Abstract
Biosynthesis of TRH, a neuropeptide involved in energy homeostasis, is modulated by glucocorticoids. TRH mRNA and peptide levels are increased upon incubation of hypothalamic cells with dexamethasone or with cAMP analogs but when combined, a mutual antagonism is observed. These effects are observed at the transcriptional level and on binding of glucocorticoid receptor (GR) or pCREB to the composite GRE (cGRE) and CRE-2 sites of TRH promoter. The present work studied the involvement of PKC and MAPK pathways on the effect of dexamethasone and on its interaction with cAMP signaling in hypothalamic cell cultures. PKC or MEK inhibition abolished dexamethasone-stimulatory effect on TRH mRNA levels, as well as its interference with the stimulatory effect of 8Br-cAMP. Binding of nuclear extracts from hypothalamic or neuroblastoma cells stimulated with dexamethasone or 8Br-cAMP to oligonucleotides containing the CRE or cGRE sites of TRH gene promoter was decreased if cells were preincubated with PKC or MEK inhibitors. Mutations on the AP-1 or the GRE half sites of cGRE showed that GR binds as an heterodimer on cGRE, and PKC or MEK inhibitors diminish binding at the AP-1 site. PKC and ERK signaling thus modulate GR activity and its interaction with CREB or AP-1 at the TRH gene promoter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyrotropin releasing hormone (TRH: pglu-his-proNH2), the first hypophysiotropic peptide discovered, was later found in various regions of the limbic system where it has a neuromodulatory function [1]. TRH is synthesized, in the rat, from a protein precursor containing five repeated sequences of gln-his-pro-gly that give rise to the active peptide [2]. TRH, released from neurons of the hypothalamic paraventricular nucleus (PVN) that project to the median eminence, controls the synthesis and release of the pituitary hormones thyrotropin and prolactin; these neurons behave as neuroendocrine transducers that play a critical role in maintaining energy homeostasis as their activity is modulated by multiple metabolic, hormonal, and neuronal signals [3, 4]. In response to a neuronal stimulus as cold exposure, TRH mRNA levels in the PVN increase very rapidly (30–60 min) and transiently as levels normalize by 2 h, despite leaving the rats in the cold for up to 6 h [5]; activation includes non-hypophysiotropic TRHergic neurons of the anterior PVN [6]. In this condition, serum corticosterone levels increase [5, 6].
In fetal hypothalamic cultures, norepinephrine, the neurotransmitter proposed to mediate the effects of cold on TRH release [7], increases TRH mRNA levels; this is mimicked by activation of protein kinase A (PKA) or C (PKC) [8–10]; levels are also enhanced by dexamethasone (dex), an analog of corticosterone that activates GR [9, 10]. The stimulatory effect of dex or 8Br-cAMP is inhibited when cells are co-incubated with both drugs. These effects occur at the transcriptional level since they are observed after transfecting a plasmid containing the minimal pro-TRH promoter bound to luciferase cDNA into either hypothalamic cells or the cell lines NIH-3T3 [10] or neuroblastoma SH-SY5Y (unpublished).
The TRH gene promoter contains response elements to various transcription factors activated by neuronal or hormonal effectors [11]. Using electrophoretic mobility shift assays (EMSA) we demonstrated that nuclear extracts from hypothalamic cultures incubated with norepinephrine or 8Br-cAMP bind to the CRE site of TRH gene promoter located at −101/−94 bp (CRE-2) [10] and not to site 4 (−59/−52) previously proposed as the CRE site [12]. We also recognized a half site GRE (GRE1/2) located at −210/−205 that is flanked by two AP-1 recognition sites forming a composite GRE (cGRE: −218/−198) [10]. GR monomer is able to bind to cGRE as an heterodimer with c-Jun or c-Fos [13, 14]. Binding of nuclear extracts from hypothalamic cells, incubated with 8Br-cAMP and dex, to either CRE or cGRE is diminished or avoided when cells are co-incubated with both compounds [10]. Involvement of pCREB, GR, or c-Jun is inferred by supershift [10] and chromatin immunoprecipitation assays (unpublished).
In contrast to the mutual antagonism between PKA signaling and glucocorticoids, dex and phorbol esters (TPA) have an additive effect on TRH mRNA levels of hypothalamic cells [9] as well as on the binding of their nuclear factors at the cGRE [10]. These results suggest that PKC stimulates AP-1 activity allowing formation of a stable GR:c-Jun or GR:c-Fos heterodimer [13–15]. Protein kinase C isoenzymes constitute a family of serine/threonine kinases that are classified into three subfamilies based on their ligand and cofactor requirements [16]. Depending on the cellular context, the isotype and its localization, PKC regulates a wide array of cellular responses including signaling of mitogen activated protein kinase (MAPK) [17]. MAPK affect the activity of transcription factors; for example, MEK1, 2 (through the extracellular regulated kinases 1 or 2 [ERK1/2]) and MEK5 phosphorylate CREB or GR, the p38 MAPK phosphorylates hGR, and JNK activates c-Jun [18–20].
Glucocorticoids in turn, through GR, enhance the expression of various components of the MAPK cascade (Ras, Raf-1, ERK1 and 2) [21, 22] but also, inhibit the activity of ERK1/2, p38, and JNK through dephosphorylation by the phosphatase MKP-1, whose expression is fastly increased by GR activation (MKP-1 mRNA at 5–15 min; protein levels at 30 min) [23]. Regulation of the MAPK pathways and MKP-1 constitute thus a negative feedback loop controlling glucocorticoid action.
The aim of this work is to study the involvement of PKC and MAPK on dex-induced TRH gene expression as both kinases constitute a convergence point with the GR mediated signaling pathway [24] and are common mediators in signal transduction pathways activated by growth factors, cytokines and stress [24–26]. The involvement of PKC and MAPK signaling pathways was evaluated on the effect of dexamethasone alone or combined with 8Br-cAMP on TRH mRNA levels in hypothalamic cells and, on the binding of activated nuclear factors at the CRE and cGRE sites of TRH promoter.
