Abstract
Neuropathic pain is induced by the injury to nervous systems and characterized by hyperalgesia, allodynia and spontaneous pain. The underlying mechanisms include peripheral and central sensitization resulted from neuronal hyperexcitability. A number of ion channels are considered to contribute to the neuronal hyperexcitability. Here, we particularly concentrate on an interesting ion channel, hyperpolarization-activated cyclic nucleotide gated (HCN) channels. We overview its biophysical properties, physiological functions, followed by focusing on the current progress in the study of its role in the development of neuropathic pain. We attempt to provide a comprehensive review of the potential valuable target, HCN channels, in the treatment of neuropathic pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic neuropathic pain, induced by injury to the nervous system, is characterized by spontaneous pain, hyperalgesia and allodynia. The mechanisms underlying the pathogenesis of neuropathic pain are complicated. Both peripheral and central sensitizations are involved. Spontaneous ectopic discharges of injured primary sensory neurons and sensitization of uninjured afferents are believed to be the major peripheral components. Centrally, enhanced nociceptive synaptic transmissions in the spinal dorsal horn and changed descending regulations in supraspinal central nervous systems are critical for the development of neuropathic pain [1–3].
Generally, the nociceptive transmission pathways exhibit hyperexcitability after injury of the nervous system, which exaggerates normal or noxious stimuli and induces chronic, abnormal painful reaction [4]. Ion channels are the primary determinants of neuronal excitability, a wide range of ion channels including sodium channels, potassium channels, calcium channels [5–7] are of great interest. Recently, hyperpolarization-activated cyclic nucleotide gated cation (HCN) channels have been identified as an important contributor and a valuable target for the treatment of neuropathic pain [8]. A series of studies from several research groups, including ours, have been examining their roles in neuropathic pain on a variety of animal models. In this review, we will give a brief introduction of this channel, followed by an extensive discussion about the current progress in the study of its role in the development of neuropathic pain.
Overview of HCN Channels
Hyperpolarization-activated cyclic nucleotide gated currents were first reported in the sino-atrial node (SAN) in rats [9]. The current can only be slowly activated under hyperpolarization of the cell membrane. Therefore it was named as I f or I q due to this unique property (“funny” and “queer”). Later, HCN current was also found widely in neurons, where it was called I h.
HCN channels belong to the super-family of voltage gated potassium channel. Similar to Shaker-type potassium channels, functional HCN channels are assembled with four subunits of the same subtypes (homotetramer) or different subtypes (heterotetromer) [10–13]. Each subunit consists of six transmembrane α-helix domains (S1–S6) (Fig. 1a). Between S6 and the C terminal is a cyclic-nucleotide binding domain (CNBD). The sequence of CNBD domain is highly homologous to that of cyclic-nucleotide gated channels (CNG) [14, 15], which can modulate the activation of HCN channels by directly binding with cAMP. Most of the transmembrane domains, including S4 voltage-sensor, pore region and CNBD domain, are highly conserved (80%–90%) among the four subtypes of HCN channels, while the N- and C-terminals are more variable [15, 16].
Structure and primary physiological functions of HCN channels. (a) A schematic drawing of the structure of single HCN channel unit (modified from [16] with permission). (b) I h, along with I T, serves as the pacemaker currents in the SAN cells and thalamic delay neurons (modified from [22]). (c) In neurons with no automatic activity, I h contributes to the maintenance of membrane resting potential. It opens when membrane potential is hyperpolarized and the inward currents draw the membrane potential toward resting condition. It closes when membrane potential is depolarized, the decreased inward currents prevent excess depolarization (modified from [22]). (d) Voltage dependence of I h activation in DRG neurons and the effects of 100 μM cAMP. The dependence was shifted toward more depolarization potential in the presence of cAMP. Curves were fitted with Boltzmann equation (our unpublished data). (Fig. 1b, c were reprinted with permission, from the Annual Review of Physiology, Volume 65 ©2003 by Annual Reviews, http://www.annualreviews.org)
Typically, HCN channels can be activated when membrane potential is hyperpolarized negative to about −50 ~ − 60 mV, suggesting the partial activation of HCN channels under resting potential. The channels are permeable to both Na+ and K+; the reversal potential of I h is about −20 ~ − 40 mV. Opened channels allow more influx of Na+ and less efflux of K+ with a net inward current, producing the depolarizing “sag” of membrane potential. Among four subtypes, HCN1 shows fastest activation and largest current compared to the other subtypes [17, 18]. The activation of HCN channels can be facilitated by the direct binding of cAMP or cGMP to the CNBD domain, which manifests as right (depolarization) shift of the activation curve (Fig. 1d). Therefore, any neurotransmitters that can up- or down-regulate the level of cAMP or cGMP are able to modulate the activation of HCN channels, such as β-adrenalin agonist [19], 5-HT [19], Ach [20], NO [21]. Interestingly, the extent of modulation by cAMP differs among the four subtypes. HCN4 can be modulated most significantly, while HCN1 is almost unaffected [22]. Similar regulation takes place when phosphoinositide, such as PIP2, directly binds to HCN channels at some presumed “PIP domain”. But this modulation is not subtype-specific [23]. In the study of HCN current, Cs+ is a useful blocker because of its rapid onset and the reversibility after washout; the drawback is that it cannot differentiate I h from some potassium channels. In comparison, another blocker, ZD7288, shows much higher specificity, thus is most widely used.
