chemiluminescence (Pierce Biotechnology, Rockford, IL) and the intensities determined using Quantity One® analysis software (Bio-Rad, Hercules, CA). The inhibition rate was calculated according to the equation: inhibition = ∆HIF-1α interference − ∆negative control/∆negative control).
Effect of HIF-1α-siRNA on the Chemotherapeutic Sensitivity of Glioma Cells
Log-phase cells were plated on 96 well plates at a density of 1.0 × 104 cells/well. The siRNA/RNAi-Mate complex was added to the wells and cells were cultured in DMEM at 37°C, 10% O2 and 5% CO2 for 6 h. The culture medium was then replaced with DMEM supplemented with 5% fetal calf serum containing either ADM (Shen Zhen Wan Le Yao Ye Co., China) or VP16 (Ji Lu Zhi Yao Co., China). The following concentrations of ADM were used: 0.0, 0.125. 0.25, 0.5, 1.0, 2.0 and 4.0 μg/ml. The following concentrations VP16 were used: 0.0, 7.5, 30.0, 60.0, 120.0 and 240.0 μg/ml. Three replicates were prepared for each concentration. Negative controls were cells exposed to FAM-siRNA. Cells were incubated under hypoxic conditions (37°C, 1% O2, 5% CO2 and 94% N2) for an additional 48 h.
Cell viability was determined using the MTT assay. Twenty μl of MTT solution (5 mg/ml) was added to each well. Four hours later, media was removed and 100 μl of DMSO was added to each well before 15 min of agitation. Absorbance of each well was then measured at 490 nm. Viability was calculated by dividing the experimental well absorbance value by the blank well absorbance value.
Statistical Analysis
Data are presented as the mean ± standard deviation (SD). Normally distributed continuous variables were compared by one-way analysis of variance (ANOVA). When a significant difference between groups was apparent, multiple comparisons of means were performed using the Bonferroni test with type-I error adjustment. Two-way ANOVA was used to compare the negative control and siRNA transfection groups with regards to different concentrations of ADM and VP16. General Lineal Model/Univariate effect analysis was used to examine the interaction of the effect of HIF-1α siRNA and ADM/VP16 on the survival rate of glioma cells. All statistical assessments were two-sided and evaluated at the 0.05 level of significance. Statistical analyses were performed using SPSS 15.0 statistical software (SPSS Inc, Chicago, IL).
Results
Effect of HIF-1α Silencing on HIF-1α and MRP1 RNA and Protein Levels
The HIF-1α and MRP1 RNA levels of glioma cells cultured under hypoxic conditions following HIF-1α silencing are presented in Table 3. Unsurprisingly, HIF-1α RNA expression was significantly lower in HIF-1α-siRNA treated cells at each time point (as indicated by higher ΔCT values) compared to both control cell groups. Inhibition was maximal at 48 h. With regards to MRP1, RNA expression was significantly decreased from control levels at 72 h only.
The post transfection silencing efficiencies for HIF-1α and MRP1 are illustrated in Fig. 1a and b, respectively. HIF-1α silencing efficiency was consistently high (>80% at each assessment point) and did not change with time. MRP1 gene silencing efficiency increased significantly with each 24 h time period (Fig. 1b) and was >60% at 72 h post-transfection.
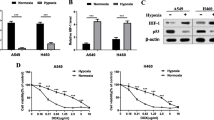
Western blotting revealed that HIF-1α siRNA inhibited expression of both HIF-1α and MRP1 protein. As shown in Fig. 2a, the inhibition rates of HIF-1α protein expression were 74.5 ± 4.2%, 61.1 ± 3.6% and 59.1 ± 2.2% at 24, 48, and 72 h post-transfection, respectively. Inhibition rates of MRP1 protein were 7.6 ± 3.8%, 36.8 ± 6.1% and 45.2 ± 4.7%) at 24, 48, and 72 h post-transfection, respectively (Fig. 2b). Representative Western blot images for HIF-1α and MRP1 are shown in Fig. 2c.