Experimental Procedure
Animals
Female Wistar rats (280–300 g) raised at the Institute's vivarium were mated on proestrous–oestrous stage of the cycle and kept in 12 h dark-light cycle, controlled temperature (23°C) and fed at libitum (Purina 2018S, Harlam). Care was taken to preserve adequate conditions following the guidelines of the Society for Neuroscience. Protocols were approved by the Institute’s Ethical commission.
Cell Culture
Primary cultures of hypothalamic cells were performed as described [9, 27]. Briefly, on the 17th day of gestation, dames were anesthesized with 0.45 ml Sedalphorte (63 mg sodium pentobarbital). The embryos were removed and the brain excised through an imaginary line between both eye and ear superior edge. The brain was kept in the skull to avoid tissue distortion, and the hypothalamus dissected by cutting the area limited by the optic chiasm and lateral sulcus including the mammillary bodies to a 2–3 mm depth that avoids the thalamic area [28]. 6 × 105 cells (yield: 2 × 106/hypothalamus) were plated in 15 mm dishes for those experiments where levels of TRH mRNA were evaluated and in 60 mm dishes for the preparation of nuclear extracts. Cultures were incubated for 14–18 days in vitro (DIV) at 90% humidity, 7% CO2, 93% air, 37°C. The effect of 10 nM TPA alone or together with 10 nM dex was tested on staurosporine-treated or PKC-depleted cells incubating the cells at 18 DIV for 3 h; cells were pretreated for 30 min with 1 μM staurosporine [29] and PKC depletion was achieved by incubating the cells at 17 DIV, for 18 h, with 5 μM TPA [30]. The following experiments were performed at 14 DIV; cells were incubated for 30 min with either 10 μM FPT III (inhibitor 3 of farnesyltransferase, enzyme that associates Ras to the membrane [31]), 50 μM PD98059 (MEK inhibitor [32]), 25 μM SB0203580 (SAPK/p38 inhibitor [33]), or 6 μM Ro-318220 (PKC inhibitor [30]) and then, for 1 h with 1 mM 8Br-cAMP, or 100 nM forskolin, 10 nM dex or combined. The dose of 10 nM dex is sub-maximal on TRH mRNA levels [9]. Cultures were observed under an inverted microscope (Zeiss Axioskop microscope); no damage was detected.
The neuroblastoma cell line SH-SY5Y (ATCC CRL-2266), kindly donated by Dra. Rosa Maria Pardós (Instituto de Fisiología, Benemérita Universidad Autónoma de Puebla, México), was grown as described [10]. Cells were differentiated at seeding with 1.6 nM TPA, cultured for 8 days with medium changes every 2 days. These cells do not express TRH mRNA (unpublished) but possess the transduction pathways involved in glucocorticoid, PKC, PKC and MAPK signals [34, 35]; nuclear factors of stimulated cells show the similar binding characteristics as those from hypothalamic cultures [10].
RT-PCR
Total RNA was purified as described [9] and analyzed in 1% agarose gels to verify its integrity. Samples showing a 260/280 nm ratio less than 1.8 were discarded. RT-PCR were carried out as described [9, 10] using 25 pM of the reported oligonucleotide primers for TRH and 50 pM for cyclophilin [10]: for TRH, sense: 5′ CCC TGG ATG GAG TCT GAT GT 3′, antisense: 5′ GAC AGC TAG TGA AGG GAA CAG G 3′ (356 bp product), 64°C, 29 cycles; for cyclophilin, sense: 5′ GGG GAG AAA GGA TTT GGC TA 3′, antisense: 5′ ACA TGC TTG CCA TCC AGC C 3′ (259 bp product), 64°C, 24 cycles. For some cultures, 50 pM of specific primers for rat G3PDH was used as control (sense primer, 5′-TGAAGGTCGGTGTCAACGGATTTGGC-3′; antisense primer, 5′-CATGTAGGCCATGAGGTCCACCAC-3′) with the amplification conditions previously described [10, 36].
Electrophoretic Mobility Shift Assay
The oligonucleotides described in Fig. 1 were synthesized at the Institute’s unit [37] and end-labeled with T4 polynucleotide kinase (Promega) and 30 μCi [γ-32P] ATP/100 ng oligonucleotide (NEN Life Science Products, Boston MA) as reported [10]. EMSA was performed as published [10, 38]; briefly, 10 μg of protein from nuclear extracts were incubated 30 min with one of the double stranded labeled oligonucleotides, and the mixture submitted to non-denaturing gel-electrophoresis (5% acrylamide:bisacrylamide 29:1) in TBE buffer (50 mM Tris HCl, 45 mM boric acid, 0.5 mM EDTA), and analyzed with a phosphorimager. The mutated sequences map-cGRE or mg-cGRE were analyzed with TRANSFAC 4.0 and Mat Inspector to verify that each would not recognize any known transcription factor.
Sequences of double stranded oligonucleotides used in EMSA. Letters in bold and italics mark the sequences on the TRH promoter, of the cAMP response element (CRE-2), the composite GRE (cGRE), with the GRE1/2 consensus sequence in the 5′-3′strand and the two AP-1 sites in the 3′-5′ strand; arrows indicate direction of the sequence. Mutations of the GRE1/2 (mg) or the AP-1 sites (map) of cGRE are marked with letters in minuscule
Statistical Analysis
Results were calculated as % of controls of each culture; data were then calculated as the mean ± S.E.M. Data of the relative mRNA levels were analyzed by ANOVA, considered significant at P < 0.01, followed by Fisher’s PLSD test. Data from densitometric analyses were calculated taking the signal from control unstimulated cells as 100%.