The most typical function of I h is its involvement in the pacemaking activity of cardiac SAN cells and a variety of neurons that have spontaneous firing or display subthreshold membrane oscillation, such as thalamocortical relay neurons [19, 24, 25], hippocampal stratum oriens-alveus interneurons [26], and injured dorsal root ganglion neurons [27, 28] (Fig. 1b). Accordingly, I h (I f) is also called pacemaker currents. Notably, I h is necessary but not sufficient for pacemaking in these neurons. In general, activation of I h during the after hyperpolarization phase of the spike trajectory drives the membrane potential toward the firing threshold; T type calcium channels, which carry more rapid depolarization currents, will elicit a spike subsequently [22]. For those neurons with no automatic activities, I h is considered to be crucial in determining their resting and passive cable properties [22]. On one hand, I h helps maintain and limit the resting potentials at a certain level (Fig. 1c). On the other hand, the partial opening of HCN channels at resting potential contributes to the resting membrane conductance, thereby set the input resistance (Rin), membrane time constant and length constant. This enables I h to modulate the response of excitable neurons to outside stimuli. For example, the presence of I h decreases Rin and dampens the response of neurons to certain depolarization input; in reverse, the absence of I h increases Rin and augments neuronal response to the same input. Such modulation happens in some postsynaptic neurons and was considered to serve as a homeostatic mechanism in response to changed synaptic activity [29]. In addition, I h is also detected in presynaptic sites and has a role in synaptic transmission through regulating presynaptic release of neurotransmitters [30, 31]. However, this function remains controversial due to some nonspecific effects of ZD7288 (see below).
Distribution of HCN Channels in the Pathway of Pain Transmission and the Their Physiological Functions
HCN channels have been found to be expressed throughout the cardiac vascular systems, sensory and motor nervous systems [17, 32, 33]. In the past decades, much attention has been paid to their role in the sensory nervous systems, e.g. vision [34]. Shortly after I h was identified in the rabbit SAN cells, Mayer and Westbrook, for the first time, reported the existence of I h in the mouse embryo DRG neurons in early 1980s [35]. Years later, it was found that the distribution of I h varied among different types of primary afferent neurons [27, 36–42] (Fig. 2a). I h shows higher current density and amplitude, faster activation, and appears more frequently in large and medium-sized (A type) primary afferent neurons than that in small (C type) neurons. It was also found that I h was mainly expressed in TTX sensitive A type neurons in contrast to the much smaller magnitude in capsaicin sensitive C type neurons [36]. It turned out that those I h-riched large neurons had shorter duration action potential and time dependent rectification under current clamp conditions, while the neurons with little or no I h showed longer duration action potential and no time-dependent rectification [40]. I h is thus believed to mainly contribute to determine the resting potential of A type primary afferent neurons in normal conditions.
Distribution of HCN channels and I h in pain pathways. (a) Typical samples of I h current in different DRG neurons [37]. The amplitude of I h is highest in large DRG neurons; relatively smaller current can be seen in medium and small-sized neurons. (b, c) HCN1 and HCN2 in DRG [51]. HCN1 is predominantly localized on the membranes of large and medium-sized DRG neurons. HCN2-ir can be detected on the membrane of large neurons (asterisk), cytoplasm of small and medium-sized neurons (arrow), and occasionally in the axons of small neurons (arrow head). Scale bar: 50 μm. (d, e) HCN1 and HCN2 in the sciatic nerve fibers [51]. HCN1 positive staining can be detected on the axolemma of myelinated axons (arrow head). HCN2 positive staining can be detected on the axolemma of unmyelinated axons (arrow head). Scale bar: 0.5 μm. (f, g) HCN1 and HCN2 in the spinal dorsal horn (unpublished data). HCN1 is mainly expressed in the lamina III to IV. HCN2 is mainly expressed in lamina I. Scale bar: 100 μm. Figure 2a was reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.
After the four cDNAs encoding HCN channels were cloned, the distributions of HCN channels in primary afferent neurons have been investigated intensively [27, 37, 43, 44]. In DRG, HCN1 is the most abundant subtype and can be detected in virtually all of large and medium-sized DRG neurons and in a few small ones. It is mainly localized on the membrane of cell soma (Fig. 2b). The expression of HCN2 is somewhat lower than that of HCN1 and can be detected in about half of all types of neurons. Interestingly, HCN2 is mainly expressed on the membrane in large neurons and sometimes colocalized with HCN1; while in small and medium-sized neurons, it is localized intracellularly and is colocalized with CGRP, the marker of peptidergic nociceptive neurons (Fig. 2c). The expression levels of HCN3/4 seem much lower than that of HCN1/2. Considering the highest current amplitude and the fastest activation of HCN1 among the four subtypes, the expression patterns of HCN subtypes in DRG are properly matched with the biophysical characteristics of I h in different sized DRG neurons.
HCN channels are also expressed in the spinal dorsal horn [37, 44, 45] (Fig. 2f, g). HCN1 can be detected primarily in myelinated axon terminals in lamina I and III to IV, consistent with their expression pattern in DRG. HCN2, on the other hand, was located in the central terminals of nonmyelinated peptidergic afferents in lamina I to out part of layer II (IIo). Further work showed that HCN2 positive terminals were predominantly apposed to excitatory interneurons in the spinal dorsal horn [37, 44, 45]. HCN3 can be found in the neurons across the spinal dorsal horn, while HCN4 positive staining can be found in both neurons and nerve fibers throughout the spinal dorsal horn (unpublished data).
So far, little attention has been paid to the expression of HCN channels in the peripheral sensory terminals. Recently, Luo et al. investigated the distribution of HCN subtypes in the glabrous skin of naïve rats’ hind paw plantar [46]. They showed that HCN1 could be detected in the Meissner’s corpuscle in dermal papillae and Merkel cells in epidermal layer. Meissner’s corpuscles are innervated by both myelinated and unmyelinated afferents, and considered to be sensitive to noxious stimuli in addition to their low threshold mechano-sensitivity. Merkel cells are mechanoreceptors innervated mainly by myelinated afferents [47]. Expression of HCN2 is similar to that of HCN1 in Meissner’s corpuscle. We additionally found abundant expression of HCN2 in the CGRP positive free nerve endings in the epidermal layer (unpublished data).
I h is also detectable in the myelinated and nonmyelinated axons of mammalian peripheral and central nervous systems when the axons are hyperpolarized by applying tetanic stimulation [48, 49]. Its effect on excitability is more prominent in sensory than in motor axons [50]. In our recent study, we found very little HCN1-ir signals and relative more HCN2-ir with linear staining pattern with immunohistochemical staining. Their subcellular distributions were also observed by immuno-electromicroscopy approach (Fig. 2d, e). We found that HCN1 positive staining can be clearly found along the axolemma of myelinated axons, HCN2-ir was seen along the membrane of unmyelinated axons in a segmental pattern. Nevertheless, the expression of HCN channels in the axon is indeed more limited than that in DRG and central nervous systems [51].