Inhibition rates of HIF-1α (a) and MRP1 (b) protein at 24, 48 and 72 h post transfection with HIF-1α siRNA as determined by Western blotting. Representative blots (c) are shown for inhibition of HIF-1α (upper panel) and MRP1 (lower panel) protein. Lanes 1, 4, and 7 were samples from cells respectively at 24, 48 and 72 h post transfection with HIF-1α siRNA; lanes 2, 5, and 8 were negative controls respectively at 24, 48 and 72 h post transfection with FAM-siRNA; lanes 3, 6, and 9 were blank controls
Effect of HIF-1α siRNA on Cell Sensitivity to ADM and VP16
The survival rates of the HIF-1α-siRNA transfected T98G cells treated with various concentrations of ADM or VP16 are presented in Fig. 3a and b. With regards to ADM, survival rates were significantly lower in transfected compared to negative control cells at all concentrations except for 0.0 and 0.5 μg/ml (P < 0.05 for all). There were significant overall effects detected for group, concentration and group × concentration (P < 0.001 for all). Survival rates were significantly lower in transfected cells at all concentrations of VP16 bar 0.0 μg/ml (P < 0.05 for all). Significant group, concentration and group × concentration effects were also detected for VP16 (P < 0.001 for all). For both drugs, the difference in viability between the negative control and treatment groups increased with increasing drug concentration. Factorial analysis of variance revealed that there was a significant relationship between the effect of HIF-1α siRNA and the effect of ADM/VP16 on the survival rate of glioma cells (Table 4). Furthermore, the combined treatments were synergistic.
Discussion
While the MDR1 gene and P-gp protein are not abundantly expressed in glioma cells, gliomas are characterized by high expression of MRP which might explain the occurrence of MDR in this tumor type [12]. In this study, we found that there was a relationship between expression of HIF-1α and MRP1 in human glioma cells. Specifically, siRNA technology was utilized to silence the HIF-1α gene in T98G cells, after which we observed decreases in MRP1 gene expression and increased cellular susceptibility to the chemotherapeutic actions of ADM and VP16.
In 2002, Comerford reported that tumor cells are capable of directly modulating MDR1/P-gp expression under hypoxic conditions [7]. Further to this, induction of HIF-1α is also known to enhance expression of MDR1/P-gp, contributing to the development of MDR. Song and colleagues later reported that under hypoxic, compared to normoxic conditions, the NSCLC cell lines SPCA1 and A549 exhibited significantly higher expression levels of HIF-1α and displayed increased MDR to cisplatin and ADM [6]. After these cells were treated with HIF-1α-siRNA, HIF-1α expression decreased and drug resistance was reversed, suggesting that the hypoxia-induced chemotherapeutic resistance was possibly mediated via the HIF pathway [6].
In this study, transfected and non-transfected human glioma cells were cultured in a hypoxic environment in medium containing ADM and VP16 at various concentrations for 48 h. Silencing of the HIF-1α gene through transfection resulted in significant decreases in MRP1 gene and protein expression over the ensuing 72 h. The importance of MRP1 in mediating MDR was evidenced by our finding that sensitivity of the transfected cells to treatment with both ADM and VP16 was heightened. Although this study only utilized a relatively small sample size, by combining the overall trend of the survival curve and specific knowledge regarding MDR, we were able to determine that resistance of HIF-1α-siRNA transfected cells to ADM and VP16 was reversed. Our findings also indicated that HIF-1α-siRNA appears to enhance the cytotoxic effects of ADM and VP16. Further studies are required to determine whether simple HIF-1α silencing is capable of inhibiting cell growth.
In a human hepatocellular carcinoma cell line HepG2 study, Zhu et al. discovered that MRP1 and HIF-1α exhibited synchronous changes in mRNA and protein levels within 6–48 h of culture under hypoxic conditions [14]. We found that MRP1 expression decreased 24–48 h after HIF-1α expression decreased, suggesting that the decrease in HIF-1α expression is not the only mediator of decreased MRP1 expression. Further investigation is warranted to determine other possible contributing mediators. Regardless of the precise mechanisms involved in MDR, in this study we demonstrated that MRP1 expression was significantly inhibited in HIF-1α silenced human glioma cells. Based on these findings, we suggest that MRP1 expression might contribute to underlying glioma drug resistance and that this resistance may be reduced through HIF-1α gene silencing. Future studies performed by our research group will aim to utilize dsRNA expressed in a recombinant vector to overcome the relatively short-lived effects of chemically synthesized siRNA. It is anticipated that the RNAi effect could then be expressed and propagated through generations of mammalian cells to maintain inhibition [19–22].