Results
Regulation of TRH mRNA Levels in Hypothalamic Cells
As co-treatment with TPA and dex results in an additive increase in TRH mRNA levels [9], we evaluated the effect of a non specific inhibitor of PKC (staurosporine) to determine the dependency of these effects on PKC activity. Cells were treated for 3 h since the effect of 10−7 M TPA on TRH mRNA levels is observed only after 2 h probably due to the time required for adequate synthesis of c-Fos [8]. The hypothesis behind this experiment was that dex may stimulate TRH mRNA levels by GR heterodimerization with c-Jun or c-Fos [13, 14], and PKC inhibition affect phosphorylation of c-Fos or c-Jun as well as AP-1 formation (c-Fos:c-Jun heterodimer). As seen in Fig. 2, staurosporine not only inhibited the stimulatory effect of TPA but also that of dex + TPA (values were below controls). Likewise, PKC depletion, by long term TPA preincubation [30], blocked the effects of TPA or dex + TPA on TRH mRNA levels. These results suggest that PKC activation of c-Jun or c-Fos is required for an adequate response of the TRH promoter to dex.
Effect of PKC inhibition on TPA and dex effects on TRH mRNA expression. Hypothalamic cells were preincubated at 18 DIV for 30 min with 1 μM staurosporine or 18 h with 5 μM TPA; cells were then incubated for 3 h with 100 nM TPA or TPA + 10 nM dexamethasone. The relative levels of TRH mRNA were evaluated by RT-PCR and calculated as the ratio of TRH cDNA signal/G3PDH cDNA signal; values were expressed as % of controls. Insert depicts a sample of the RT-PCR products, with arrows signaling G3PDH and TRH cDNAs. *Significant difference against control, n = 6/group; letters: f, h, significant difference against F, G; e, h = between these two groups; P < 0.01 by Post-hoc Fisher analysis
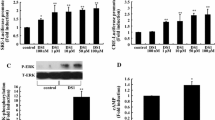
The extent and loci of GR phosphorylation determines its stability, nuclear translocation and, in a promoter-dependent manner, some of its genomic effects [18–20]. Since various MAPKs phosphorylate GR [22, 24] reducing its activity, we studied their involvement in the stimulatory effect of dex and its interaction with cAMP-stimulated factors. As previously reported [9, 10], hypothalamic cells cultured for 1 h with either 8Br-cAMP or dex increased TRH mRNA levels, but when both drugs were added simultaneously the stimulation was lower that caused by any of them (Fig. 3a). Preincubation with the MEK inhibitor PD98059 did not change the stimulatory effect of 8Br-cAMP but inhibited that of dex, allowing the full effect of 8Br-cAMP when both drugs were added to the culture (Fig. 3a) (ANOVA: F 7,86 = 13.9, P < 0.0001; n = 6/group). These results support the involvement of the ERK pathway in the effect of dex on TRH mRNA levels as well as on its interference with that of 8Br-cAMP.
Effect of inhibitors of the MAPK pathway on TRH mRNA expression. Primary cultures of hypothalamic cells were preincubated at 18 DIV for 30 min with one of the following inhibitors: PD98059 (PD) for MEK, FPT III for Ras, and SB203580 for p38. Cells were then incubated with 1 mM 8Br-cAMP (A), or 10 nM dexamethasone (D), or 8Br-cAMP + dexamethasone (AD). The relative levels of TRH mRNA were evaluated by RT-PCR and calculated as the ratio of TRH cDNA signal/cyclophilin cDNA signal; values were expressed as % of controls. Insert depicts a sample of the RT-PCR products, with arrow signaling TRH cDNA. *Significant difference against control; P < 0.01 by Post-hoc Fisher analysis. Equal letters represent significant difference between these groups; in B: f are significantly different against F (P < 0.01, n = 4)
To evaluate different stages of activation of the ERK and p38 pathways, other inhibitors were used on cells incubated with dex with or without 8Br-cAMP present; the 8Br-cAMP group was not included to be able to have at least three dishes/group and evaluate the effect of these inhibitors within the same culture. Results are the mean of two independent cultures (F 11,82 = 7.4, P < 0.0001, n = 6–8/group). As shown in Fig. 3b, the Ras inhibitor FPT III caused a small but significant increase in the basal levels of TRH mRNA suggesting a basal inhibitory effect of Ras; addition of dex or dex + 8Br-cAMP caused no further increase. The inhibitor of p38 kinase (SB203580) did not alter the basal TRH mRNA levels but reduced dex stimulatory effect, albeit to a lower extent than that observed with the MEK inhibitor PD98059 (Fig. 3a, b). In SB203580-treated cultures, co-stimulation with 8Br-cAMP and dex increased TRH mRNA levels compared to cells treated with the inhibitor but non stimulated (Fig. 3b), suggesting that the p38 inhibitor impeded the interfering effect of dex on 8Br-cAMP stimulation; the stimulatory effect of dex did not achieve significance due to the slight enhancement in TRH mRNA levels of unstimulated cells (compared to the control group). The effect of dex to increase TRH mRNA levels, and on its interference with cAMP activated pathways on TRH mRNA levels, seems thus to depend on the activation of ERK (1, 2 or 5); this latter event can be influenced by p38.
Binding of Nuclear Extracts from Hypothalamic Cells to Response Elements of the TRH Promoter
To further characterize the role of PKC and MEK, the ability of nuclear extracts from hypothalamic cells to bind to CRE-2 or cGRE sites was studied by EMSA, using a more specific PKC inhibitor (Ro-318220) than staurosporine [30, 39], and the MEK inhibitor PD98059. Nuclear extracts from controls or 8Br-cAMP-treated cells show two bands bound to oligonucleotides containing the CRE site that can be displaced with an oligonucleotide containing the consensus CRE sequence, or with anti-pCREB or anti-c-Jun [10] suggesting that the proteins bound are either pCREB homodimers, pCREB heterodimers with c-Jun or other unidentified factors of the ATF family. Similar results were obtained in these new EMSA experiments, being the lower-mobility band (a) considerably more intense than band (b). 8Br-cAMP or dex treatment increased binding of nuclear extracts compared to controls (Fig. 4a). Pretreatment with the MEK inhibitor increased the signal of band (a) from non stimulated cells to a similar intensity as those treated with 8Br-cAMP. Compared to the binding of nuclear extracts from cells incubated with dex only, a significant decrease in binding, and blurring of the bands, was observed using nuclear extracts from dex treated cells in presence of PD98059; this blurring indicates loss of binding stability [40]. This latter effect was more pronounced with Ro-318220 (Fig. 4a) (ANOVA: F 6,18 = 3.7, P = 0.01, n = 4). Results suggest that dex effects on nuclear factors capable of binding to CRE-2 require the activity of ERK or PKC.