Although much work has highlighted the involvement of cerebral I h in a variety of physiological and pathological conditions, such as slow wave sleep and epilepsy [22], so far there is little work concerning about its expression and function in the supra-spinal pain pathways. Recent studies showed the existence of I h in the ascending and descending regulation pathways of pain transmission, including μ-receptor containing neurons in dorsal raphe nucleus [52], and GABAergic neurons in raphe nucleus magnus and periaqueductal gray (PAG) [53]. The regulation of I h by cAMP in these areas was found to participate in the withdrawal pain sensation after chronic morphine treatment. Additionally, both I h and HCN-ir were found in the neurons in trigeminal nuclei and were believed to contribute to the trigeminal neuralgia [54].
Role of I h in Neuropathic Pain
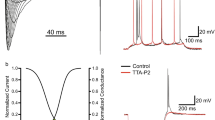
Recently, a series of behavioral studies on a variety of animal models consistently showed the contribution of I h to neuropathic pain. When ZD7288 was given to the neuropathic pain models either intraperitoneally [27], or intrathecally [55], or perineuronally to the injured sciatic nerve [51], or intraplantarly to the injured hind paw [46], or topically to the injured DRG (our unpublished data), significant analgesic effects were observed with no disturbance in motor function (Fig. 3b–d). Besides, several clinically used analgesics were suggested to function partially through the inhibition of I h, such as loperamide [56], propofol [57] and clonidine [58]. In this section, we attempt to discuss extensively about potential analgesic mechanisms of I h.
HCN channels contribute to the development of neuropathic pain. (a) In SNL model of rats, topical application of ZD7288 to the injured DRG significantly suppresses ectopic discharges in a concentration-dependent manner [28]. (b) Topically application of ZD7288 to the injured DRG inhibits the mechanical allodynia in SNL rats (unpublished data). (c) Perineuronal perfusion of ZD7288 markedly alleviates mechanical allodynia in CCI rats [51]. (d) Intrathecal injection of ZD7288 significantly reverses mechanical allodynia in SNL rats
Pacemaker Currents in Spontaneous Ectopic Discharges
Ectopic discharges were initially recorded in the sciatic nerve neuroma and later in all other neuropathic pain models, and were suggested to be involved in the development of mechanical allodynia and spontaneous pain [59–63]. This kind of spontaneous activity was generated from injured DRG neuronal soma or axons, as well as adjacent uninjured nerves. DRG neurons and their axons barely fire action potential on their owns under normal condition, therefore, those activities are called “ectopic discharges”. There are three typical patterns of ectopic discharges, tonic (regular), burst (on and off), and irregular discharges; some of them are initiated and companied by subthreshold oscillation, some are not [64, 65]. The spontaneous property of ectopic discharges is similar to that of SAN cells, thalamic relay interneurons and other neurons with rhythmic automatic activity, thus I h likely has a role in ectopic discharges.
There are three pieces of evidence supporting the role of I h in ectopic dischares. First, ectopic discharges are essentially generated from large and medium-sized DRG neurons in neuropathic rats of the spinal nerve ligation (SNL) [60], the sciatic nerve chronic constriction injury (CCI) [66], the DRG chronic compression (CCD) [67] and the early stage of neuroma [63], consistent with the fact that I h is present primarily in these large and medium-sized neurons. Second, an obvious upregulation of I h density and activation rate were observed in large and/or medium-sized DRG neurons 1–3 weeks after SNL injury [27] and within 1 week after CCD injury [68]. Same changes were also found in large and medium-sized TRG neurons 3 days after CCI of infraorbital nerve [69]. In sciatic nerve CCI model, we detected significantly increased expression of both HCN1 and HCN2 at the site of injury [51]. However, I h was decreased in the DRG neurons 2–7 weeks after transection of sciatic nerves in neuroma model [70]. We also observed a remarkable reduction of both I h and HCN proteins 2–5 weeks after SNL (unpublished observation). Third, low concentration of ZD7288 significantly suppresses ectopic discharges generated from either injured DRG neuronal soma or axons in both in vivo and in vitro studies [27, 28, 51, 71] (Fig. 3a). The inhibition efficiency is much higher in large neurons than that in small neurons [27].
How does I h participate in the generation of ectopic discharges? It was proposed that pacemaking property of I h might contribute. However, if this is the case, it would be hard to explain the slower activation rate of HCN channels (hundreds of milliseconds around resting potential) versus the high frequency of some of the ectopic discharges (up to 100 Hz) [5]. Although Yao et al. detected that the time constant of fast activation of I h was significantly increased (to near 200 ms under −70 mv) in the dissociated DRG neurons in the CCD model [68], it is still unlikely that I h will be able to trigger such fast firing. Chaplan et al. found no change of fast activation constant in SNL models under −140 mV in DRG neurons. Aβ subunit for HCN channels, Mink-related peptide (MiRP1), was found recently to be able to accelerate the activation of HCN channels when it was co-expressed with HCN1 or HCN2 in Xenopus oocytes [72]. Besides, in vivo and in vitro studies showed that the activation rate of HCN channels, as well as that of other ion channels, could be slowed down by the inhibition of tyrosine protein kinase [73]. It was also reported that N-glycosylation of potassium channels and sodium channels could increase their activation kinetics [74, 75]. Since functional HCN channels are also N-glycosylated proteins [76], similar modification could take place on them. Therefore, it is possible that the change of channel assembly among HCN subunits and non-HCN subunits, or the regulation by protein kinases and glycosylation after nerve injury will speed the activation of HCN channels. These changes together likely enable I h to contribute to the rapid ectopic discharges.
A number of other unsolved problems are yet to be investigated. For example, it is hard to reconcile the increased density of I h with paradoxically decreased HCN1/2 mRNAs and proteins in DRG 1–3 weeks after SNL injury [27]. Although it is likely due to the change of subunit polymerization or regulation of other accessory proteins, there is no direct evidence at this point. Besides, recent studies have indicated the critical role of ectopic discharges in the trigger of central sensitization and neuropathic pain behaviors in the early stage (within 1 week) after peripheral nerve injury [77, 78]. Therefore, the change of HCN channels and I h in the early stage and their contribution to trigger and maintain neuropathic pain are worthy further investigation.