Compared to the conventional antisense nucleotide technique, RNAi has a higher specificity and silencing efficiency. It is widely applied in functional genomics, gene therapy, drug discovery, and other areas. Chemically synthesized siRNA is currently the simplest RNAi application. Typical RNAi sequences are 19–21 nucleotides long and do not cause non-specific gene silencing [23]. Unfortunately, chemically synthesized siRNA can only produce temporary RNAi effects.
In summary, this study employed RNAi technology to inhibit the expression of HIF-1α in human glioma cells. The key findings of this study were significantly decreased MRP1 expression 24–48 h after HIF-1α-siRNA transfection and subsequent increased sensitivity of glioma cells to ADM and VP16. Although further study of other glioma cells lines is warranted, the current findings suggest that the HIF-1α gene plays a role in mediating chemotherapeutic drug resistance in glioma cells. Given this, treatment strategies involving targeted downregulation of this gene may ultimately prove to be efficacious in the clinical setting. Such treatment could be employed as an alternative to conventional therapy targeting MRP or used as an adjuvant for human glioma.
References
Yamanaka R (2008) Cell- and peptide-based immunotherapeutic approaches for glioma. Trends Mol Med 14:228–235
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (2008) Central Nervous System Cancers V.1
DeAngelis LM (2001) Brain tumors. N Engl J Med 344:114–123
Davis FG, Kupelian V, Freels S et al (2001) Prevalence estimates for primary brain tumors in the United States by behavior and major histology groups. NeuroOncol 3:152–158
Curran WJ Jr, Scott CB, Horton J et al (1993) Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Natl Cancer Inst 85:704–710
Song X, Liu X, Chi W et al (2006) Hypoxia-induced resistance to cisplatin and doxorubicin in non-small cell lung cancer is inhibited by silencing of HIF-1alpha gene. Cancer Chemother Pharmacol 58:776–784
Comerford KM, Wallace TJ, Karhausen J et al (2002) Hypoxia-inducible factor-1- dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 62:3387–3394
Goldman B (2003) Multidrug resistance: can new drugs help chemotherapy score against cancer? J Natl Cancer Inst 95:255–257
Mattern J (2003) Drug resistance in cancer: a multifactorial problem. Anticancer Res 23:1769–1772
Fardel O, Lecureur V, Guillouzo A (1996) The P-glycoprotein multidrug transporter. Gen Pharmacol 27:1283–1291
Cole SP, Bhardwaj G, Gerlach JH et al (1992) Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258:1650–1654
Decleves X, Fajac A, Lehmann-Che J et al (2002) Molecular and functional MDR1-Pgp and MRPs expression in human glioblastoma multiforme cell lines. Int J Cancer 98:173–180
Mohri M, Nitta H, Yamashita J (2000) Expression of multidrug resistance- associated protein (MRP) in human gliomas. J Neurooncol 49:105–115
Zhu H, Chen XP, Luo SF et al (2005) Involvement of hypoxia-inducible factor-1-alpha in multidrug resistance induced by hypoxia in HepG2 cells. J Exp Clin Cancer Res 24:565–574
Kizaka-Kondoh S, Inoue M, Harada H et al (2003) Tumor hypoxia: a target for selective cancer therapy. Cancer Sci 94:1021–1028
Han HK, Han CY, Cheon EP et al (2007) Role of hypoxia-inducible factor-alpha in hepatitis-B-virus X protein-mediated MDR1 activation. Biochem Biophys Res Commun 357:567–573
Vezmar M, Georges E (2000) Reversal of MRP-mediated doxorubicin resistance with quinoline-based drugs. Biochem Pharmacol 59:1245–1252
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408
Yu JY, DeRuiter SL, Turner DL (2002) RNA interference by expression of short- interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci USA 99:6047–6052
Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550–553
Lee NS, Dohjima T, Bauer G et al (2002) Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat Biotechnol 20:500–505
Miyagishi M, Taira K (2002) U6 promoter-driven siRNAs with four uridine 3′ overhangs efficiently suppress targeted gene expression in mammalian cells. Nat Biotechnol 20:497–500
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494–498
Acknowledgments
This study was supported by the Country Natural Science Fund and West China Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, L., Feng, P., Li, S. et al. Effect of Hypoxia-inducible Factor-1α Silencing on the Sensitivity of Human Brain Glioma Cells to Doxorubicin and Etoposide. Neurochem Res 34, 984–990 (2009). https://doi.org/10.1007/s11064-008-9864-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9864-9