Effect of MEK or PKC inhibition on binding of hypothalamic nuclear extracts to CRE or cGRE. Primary cultures of hypothalamic cells, preincubated at 18 DIV for 30 min with 50 μM PD98059 (pd), or 6 μM Ro-318220 (Ro) and then incubated 1 h with 1 mM 8Br-cAMP (A), or 10 nM dexamethasone (D). Their nuclear extracts were incubated with double stranded oligonucleotides containing CRE-2 (A) or cGRE sequences (B) (see Fig. 1) for EMSA analysis. (C) Histograms show the mean ± S.E.M. of the densitometric values of band (A) of CRE-2 or band (C) of cGRE calculated as % of controls (=100%)
A similar analysis was performed using the same nuclear extracts with the oligonucleotide containing cGRE (Fig. 1b). Our previous work demonstrated that the lower band (c) detected using this oligonucleotide is displaced with excess of unlabeled oligonucleotides containing the consensus GRE, while the upper bands, with oligonucleotides containing consensus CRE or AP-1 suggesting binding of GR and AP-1 respectively [10]. As previously observed with hypothalamic cells [10], the main band was that of higher mobility band (c) whose signal was increased using nuclear extracts from cells treated with 8Br-cAMP similarly if they were preincubated with the MEK inhibitor; increased signal was observed with nuclear extracts from dex incubated cells which was further increased by PD98059 preincubation (Fig. 4b) (ANOVA: F 6,20 = 4.3, P = 0.006, n = 4). These results suggest that binding to the GRE site was not diminished by MEK inhibitor. The signal of bands (a) and (b) were very faint and difficult to quantify; the intensity of band (a) was slightly increased only with nuclear extracts from 8Br-cAMP treated cells and from unstimulated cell pretreated with PD98059 (Fig. 4b).
Effect of Mutations of the Composite GRE
To evaluate the role of each site in cGRE, we mutated the GRE1/2 site (mg-GRE) or the 2 AP-1 sites (map-GRE) (Fig. 1). As hypothalamic cell cultures contain many cell populations including glia, we used the differentiated neuroblastoma cell line SH-SY5Y. This line does not express TRH mRNA (unpublished) but its nuclear extracts have similar binding specificities to CRE or cGRE containing oligonucleotides, as hypothalamic cell cultures [10]. An exception is that the lower mobility band (a) of cGRE is more intense due to higher concentrations of AP-1 factors as cells are differentiated with TPA treatment [10]; band (b) behaves as with hypothalamic cells, very faint and without changes upon cell-treatments probably due to non-specific binding. We first stimulated cells with forskolin (FK) or dex that induced increased binding, of similar intensity, to both bands using cGRE (Fig. 5). Mutation of the AP-1 sites (map-cGRE) diminished binding compared to cGRE although, nuclear extracts from FK stimulated cells still showed increased signal compared to binding of control cells. Mutation of the GRE1/2 site (mg-cGRE) favored binding to band (a) of nuclear extracts from FK stimulated cells (Fig. 5; ANOVA: F 17,99 = 6, P < 0.0001; n = 6, two independent experiments). These results suggest that when the AP-1 sites are mutated, binding of nuclear extracts is considerably diminished, even at the GRE1/2 site suggesting that GR binding as a monomer is not stable.
Binding of nuclear extracts of neuroblastoma SH-SY5Y cells to cGRE and mutated cGREs. Differentiated neuroblastoma cells were incubated for 1 h with forskolin (FK) or dexamethasone (D) and their nuclear extracts analyzed by EMSA using the double stranded oligonucleotides shown in Fig. 1. Results of densitometric analyses of bands (a) and (c) from two independent experiments are shown in the table, calculated as % of control values (=100%; SEM was less than 9%, not shown), n = 4–6, *P < 0.005, +P < 0.05 against controls; e: P < 0.05 between them
Neuroblastoma cells were then treated as the hypothalamic cell cultures, with 8Br-cAMP, dex, +/− MEK inhibitor. Results were similar as binding to band (a) at the CRE-oligonucleotide increased when cells were incubated with 8Br-cAMP or dex. Treatment with MEK inhibitor slightly increased binding compared to nuclear extracts from unstimulated cells but impeded the effect of 8Br-cAMP or dex treatment (Fig. 6a).
Effect of MEK inhibition on binding of nuclear extracts of neuroblastoma SH-SY5Y cells to CRE or cGRE. Differentiated neuroblastoma cells were preincubated for 30 min with PD98059 (pd or vehicle), followed by 1 h incubation with 1 mM 8Br-cAMP (A), or 10 nM dexamethasone (D). Nuclear extracts were incubated with double stranded oligonucleotides with CRE, cGRE or the mutated AP-1 (map-cGRE) or GRE1/2 sites (mg-cGRE) (see Fig. 1) for EMSA analysis. Histograms show the mean ± S.E.M. of the densitometric values of the band indicated on the abscissa, n = 3
The signal of band (a) was increased using cGRE and nuclear extracts from cells treated with 8Br-cAMP, dex, MEK inhibitor alone, or with 8Br-cAMP, and only slightly with dex + PD98059. In contrast, the intensity of band (c) was increased only with dex treatment in presence of PD98059 (Fig. 6b).