Subthreshold membrane oscillation (STMO) is considered to be a trigger of ectopic discharges [64, 65, 79, 80]. It has been reported that inhibition of I h could suppress STMO in a variety of central neurons, such as thalamic relay neurons and inferior olive neurons [25, 81, 82], I h hereby contributes to the development of STMO in these neurons. However, to our knowledge, there are no studies investigating this issue in DRG neurons till now. Some inconsistency indeed exists between the characteristics of I h and that of STMO generated from injured DRG neurons [5–7]. One is that much higher frequency of STMO in DRG neurons is in contrast to the slow activation rate of I h. Similar to what we discussed above about the involvement of I h in some fast firing ectopic discharges, it is likely that the activation rate of I h could be regulated by some post-translation modulations. Second, STMO sustains under resting potential in which very little HCN channels are activated, yet this discrepancy could be partly explained by the finding of increased I h density as well as the shift of its activation curve toward depolarization potential after nerve injury [27, 28]. The most prominent contradiction lies in their opposite voltage sensitivity, i.e., depolarization-facilitation for STMO [65] versus hyperpolarization-activation for I h. Although intracellular recording in some central neurons confirmed that inhibition of I h suppressed STMO by affecting the after-hyperpolarization [25, 81, 82], so far there is no evidence to exclude the possibility that hyperpolarization of membrane potential, which can be induced by inhibition of I h, per se participated in the suppression of STMO. Further work needs to be done to clarify the mechanisms involved in the regulation of I h in STMO of injured DRG neurons.
Involvement in the Sensitization of Nociceptors
In addition to the abnormal discharges of injured neurons, adjacent uninjured afferents also undergo hyperexcitability after peripheral nerve injury. A most remarkable change is the sensitization of peripheral nociceptors which characterized by the decreased threshold for activation, increased or exaggerated response to noxious stimuli, as well as spontaneous activity [83]. A number of ion channels, such as Nav1.8, T-type calcium channels, TRPV1 and acid sensitive ion channels [4], are suggested to contribute to the sensitization of peripheral nociceptors.
Up to date, there are only a few studies addressing the functional role of peripheral HCN channels. A behavioral test recently demonstrated an obvious but insignificant reversal of mechanical allodynia by intraplantar injection of 100 μM ZD7288 to the partial sciatic nerve injury rats [84]. Recently, another study showed that HCN channels were expressed in the peripheral receptors [46] as mentioned above. In addition, they also found that the mechanical allodynia of SNL rats and spontaneous pain of mild thermal injury (MIT) rats were significantly alleviated by intraplantar application of 30 mM ZD7288, while the thermal hyperalgesia of MIT rats was unaffected. These findings indicate that HCN channels in the peripheral terminals are pro-nociceptive in the neuropathic pain models. However, so far there is no report examining the change of expression or biophysical properties of HCN channels in nociceptors after peripheral nerve injury. Two points should be noted with regard to these studies. First, in both studies, the analgesic effect of ZD7288 was transient and completely disappeared within 1 h after administration. However, it is well known that the inhibition of HCN channels by ZD7288 is irreversible. In agreement with that, we also found that suppression of ectopic discharges by ZD7288 lasted throughout our experiment (at least 1 h) without recovery in both in vivo and ex vivo recording. Also, analgesic effect of ZD7288 by systemic or perineuronal application in neuropathic pain rats lasted for nearly 24 h [27, 51]. This discrepancy could be due to the different metabolism mechanisms of ZD7288 in different systems. Second, the concentration of ZD7288 used in one study was 30 mM, which is hundreds of times higher than the effective concentration used in previous studies of I h. Therefore, we can hardly exclude its nonspecific effects.
On the other hand, an interesting study regarding the role of HCN channels in the aortic baroreceptors might provide us some hint about its potential role in peripheral nociceptors [43]. Like other sensors, baroreceptors are activated by certain stimuli, for example, pressure. It was found that inhibition of I h in baroreceptors upregulated the excitability of baroreceptors through increasing the membrane input resistance, leading to a decreased pressure threshold for activation and increased discharges in response to supra-threshold pressure. These characteristics are reminiscent of sensitized nociceptors after peripheral nerve injury. In this regard, peripheral I h likely has an anti-nociceptive role, which still remains an open question.
Regulator in the Axonal Conduction
I h is expressed in both myelinated and unmyelinated axons. It was found that activation of I h inhibited the membrane hyperpolarization and the slowing of axonal conduction induced by tetanic stimulation with low frequency in the ex vivo study (activity-dependent slowing of conduction) [85, 86]. Recently, we also detected the relatively lower expression of HCN1 and HCN2 channels on the axolemma of axons. Previous work found that clonidine was able to strengthen and prolong the peripheral nerve blocking effect of local analgesics through inhibiting axonal I h [58, 87]. Consistent with the role of I h in axonal conduction, Dalle et al. reported that perineuronal administration of ZD7288 significantly alleviated the mechanical allodynia of partial sciatic nerve injury rats [84, 88]. However, other studies indicated that ZD7288 was not able to block axonal conduction [27, 51]. It is likely, in this case, that the analgesic effect of ZD7288 and clonidine is partially due to the inhibition of ectopic discharges generated from the injury sciatic nerves rather than their effects on axonal conduction. In agreement with this idea, we recently found that perineuronal ZD7288 perfusion can markedly relieve mechanical allodynia via inhibiting ectoptic discharges in CCI model [51]. The properties of activity-dependent slowing of conduction in uninjured sciatic nerves were found to be increased in SNL models [87], presumably due to the decreased expression of I h in the uninjured axons. However, whether and how this change contributes to the development of neuropathic pain behaviors is yet to be studied.
Role in the Synaptic Transmission in Spinal Dorsal Horn
The strengthened nociceptive synaptic transmission in the spinal dorsal horn, which is part of the central sensitization, is another critical inducer of neuropathic pain behaviors [89]. Increased release of exciting neurotransmitters, such as substance P (SP) and glutamate, from the nociceptive central terminals contributes a great deal to neuropathic pain. As mentioned above, HCN channels are expressed in the nociceptive and mechano-sensitive central terminals in the spinal dorsal horn. Moreover, HCN2-positive terminals also contain SP and glutamate. They formed synaptic connections with excitatory interneurons. These kinds of distribution likely enable HCN channels to participate in the regulation of presynaptic release of neurotransmitters and subsequently to regulate the excitability of interneurons in the spinal dorsal horn. Consistent with this idea, the in vitro recording on the spinal cord slices showed that the number of monosynaptic excitatory postsynaptic potential (mEPSP) induced by electrical stimulation of primary afferents was slightly reduced by ZD7288, suggesting the inhibition of synaptic transmission by ZD7288 [45]. Accordingly, we found that intrathecal administration of ZD7288 reversed mechanical allodynia significantly [55]. However, the role of I h in the synaptic transmission was questioned because of nonspecific effects of ZD7288 on AMPA and NMDA receptors, which will be discussed below.
I h Blockers and Transgenic Approaches
So far a number of HCN blockers have been discovered, including Cs+, ZD7288, alinidine (ST-567), zatabradine (UL-FS49), DK-AH 268 and ivabradine (S16257) [22]. Inhibition of I h by these blockers can reduce the rate of heart beat, hence I h blockers are also called bradycardics [90]. ZD7288 is the most widely used in the study of I h due to its high specificity and commercial availability. Therefore, most of our knowledge about the functions of I h, including its role in pain, has been obtained by using ZD7288.
However, some of these findings are recently questioned as a result of the discovery of the nonspecific effects of ZD7288. For example, T-type calcium channels were found to be inhibited by relatively higher concentration of ZD7288 in the mouse spermatogenic cells [91]. It was considered that I h and I T cooperated in the pacemaking process in both SAN cells and thalamic relay neurons. It would be very hard to differentiate the role of these two channels by ZD7288. However, there are two clues that might be helpful in clarifying this issue. First, the inhibition of I h by ZD7288 is much more effective than that of T-type calcium channels (IC50 was 2 μm and 100 μm for inhibition of I h and I T respectively). It is reasonable to assume that ZD7288 will preferentially inhibit I h rather than I T. Second, the effect of ZD7288 on I h is ir-reversible, in contrast, I T showed partial recovery after washout. In view of the sustained suppression of ectopic discharges by low dose of ZD7288, it is more likely that ZD7288 targets at I h.
Other nonspecific target of ZD7288 involves the controversy surrounding whether I h participates in the regulation of synaptic transmission. It was found that the inhibition of LTP or EPSPs by low dose of ZD7288 (20–50 μM) was likely dependent on the inhibition of postsynaptic NMDA or AMDA receptors [92] or other pre-synatpic mechanisms [93]. These findings challenged the proposed role of I h in the nociceptive synaptic transmission of spinal dorsal horn in the development of neuropathic pain. Thus it remains unclear whether I h was convincingly involved in the alleviation of mechanical allodynia when ZD7288 was applied intraperitoneally or intrathecally.
Ivabradine, known as Procarralan commercially, is considered to be the most specific blocker of I h without effect on T-type, L-type calcium channels and delayed outward potassium channels [90]. Recently it was approved by European Medicines Evaluation Agency as a new treatment drug for patients with chronic stable angina pectoris. This drug can slow the heart rate by inhibiting I h. It is of great interest to confirm the behavioral and electrophysiological studies of neuropathic pain with this drug.
Very recently, HCN1, 2 and 4 transgenic mice have been successfully established and widely used in understanding various physiological and pathological functions of I h in cardiovascular and nervous systems. HCN knockout mice consistently exhibit the disturbed heart beat, recapitulating the pharmacological results obtained in the past decades [94, 95]. Interestingly, over-expression of HCN2 and 4 had been found to accelerate the heart rates [96]. These studies strongly suggest the pacemaking function of I h in SAN and central neurons. However, their applications in the study of pain are still in absence. It would be interesting to examine if there are any alterations of ectopic discharges and neuropathic pain behaviors on these transgenic mice.
Conclusions and Future Directions
In the past decade, following the cloning of HCN channels, rapid progress has been made in the study of the structure, biophysical properties as well as functions of HCN channels. I h shows broad physiological functions and participates critically in many pathological conditions. While the role of I h in pain has also attracted much attention recently, a number of unsolved problems remain to be explored. For example, the exact biophysical mechanisms of I h in ectopic discharges is unclear. The role of I h in spinal central sensitization is yet to be defined. Nevertheless, with the development of more specific blockers and transgenic methods, more progresses will be made in understanding mechanisms concerning how I h participates in the development of neuropathic pain, and then hopefully, in the exploitation of new clinical analgesics. However, the broad distribution of HCN channels throughout peripheral and central nervous system inevitably brings problems to the clinical application of HCN blockers. Mild vision disorder, for example, was found occasionally during the treatment of angina patients with ivabradine [90]. The application of HCN blockers as analgesic will definitely face the same question when they are taken orally or in vein. However, some remarkable features of the involvement of HCN channels in neuropathic pain are likely to provide some clues in designing drugs. For instance, HCN channels mainly function in the peripheral pain pathways especially in the ectopic discharges. Besides, the predominant subtypes in pain pathways are HCN1 followed by HCN2, contrast to HCN4 in SAN cells [16]. Hereby, subtype specific, blood brain barrier impermeable synthetic agents will be much more effective in analgesia and accompanied by fewer side effects ideally. Alternatively, local application might be a feasible approach as well. We have witnessed the approval of ivabradine in the clinical treatment of angina. There is no reason to doubt that, although there is a long way ahead, I h could also become a valuable target in the clinical treatment of chronic pain.