Using the mutated cGRE lacking the two AP-1 sites (map-cGRE) lowered the intensity of bands compared to those of cGRE, as shown above; increased signal was observed on band (a) with nuclear extracts from 8Br-cAMP or dex treated cells which was completely abolished (signal was below that of controls) with the MEK inhibitor (Fig. 6c). Although there were no differences in the intensity of band (c) upon 8Br-cAMP or dex incubation, when these compounds were added to cells pretreated with PD98059, signal diminished below that of non stimulated cells treated or not with the inhibitor (Fig. 6c).
The signal of the DNA-protein complex corresponding to band (a) of the mutated cGRE lacking the GRE1/2 site (mg-cGRE) was increased using nuclear extracts from 8Br-cAMP or dex treated cells and, considerably higher than with cGRE; the duplet of band (a) suggests a different composition of the nuclear proteins (Fig. 6d). Pre-treatment with MEK inhibitor increased binding to (a) compared to untreated cells, this signal slightly decreased when 8Br-cAMP or dex was added in presence of the inhibitor (Fig. 6d). The increased signal of the inferior band (c) of 8Br-cAMP or dex treated cells was not observed if cells were preincubated with PD98058. The augmented intensity of mg-cGRE compared to cGRE points for binding of AP-1 complex with higher affinity than the heterodimer c-Fos:GR or c-Jun:GR.
Discussion
Through the combined approach of measuring TRH mRNA levels in hypothalamic cultured cells, and binding of their nuclear extracts to the CRE-2 and cGRE sequences of TRH promoter, we previously demonstrated a negative interaction between GR and PKA signaling pathways while a positive, between GR and PKC [9, 10]. It is well recognized that the specificity of the response depends on the tissue and cell type, as well as on the characteristics of the promoter. As glucocorticoid–PKA negative interactions are reproduced in an heterologous cell line (NIH-3T3) transfected with TRH-luc [10] or neuroblastoma cells (unpublished), and their nuclear extracts present similar binding responses in EMSA, these approaches allow recognition of cross-talk of intracellular pathways interacting with TRH promoter. The mutual antagonism between PKA and GR pathways occurs at the transcriptional level, and on the binding to CRE-2 (−101/−94) or cGRE (−218/−198) sites (observed by EMSA [10) and recently, using DNase protection assays [unpublished]).
In this work we provided evidence for the requirement of PKC and ERK/MAPK activities on the stimulatory effect of dex, and on its interference with cAMP-induced increase of TRH mRNA levels in hypothalamic cultured cells. As the stimulatory effect of dex on TRH mRNA levels, and its antagonism of cAMP signaling-induced transcription probably involve ligand-activated GR [10, and unpublished], the simplest explanation of our data could be that PKC, ERK, and partially p38, are required for GR action. However, activation of MAPK pathways provokes GR dysregulation [41] so their inhibition should increase GR function.
Unliganded GR is bound in the cytoplasm to a multiprotein complex containing heat-shock proteins (hsp90) and several kinases of the MAPK signaling system, including Src [42, 43]. Upon ligand binding, GR is released, phosphorylated and translocated to the nucleus where it acts as a transcription factor [42–44]. The activity and stability of the receptor varies depending on the site and degree of phosphorylation. Ligand binding induces phosphorylation at ser-211 (human GR) and translocation to the nucleus (within 5 min) [42]; translocation and transactivation is further enhanced with inhibitors of JNK or ERK, but decreased with inhibitors of p38 [20, 42–44]. Our results on loss of dex effect with p38 inhibition can be explained by p38 phosphorylating specific coactivators required for GR action and, indirectly inhibiting the ligand binding domain [45]. In contrast to the reported enhanced transactivation produced by GR after ERK inhibition [46], dex stimulatory effect on TRH mRNA levels was lost with PD98059, a result coincident with other examples in which PKC and ERK-MAPK inhibitors attenuate the corticosterone-mediated gene transcription [47]. We thus propose that the effect of PKC and ERK inhibition on dex effects on TRH transcription might be occurring at different levels: (1) the stimulatory effect of dex being impeded by inhibition of AP-1 components that are required for the formation of a stable GR heterodimer, and the appropriate recruitment of coactivators; (2) the interfering effect of dex on cAMP signaling to be due to effects on GR translocation (see below).
We previously suggested that dexamethasone may induce TRH transcription, and binding of nuclear factors to CRE-2 [10, unpublished], by increasing CREB phosphorylation (deduced by CHIP, unpublished) through increased synthesis of serum and glucocorticoid inducible kinase (SgK), that is able to phosphorylate CREB [48, 49]. SgK activity is regulated not only by glucocorticoids but also by TPA (in 1 h) and ERK at the transcriptional and posttranscriptional levels [50]. Furthermore, corticosterone treatment of AtT20 cells increases, in 1h, the amount of Ras, Raf-1 and Erk1/2 protein [21] which could also increase pCREB. The decreased binding to CRE-2 by PKC and MEK inhibitors in dex-stimulated cells can be explained by inhibition of ERK and SgK activities, since it has recently been shown that PKC can activate SgK, which in turn phosphorylates CREB via an ERK mediated mechanism [51].
The TRH gene promoter has a half site GRE, so GR can only bind to the cGRE that contains two flanking AP-1 sites as a monomer to the GRE1/2, which is stabilized by its union to either c-Jun, c-Fos, or even c-Jun:c-Jun or c-Jun:c-Fos, [10]. Mutations on cGRE showed that the AP-1 sites are essential for the stability of GR binding. PD98059 treatment inhibited the binding of nuclear extracts from dex stimulated cells to either CRE or the mutated cGREs (lacking either the AP-1 sites or the GRE1/2 site). The interaction of GR with AP-1 components involves the three-dimensional structure of GR [20], that could be affected by altering its phosphorylation state with the MEK inhibitor. This inhibitor caused a strong decrease on binding to the AP-1 sites of cGRE and, of mg-cGRE, suggesting diminished levels of activated c-Jun or c-Fos. This is supported by the effect of ERK1/2 activation on c-Fos induced transcription (observed within minutes), and its ability to determine the pattern of expression of other components of AP-1 [52], as well as their binding [53]. The stimulatory or inhibitory effects of the heterodimer formed, whether c-Fos:GR or c-Jun:GR depends on the promoter and cellular context [12, 13, 20]. At the short times of incubation studied, the effect of dexamethasone would require activation of GR, and heterodimerization with existing components of the AP-1 family as the MAPK pathways could be partially stimulated in the conditions used: cells are grown in presence of 10 % fetal bovine serum (FBS) and with conditioned medium, which contains BDNF and other growth factors, that allow higher basal levels of TRH [54].