References
Devor M (1991) Neuropathic pain and injured nerve: peripheral mechanisms. Br Med Bull 47:619–630
Campbell JN, Meyer RA (2006) Mechanisms of neuropathic pain. Neuron 52:77–92
Scholz J, Woolf CJ (2002) Can we conquer pain? Nat Neurosci 5(Suppl):1062–1067
Woolf CJ, Salter MW (2000) Neuronal plasticity: increasing the gain in pain. Science 288:1765–1769
Amir R, Argoff CE, Bennett GJ, Cummins TR, Durieux ME, Gerner P, Gold MS, Porreca F, Strichartz GR (2006) The role of sodium channels in chronic inflammatory and neuropathic pain. J Pain 7:S1–S29
McGivern JG (2006) Targeting N-type and T-type calcium channels for the treatment of pain. Drug Discov Today 11:245–253
Kim DS, Choi JO, Rim HD, Cho HJ (2002) Downregulation of voltage-gated potassium channel alpha gene expression in dorsal root ganglia following chronic constriction injury of the rat sciatic nerve. Mol Brain Res 105:146–152
Brown SM, Dubin AE, Chaplan SR (2004) The role of pacemaker currents in neuropathic pain. Pain Pract 4:182–193
Noma A, Irisawa H (1976) Membrane currents in the rabbit sinoatrial node cell as studied by the double microelectrode method. Pflugers Arch 364:45–52
Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M (1998) A family of hyperpolarization-activated mammalian cation channels. Nature 393:587–591
Santoro B, Baram TZ (2003) The multiple personalities of Ih-channels. Trends Neurosci 26:550–554
Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR (1998) Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell 93:717–729
Santoro B, Grant SG, Bartsch D, Kandel ER (1997) Interactive cloning with the SH3 domain of N-src identifies a new brain specific ion channel protein, with homology to eag and cyclic nucleotide-gated channels. Proc Natl Acad Sci USA 94:14815–14820
Finn JT, Grunwald ME, Yau KW (1996) Cyclic nucleotide-gated ion channels: an extended family with diverse functions. Annu Rev Physiol 58:395–426
Kaupp UB, Seifert R (2001) Molecular diversity of pacemaker ion channels. Annu Rev Physiol 63:235–257
Biel M, Schneider A, Wahl C (2002) Cardiac HCN channels: structure, function, and modulation. Trends Cardiovasc Med 12:206–212
Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A (2001) Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem 268:1646–1652
Santoro B, Tibbs GR (1999) The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels. Ann N Y Acad Sci 868:741–764
Pape HC, McCormick DA (1989) Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature 340:715–718
DiFrancesco D, Tromba C (1987) Acetylcholine inhibits activation of the cardiac hyperpolarizing-activated current, If. Pflugers Arch 410:139–142
Musialek P, Lei M, Brown HF, Paterson DJ, Casadei B (1997) Nitric oxide can increase heart rate by stimulating the hyperpolarization-activated inward current, If. Circ Res 81:60–68
Robinson RB, Siegelbaum SA (2003) Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 65:453–480
Zolles G, Klocker N, Wenzel D, Weisser-Thomas J, Fleischmann BK, Roeper J, Fakler B (2006) Pacemaking by HCN channels requires interaction with phosphoinositides. Neuron 52:1027–1036
Luthi A, McCormick DA (1999) Ca2+-mediated up-regulation of Ih in the thalamus. How cell-intrinsic ionic currents may shape network activity. Ann N Y Acad Sci 868:765–769
McCormick DA, Pape HC (1990) Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol 431:291–318
Maccaferri G, McBain CJ (1996) The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J Physiol 497:119–130
Chaplan SR, Guo HQ, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM, Dubin AE (2003) Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci 23:1169–1178
Sun Q, Xing GG, Tu HY, Han JS, Wan Y (2005) Inhibition of hyperpolarization-activated current by ZD7288 suppresses ectopic discharges of injured dorsal root ganglion neurons in a rat model of neuropathic pain. Brain Res 1032:63–69
van Welie I, van Hooft JA, Wadman WJ (2004) Homeostatic scaling of neuronal excitability by synaptic modulation of somatic hyperpolarization-activated Ih channels. Proc Natl Acad Sci USA 101:5123–5128
Beaumont V, Zucker RS (2000) Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci 3:133–141
Mellor J, Nicoll RA, Schmitz D (2002) Mediation of hippocampal mossy fiber long-term potentiation by presynaptic Ih channels. Science 295:143–147
Monteggia LM, Eisch AJ, Tang MD, Kaczmarek LK, Nestler EJ (2000) Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated channel family in rat brain. Mol Brain Res 81:129–139
Moosmang S, Biel M, Hofmann F, Ludwig A (1999) Differential distribution of four hyperpolarization-activated cation channels in mouse brain. Biol Chem 380:975–980
Kawai F, Horiguchi M, Suzuki H, Miyachi E (2002) Modulation by hyperpolarization-activated cationic currents of voltage responses in human rods. Brain Res 943:48–55
Mayer ML, Westbrook GL (1983) A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol 340:19–45
Doan TN, Kunze DL (1999) Contribution of the hyperpolarization-activated current to the resting membrane potential of rat nodose sensory neurons. J Physiol 514:125–138
Tu H, Deng L, Sun Q, Yao L, Han JS, Wan Y (2004) Hyperpolarization-activated, cyclic nucleotide-gated cation channels: roles in the differential electrophysiological properties of rat primary afferent neurons. J Neurosci Res 76:713–722
Ingram SL, Williams JT (1996) Modulation of the hyperpolarization-activated current (Ih) by cyclic nucleotides in guinea-pig primary afferent neurons. J Physiol 492:97–106
Tokimasa T, Akasu T (1990) Cyclic AMP regulates an inward rectifying sodium-potassium current in dissociated bull-frog sympathetic neurones. J Physiol 420:409–429
Scroggs RS, Todorovic SM, Anderson EG, Fox AP (1994) Variation in IH, IIR, and ILEAK between acutely isolated adult rat dorsal root ganglion neurons of different size. J Neurophysiol 71:271–279