The second effect, the interference of dex with 8Br-cAMP stimulation of TRH mRNA levels was avoided with inhibitors of p38 (SB0203580) and MEK (PD98059). Although ERK1/2 increases pCREB [55], its inhibition did not alter 8Br-cAMP induced enhancement of TRH mRNA levels, neither the binding of nuclear extracts of stimulated cells to CRE-2, suggesting PKA activation was sufficient for pCREB to increase TRH transcription independent of MEK activity. We have proposed that the mutual antagonism, between dex and 8Br-cAMP signaling pathways, involves the activated GR interacting with either pCREB [56] or cPKA [57] before binding of these transcription factors to their response elements [10]. Activation of GR depends, as mentioned above, not only on ligand binding but also, on the phosphorylation state of the receptor which can accelerate its nuclear translocation or, its export. MEK inhibition restores the nuclear translocation of GR in some systems [58, 59]; however, PD98059 treatment also promotes nuclear export of ERK/MAPK + Sgk into the cytoplasm [60]. As the transrepressive effect of glucocorticoids occurs only if glucocorticoids are present during the phase of active transcription [48], and we have shown that for mutual interference to occur, dex and 8Br-cAMP (or noradrenaline) must be added simultaneously (if dex is added 10 min before PKA stimulation, the interference is no longer observed) [61], the present results suggest that inhibition of ERK or p38 affects the nucleocytoplasmic traffic of activated GR, eliminating the interaction with pCREB (or PKA) leaving pCREB able to induce TRH transcription.
The results presented herein support a cross talk between various pathways that, depending on the stimulus could differentially modulate TRH transcription. The ERK1/2 pathway can be stimulated by growth factors as BDNF [25], that increases TRH mRNA levels in immature hypothalamic cultures and this effect is antagonized by PD98059 [62, 63]. In vivo, stress increases BDNF expression in the PVN in a fast and transient manner [64], which could activate TRH neurons expressing TrkB receptor [63]. One could envisage situations where the TRHergic neuron receives diverse stimuli [65, 66], simultaneously or in succession, adjusting the levels of immediate early genes expression, differentially modulating transcription factors as CREB, GR of AP-1 affecting thus, the inhibitory effect of rising corticosterone levels and, depending on the type of stress or previous history of the animal, alter the response to an incoming stimulus.
References
Gary KA, Sevarino KA, Yarbrough GC, Prange AJ, Winokur A (2003) The thyrotropin-releasing hormone (TRH) hypothesis of homeostatic regulation: implications for TRH-based therapeutics. J Pharmacol Exp Ther 305:410–416
Perello M, Nillni EA (2007) The biosynthesis and processing of neuropeptides: lessons from prothyrotropin releasing hormone (proTRH). Front Biosci 12:3554–3565
Joseph-Bravo P (2004) Hypophysiotropic thyrotropin-releasing hormone neurons as transducers of energy homeostasis. Endocrinology 145:4813–4815
Lechan RM, Fekete C (2006) The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res 153:209–235
Uribe RM, Redondo JL, Charli JL, Joseph-Bravo P (1993) Suckling and cold stress rapidly and nucleus of lactating rats exposed to suckling or cold stimulation. Brain Res 1132:120–128
Sánchez E, Uribe RM, Corkidi G, Zoeller RT, Cisneros M, Zacarias M, Morales-Chapa C, Charli JL, Joseph-Bravo P (2001) Differential responses of thyrotropin-releasing hormone (TRH) neurons to cold exposure or suckling indicate functional heterogeneity of the TRH system in the paraventricular nucleus of the rat hypothalamus. Neuroendocrinology 74:407–422
Arancibia S, Rage F, Astier H, Tapia Arancibia L (1996) Neuroendocrine and autonomous mechanisms underlying thermoregulation in cold environment. Neuroendocrinology 64:257–267
Uribe RM, Pérez-Martínez L, Covarrubias ML, Gómez O, Covarrubias L, Charli JL, Joseph-Bravo P (1995) Phorbol ester or cAMP enhance thyrotropin-releasing hormone mRNA in primary cultures of hypothalamic cells. Neurosci Lett 201:41–44
Pérez-Martínez L, Carreón-Rodríguez A, González-Alzati ME, Morales C, Charli JL, Joseph-Bravo P (1998) Dexamethasone rapidly regulates TRH mRNA levels in hypothalamic cell cultures: interaction with the cAMP pathway. Neuroendocrinology 68:345–354
Cote-Vélez A, Pérez-Martínez L, Diaz-Gallardo MY, Pérez-Monter C, Carreón-Rodríguez A, Charli JL, Joseph-Bravo P (2005) Dexamethasone represses cAMP rapid upregulation of TRH gene transcription: identification of a composite glucocorticoid response element and a cAMP response element in TRH promoter. J Mol Endocrinol 34:177–197
Lee SL, Stewart K, Goodman RH (1988) Structure of the gene encoding rat thyrotropin releasing hormone. J Biol Chem 263:16604–16609
Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjorbaek C, Elmquist JK, Flier JS, Hollenberg AN (2001) Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest 107:111–120
Diamond MI, Miner JN, Yoshinaga SK, Yamamoto KR (1990) Transcription factor interactions selectors of positive or negative regulation from a single DNA element. Science 249:1266–1272
Yang-Yen HF, Chambard JC, Sun YL, Smeal T, Schmidt TJ, Drouin J, Krin M (1990) Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell 62:1205–1215
Pearce D, Matsui W, Miner J, Yamamoto KR (1998) Glucocorticoid receptor transcriptional activity determined by spacing of receptor and nonreceptor DNA sites. J Biol Chem 273:30081–30085
Spitaler M, Cantrell DA (2004) Protein kinase C and beyond. Nat Immunol 5:785–790
Schonwasser DC, Marais RM, Marshall CJ, Parker PJ (1998) Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol 18:790–798
Ismaili N, Garabedian MJ (2004) Modulation of glucocorticoid receptor function via phosphorylation. Ann NY Acad Sci 1024:86–101
Krstic MD, Rogatsky I, Yamamoto KR, Garabedian MJ (1997) Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol Cell Biol 17:3947–3954
Miller AL, Webb MS, Copik AJ, Wang YX, Johnson BH, Kumar R, Thompson EB (2005) p38 mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: Correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol Endocrinol 19:1569–1583
Revest JM, Di Blasi F, Kitchener P, Rougé-Pont F, Desmendt A, Turiault M, Tronche F, Piazza PV (2005) The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat Neurosci 8:664–672
Kassel O, Herrlich P (2007) Crosstalk between the glucocorticoid receptor and other transcription factors: molecular aspects. Mol Cell Endocrinol 275:13–29
Lasa M, Abraham SM, Boucheron C, Saklatvala J, Clark AR (2002) Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol Cell Biol 22:7802–7811
Clark AR, Lasa M (2003) Crosstalk between glucocorticoids and mitogen-activated protein kinase signaling pathways. Curr Opin Pharmacol 3:404–411
Dickinson RJ, Keyse SM (2006) Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci 119:4607–4615
Grewal JS, Randall DY, Stork P (1999) Extracellular-signal-regulated kinase signaling in neurons. Curr Opin Neurobiol 9:544–553
Joseph-Bravo P, Pérez-Martínez L, Lezama L, Morales-Chapa C, Charli JL (2002) An improved method for the expression of TRH in serum-supplemented primary cultures of fetal hypothalamic cells. Brain Res Protoc 9:93–104
Paxinos G, Ashwell KWS, Tork I (1995) Atlas of the developing rat nervous system, Second edn. Academic Press, Sydney, p xi
Meszaros JG, Roberth R, Lio FM, Brunton L (2000) Protein kinase C contributes to desensitization of ANG II signaling in adult rat cardiac fibroblast. Am J Physiol Cell Physiol 279:C1978–C1985
Isonishi S, Ohkawa K, Tanaka T, Howell SB (2000) Depletion of protein kinase C (PKC) by 12-O-tetradecanoylphorbol-13-acetate (TPA) enhances platinum drug sensitivity in human ovarian carcinoma cells. Can Res Camp 82:34–38
Wang D, Yu X, Brecher P (1998) Nitric acid and N-acetylcysteine inhibit the activation of mitogen-activated protein kinases by angiotensin II in rat cardiac fibroblasts. J Biol Chem 273:33027–33034
Watters J, Cambell J, Cunninham M, Krebs E, Dorsa D (1997) Rapid membrane effects of steroids in neuroblastoma cells: effects of estrogen on mitogen activated protein kinase signaling cascade and c-fos immediate early gene transcription. Endocrinology 138:4030–4033
Deak M, Clifton A, Lucocq J, Alessi D (1998) Mitogen- and stress-activated protein kinase-1(MSK1) is directly activated by MAPK and SAPK/p38 and may mediate activation of CREB. EMBO J 17:4426–4441
O’Carrol AM, Lolait SJ, Howell GM (2006) Transcriptional regulation of the rat apelin receptor gene: promoter cloning and identification of and Sp1 site necessary for promoter activity. J Mol Endocrinol 36:221–235
Olsson AK, Vadhammar K, Nanberg E (2000) Activation and protein kinase C-dependent nuclear accumulation of ERK in differentiating human neuroblastoma cells. Exp Cells Res 256:454–467
de Gortari P, Uribe RM, Garcia-Vázquez A, Aguilar-Valles A, Martínez A, Valdes A, Charli JL, Fernandez-Guardiola A, Joseph-Bravo P (2006) Amygdala kindling differentially regulates the expression of the elements involved in TRH transmission. Neurochem Int 48:31–42
Atkinson T, Smith M (1984) Solid-phase synthesis of oligodeoxyribo-nucleotides by the phosphite-triester method. In: Gait MJ (ed) Oligonucleotide synthesis a practical approach. Practical approach series. IRL Press, Oxford Washington DC, EEUU
Pedraza-Alva G, Zingg JM, Jos JP (1994) AP-1 binds to a putative cAMP response element of the MyoD1 promoter and negatively modulates MyoD1 expression in dividing myoblast. J Biol Chem 269:6978–6985
Persaud SJ, Jones PM, Howell SL (1993) Staurosporine inhibits protein kinases activated by Ca2+ and cyclic AMP in addition to inhibiting protein kinase C in rat islets of Langerhans. Mol Cell Endocrinol 94:55–60
Dahlman-Wright K, Wright A, Gustafsson JA, Carlstedt-Duke J (1991) Interaction of the glucocorticoid receptor DNA-binding domain with DNA as a dimer is mediated by a short segment of five amino acids. J Biol Chem 266:3107–3112
Pace TWW, Hu F, Miller AH (2006) Cytokine-effects of glucocorticoid receptor function: relevance of glucocorticoid resistence and the pathophysiology and treatment of major depression. Brain Behav Immun 21:9–19
de Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475
Chrousos GP, Kino T (2005) Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE 304:1–6
Webster CJ, Cidlowski AJ (1999) Mechanisms of glucocorticoid-receptor-mediated repression of gene expression. Trends Endocrinol Metab 10:396–402