Pearce RJ, Duchen MR (1994) Differential expression of membrane currents in dissociated mouse primary sensory neurons. Neuroscience 63:1041–1056
Ingram SL, Williams JT (1994) Opioid inhibition of Ih via adenylyl cyclase. Neuron 13:179–186
Doan TN, Stephans K, Ramirez AN, Glazebrook PA, Andresen MC, Kunze DL (2004) Differential distribution and function of hyperpolarization-activated channels in sensory neurons and mechanosensitive fibers. J Neurosci 24:3335–3343
Antal M, Papp I, Bahaerguli N, Veress G, Vereb G (2004) Expression of hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 in axon terminals of peptidergic nociceptive primary sensory neurons in the superficial spinal dorsal horn of rats. Eur J NeuroSci 19:1336–1342
Papp I, Szucs P, Hollo K, Erdelyi F, Szabo G, Antal M (2006) Hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 ion channels modulate synaptic transmission from nociceptive primary afferents containing substance P to secondary sensory neurons in laminae I-IIo of the rodent spinal dorsal horn. Eur J NeuroSci 24:1341–1352
Luo L, Chang L, Brown SM, Ao H, Lee DH, Higuera ES, Dubin AE, Chaplan SR (2007) Role of peripheral hyperpolarization-activated cyclic nucleotide-modulated channel pacemaker channels in acute and chronic pain models in the rat. Neuroscience 144:1477–1485
Pare M, Elde R, Mazurkiewicz JE, Smith AM, Rice FL (2001) The Meissner corpuscle revised: a multiafferented mechanoreceptor with nociceptor immunochemical properties. J Neurosci 21:7236–7246
Baker M, Bostock H, Grafe P, Martius P (1987) Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J Physiol 383:45–67
Baker M, Bostock H (1989) Depolarization changes the mechanism of accommodation in rat and human motor axons. J Physiol 411:545–561
Bostock H, Burke D, Hales JP (1994) Differences in behavior of sensory and motor axons following release of ischemia. Brain 117(Pt 2):225–234
Jiang YQ, Xing GG, Wang SL, Tu HY, Chi YN, Li J, Liu FY, Han JS, Wan Y (2008) Axonal accumulation of hyperpolarization-activated cyclic nucleotide-gated cation channels contributes to mechanical allodynia after peripheral nerve injury in rat. Pain [Epub ahead of print]. doi:10.1016/j.pain.2007.10.011
Bie B, Peng Y, Zhang Y, Pan ZZ (2005) cAMP-mediated mechanisms for pain sensitization during opioid withdrawal. J Neurosci 25:3824–3832
Jolas T, Nestler EJ, Aghajanian GK (2000) Chronic morphine increases GABA tone on serotonergic neurons of the dorsal raphe nucleus: association with an up-regulation of the cyclic AMP pathway. Neuroscience 95:433–443
Khakh BS, Henderson G (1998) Hyperpolarization-activated cationic currents (Ih) in neurones of the trigeminal mesencephalic nucleus of the rat. J Physiol 510(Pt 3):695–704
Tu H, Jiang YQ, Liu FY, Xing GG, Shi YS, Li T, Yao L, Han JS, Wan Y (2006) The effects of intrathecal application of ZD7288, and HCN blocker, on mechanical allodynia of neuropathic pain model rats. Chin J Pain Med 12:228–233
Vasilyev DV, Shan Q, Lee Y, Mayer SC, Bowlby MR, Strassle BW, Kaftan EJ, Rogers KE, Dunlop J (2007) Direct inhibition of Ih by analgesic loperamide in rat DRG neurons. J Neurophysiol 97:3713–3721
Lyashchenko AK, Redd KJ, Yang J, Tibbs GR (2007) Propofol inhibits HCN1 pacemaker channels by selective association with the closed states of the membrane embedded channel core. J Physiol 583:37–56
Yagi J, Sumino R (1998) Inhibition of a hyperpolarization-activated current by clonidine in rat dorsal root ganglion neurons. J Neurophysiol 80:1094–1104
Devor M, Raber P (1983) Autotomy after nerve injury and its relation to spontaneous discharge originating in nerve-end neuromas. Behav Neural Biol 37:276–283
Liu CN, Wall PD, Ben Dor E, Michaelis M, Amir R, Devor M (2000) Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain 85:503–521
Tal M, Eliav E (1996) Abnormal discharge originates at the site of nerve injury in experimental constriction neuropathy (CCI) in the rat. Pain 64:511–518
Xie YK, Xiao WH, Li HQ (1993) The relationship between new ion channels and ectopic discharges from a region of nerve injury. Sci China B 36:68–74
Wall PD, Gutnick M (1974) Ongoing activity in peripheral nerves: the physiology and pharmacology of impulses originating from a neuroma. Exp Neurol 43:580–593
Amir R, Kocsis JD, Devor M (2005) Multiple interacting sites of ectopic spike electrogenesis in primary sensory neurons. J Neurosci 25:2576–2585
Amir R, Michaelis M, Devor M (1999) Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J Neurosci 19:8589–8596
Kajander KC, Bennett GJ (1992) Onset of a painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in A beta and A delta primary afferent neurons. J Neurophysiol 68:734–744
Song XJ, Hu SJ, Greenquist KW, Zhang JM, LaMotte RH (1999) Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol 82:3347–3358
Yao H, Donnelly DF, Ma C, LaMotte RH (2003) Upregulation of the hyperpolarization-activated cation current after chronic compression of the dorsal root ganglion. J Neurosci 23:2069–2074
Kitagawa J, Takeda M, Suzuki I, Kadoi J, Tsuboi Y, Honda K, Matsumoto S, Nakagawa H, Tanabe A, Iwata K (2006) Mechanisms involved in modulation of trigeminal primary afferent activity in rats with peripheral mononeuropathy. Eur J Neurosci 24:1976–1986
Abdulla FA, Smith PA (2001) Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J Neurophysiol 85:644–658
Lee DH, Chang L, Sorkin LS, Chaplan SR (2005) Hyperpolarization-activated, cation-nonselective, cyclic nucleotide-modulated channel blockade alleviates mechanical allodynia and suppresses ectopic discharge in spinal nerve ligated rats. J Pain 6:417–424