Szatmary Z, Garabedian MJ, Vilcek J (2004) Inhibition of glucocorticoid receptor-mediated transcriptional activation by p38 mitogen-activated protein (MAP) kinase. J Biol Chem 279:43708–43715
Miller AL, Garza AS, Johnson BH, Thompson EB (2007) Pathway interactions between MAPK, mTOR, PKA, and the glucocorticoid receptor in lymphoid cells. Cancer Cell Int 7:3
Basta-Kaim A, Budziszewaska B, Jawarska-Feil L, Leskiewicz M, Tetich M, Otczyk M, Kubera M, Lason W (2006) Effects of neurosteroids on glucocorticoid receptor-mediated gene transcription in LMCAT cells- a possible interaction with psychotropic drugs. Eur Neuropsychopharmacol 17:37–45
David S, Kalb RG (2005) Serum/glucocorticoid-inducible kinase can phosphorylate the cyclic AMP response element binding protein, CREB. FEBS Lett 579:1534–1538
Heck S, Kullmann M, Gast A, Ponta H, Rahmsdorf HJ, Herrlich P, Cato AC (1994) A distinct modulating domain in glucocorticoid receptor monomer in the repression of activity of the transcription factor AP-1. Embo J 13:4087–4095
Mizuno H, Nishida E (2001) The ERK MAP kinase pathway mediates induction of SGK (serum- and glucocorticoid-inducible kinase) by growth factors. Genes Cells 6:261–268
Dallman MF, Warne JP, Foster MT, Pecoraro NC (2007) Glucocorticoids and insulin both modulate caloric intake through actions on the brain. J Physiol 583:431–436
Chalmers CJ, Gilley R, March HN, Balmanno K, Cook SJ (2007) The duration of ERK 1/2 activity determines the activation of c-Fos and Fra-1 and the composition and quantitative transcriptional output of AP-1. Cell Signal 19:695–704
Hu Y, Peng J, Feng D, Chu L, Li X, Jin Z, Lin Z, Zeng Q (2006) Role of extracellular signal-regulated kinase, p38 kinase, and activator protein-1 in transforming growth factor-β1-induced alpha smooth muscle actin expression in human fetal lung fibroblast in vitro. Lung 184:33–42
Charli JL, Cruz C, Redondo JL, Guerra C, Joseph-Bravo P (1995) Homologous conditioned medium enhances expression of TRH in hypothalamic neurons in primary culture. Dev Brain Res 89:155–160
Impey S, Goodman RH (2001) CREB signaling: time is everything. Sci STKE 82:PE1
Imai E, Miner JN, Mitchell JA, Yamamoto KR, Granner DK (1993) Glucocorticoid receptor-cAMP response element-binding protein interaction and the response of the phosphoenolpyruvate carboxykinase gene to glucocorticoids. J Biol Chem 268:5353–5356
Doucas V, Shi Y, Miyamoto S, West A, Verma I, Evans RM (2000) Cytoplasmic catalytic subunit of protein kinase A mediates cross-repression by NF-kappa B and the glucocorticoid receptor. PNAS 97:11893–11898
Kumar R, Thompson EB (2005) Gene regulation by the glucocorticoid receptor: structure: function relationship. J Ster Biochem and Mol Biol 94:383–394
Onda K, Nagashima M, Kawakubo Y, Inoue S, Hirano T, Oka K (2006) Mitogen-activated protein kinase kinase 1/extracellular signal-regulated kinase (MEK-1/ERK) inhibitors sensitize reduced glucocorticoid response mediated by TNF alpha in human epidermal keratinocytes (HaCaT). Biochem Biophys Res Comm 351:266–272
Buse P, Maiyar AC, Failor KL, Tran S, Leong MLL, Firestone GL (2007) The stimulus-dependent co-localization of serum- and glucocorticoid-regulated protein kinase (Sgk) and Erk/MAPK in mammary tumor cells involves the mutual interaction with the importin-alpha nuclear import protein. Exp Cell Res 313:3261–3275
Joseph-Bravo P, Cote-Vélez A, Pérez-Martínez L (2006) Integration of neuroendocrine signals that regulate the activity of hypophysiotropic peptides. In: Joseph-Bravo P (ed) Molecular endocrinology. Research Signpost Ed, Kerala, India, pp 1–24
Guerra-Crespo M, Ubieta R, Joseph-Bravo P, Charli JL, Pérez-Martínez L (2001) BDNF increases the early expression of TRH rnRNA in fetal TrkB(+) hypothalamic neurons in primary culture. Eur J Neurosci 14:483–494
Ubieta R, Uribe RM, González JA, García-Vázquez A, Pérez-Monter C, Pérez-Martínez L, Joseph-Bravo P, Charli JL (2007) BDNF up-regulates pre-pro-TRH mRNA expression in the fetal/neonatal paraventricular nucleus of the hipothalamus. Properties of the transduction pathway. Brain Res 1174:28–38
Tapia-Arancibia L, Rage F, Givalois L, Arancibia S (2004) Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol 25:77–107
Gutiérrez-Mariscal M, de Gortari P, López-Rubalcava C, Martínez A, Joseph-Bravo P (2008) Analysis of the anxiolytic-like effect of TRH and the response of amigdalar TRHergic neurons in anxiety. Psychoneuroendocrinology 33:198–213
Jaimes-Hoy L, Joseph-Bravo P, de Gortari P (2008) Differential response of TRHergic neurons of the hipothalamic paraventricular nucleus (PNV) in animals submitted to food-restriction or dehydration-induced anorexia and cold exposure. Horm Behav 53:366–377
Acknowledgements
We thank the technical support of F. Romero, M Villa, E. Martel, and E. Bustos, as well as S. González for animal care, and S. Ainsworth for bibliographic assistance. This work was supported by grants from CONACYT 43503 and DGAPA-UNAM 215507.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue article in honor of Dr. Ricardo Tapia.
Rights and permissions
About this article
Cite this article
Cote-Vélez, A., Pérez-Martínez, L., Charli, JL. et al. The PKC and ERK/MAPK Pathways Regulate Glucocorticoid Action on TRH Transcription. Neurochem Res 33, 1582–1591 (2008). https://doi.org/10.1007/s11064-008-9698-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9698-5