Yu H, Wu J, Potapova I, Wymore RT, Holmes B, Zuckerman J, Pan Z, Wang H, Shi W, Robinson RB, El Maghrabi MR, Benjamin W, Dixon J, McKinnon D, Cohen IS, Wymore R (2001) MinK-related peptide 1: a beta subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circ Res 88:E84–E87
Zong X, Eckert C, Yuan H, Wahl-Schott C, Abicht H, Fang L, Li R, Mistrik P, Gerstner A, Much B, Baumann L, Michalakis S, Zeng R, Chen Z, Biel M (2005) A novel mechanism of modulation of hyperpolarization-activated cyclic nucleotide-gated channels by Src kinase. J Biol Chem 280:34224–34232
Ufret-Vincenty CA, Baro DJ, Lederer WJ, Rockman HA, Quinones LE, Santana LF (2001) Role of sodium channel deglycosylation in the genesis of cardiac arrhythmias in heart failure. J Biol Chem 276:28197–28203
Watanabe I, Wang HG, Sutachan JJ, Zhu J, Recio-Pinto E, Thornhill WB (2003) Glycosylation affects rat Kv1.1 potassium channel gating by a combined surface potential and cooperative subunit interaction mechanism. J Physiol 550:51–66
Much B, Wahl-Schott C, Zong X, Schneider A, Baumann L, Moosmang S, Ludwig A, Biel M (2003) Role of subunit heteromerization and N-linked glycosylation in the formation of functional hyperpolarization-activated cyclic nucleotide-gated channels. J Biol Chem 278:43781–43786
Xie W, Strong JA, Li H, Zhang JM (2007) Sympathetic sprouting near sensory neurons after nerve injury occurs preferentially on spontaneously active cells and is reduced by early nerve block. J Neurophysiol 97:492–502
Sun Q, Tu H, Xing GG, Han JS, Wan Y (2005) Ectopic discharges from injured nerve fibers are highly correlated with tactile allodynia only in early, but not late, stage in rats with spinal nerve ligation. Exp Neurol 191:128–136
Amir R, Michaelis M, Devor M (2002) Burst discharge in primary sensory neurons: triggered by subthreshold oscillations, maintained by depolarizing after potentials. J Neurosci 22:1187–1198
Ma C, LaMotte RH (2007) Multiple sites for generation of ectopic spontaneous activity in neurons of the chronically compressed dorsal root ganglion. J Neurosci 27:14059–14068
Bal T, McCormick DA (1997) Synchronized oscillations in the inferior olive are controlled by the hyperpolarization-activated cation current I(h). J Neurophysiol 77:3145–3156
Gauss R, Seifert R (2000) Pacemaker oscillations in heart and brain: a key role for hyperpolarization-activated cation channels. Chronobiol Int 17:453–469
Shim B, Kim DW, Kim BH, Nam TS, Leem JW, Chung JM (2005) Mechanical and heat sensitization of cutaneous nociceptors in rats with experimental peripheral neuropathy. Neuroscience 132:193–201
Dalle C, Eisenach JC (2005) Peripheral block of the hyperpolarization-activated cation current (Ih) reduces mechanical allodynia in animal models of postoperative and neuropathic pain. Reg Anesth Pain Med 30:243–248
Takigawa T, Alzheimer C, Quasthoff S, Grafe P (1998) A special blocker reveals the presence and function of the hyperpolarization-activated cation current IH in peripheral mammalian nerve fibres. Neuroscience 82:631–634
Grafe P, Quasthoff S, Grosskreutz J, Alzheimer C (1997) Function of the hyperpolarization-activated inward rectification in nonmyelinated peripheral rat and human axons. J Neurophysiol 77:421–426
Kroin JS, Buvanendran A, Beck DR, Topic JE, Watts DE, Tuman KJ (2004) Clonidine prolongation of lidocaine analgesia after sciatic nerve block in rats is mediated via the hyperpolarization-activated cation current, not by alpha-adrenoreceptors. Anesthesiology 101:488–494
Dalle C, Schneider M, Clergue F, Bretton C, Jirounek P (2001) Inhibition of the I(h) current in isolated peripheral nerve: a novel mode of peripheral antinociception? Muscle Nerve 24:254–261
Ji RR, Kohno T, Moore KA, Woolf CJ (2003) Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 26:696–705
Bucchi A, Barbuti A, Baruscotti M, Difrancesco D (2007) Heart rate reduction via selective ‘funny’ channel blockers. Curr Opin Pharmacol 7:208–213
Felix R, Sandoval A, Sanchez D, Gomora JC, Vega-Beltran JL, Trevino CL, Darszon A (2003) ZD7288 inhibits low-threshold Ca2+ channel activity and regulates sperm function. Biochem Biophys Res Commun 311:187–192
Chen C (2004) ZD7288 inhibits postsynaptic glutamate receptor-mediated responses at hippocampal perforant path-granule cell synapses. Eur J Neurosci 19:643–649
Chevaleyre V, Castillo PE (2002) Assessing the role of Ih channels in synaptic transmission and mossy fiber LTP. Proc Natl Acad Sci USA 99:9538–9543
Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, Feil S, Feil R, Lancel M, Chien KR, Konnerth A, Pape HC, Biel M, Hofmann F (2003) Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J 22:216–224
Stieber J, Herrmann S, Feil S, Loster J, Feil R, Biel M, Hofmann F, Ludwig A (2003) The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci USA 100:15235–15240
Er F, Larbig R, Ludwig A, Biel M, Hofmann F, Beuckelmann DJ, Hoppe UC (2003) Dominant-negative suppression of HCN channels markedly reduces the native pacemaker current I(f) and undermines spontaneous beating of neonatal cardiomyocytes. Circulation 107:485–489
Acknowledgements
This work was supported by the grants from Beijing Natural Science Foundation (7052039), National Natural Science Foundation of China (30600173, 30570566, 30470559 and 30330230), “111” Project of the Ministry of Education of China and “973” program of the Ministry of Science and Technology of China (2007CB512501).
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue article in honor of Dr. Ji-Sheng Han.
Yu-Qiu Jiang, Qian Sun, and Hui-Yin Tu—contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Jiang, YQ., Sun, Q., Tu, HY. et al. Characteristics of HCN Channels and Their Participation in Neuropathic Pain. Neurochem Res 33, 1979–1989 (2008). https://doi.org/10.1007/s11064-008-9717-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9717-6