Abstract
The anti-neoplastic drug taxol binds to β-tubulin to prevent tumor cell division, promoting cell death. However, high dose taxol treatment may induce cell death in normal cells too. The anti-apoptotic molecule Bcl-2 is upregulated in many cancer cells to protect them from apoptosis. In the current study, we knocked down Bcl-2 expression using cognate siRNA during low-dose taxol treatment to induce apoptosis in two human glioblastoma U138MG and U251MG cell lines. The cells were treated with either 100 nM taxol or 100 nM Bcl-2 siRNA or both for 72 h. Immunofluorescent stainings for calpain and active caspase-3 showed increases in expression and co-localization of these proteases in apoptotic cells. Fluorometric assays demonstrated increases in intracellular free [Ca2+], calpain, and caspase-3 indicating augmentation of apoptosis. Western blotting demonstrated dramatic increases in the levels of Bax, Bak, tBid, active caspases, DNA fragmentation factor-40 (DFF40), cleaved fragments of lamin, fodrin, and poly(ADP-ribose) polymerase (PARP) during apoptosis. The events related to apoptosis were prominent more in combination therapy than in either treatment alone. Our current study demonstrated that Bcl-2 siRNA significantly augmented taxol mediated apoptosis in different human glioblastoma cells through induction of calpain and caspase proteolytic activities. Thus, combination of taxol and Bcl-2 siRNA offers a novel therapeutic strategy for controlling the malignant growth of human glioblastoma cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma is the most malignant primary brain tumor, which is hardly amenable to conventional therapies. The highly invasive nature of glioblastomas makes them highly resistant to the conventional treatment strategies [26]. The prognosis of patients diagnosed with this brain cancer is very poor, because patients usually do not survive more than 9–12 months even after mass-reductive surgery, radiation therapy, and chemotherapy [16]. Since malignant brain tumors remain refractory, the current treatment approaches produce no long-term survivors of these tumors. The traditional means of cancer therapy are plagued with numerous side effects and subsequent poor quality of life in course of treatment. Since the current conventional therapeutic strategies fail to address the inherent biologic properties of brain tumors, it is important to develop new treatment modalities incorporating conventional chemotherapy and emerging gene therapy.
Apoptosis is regulated by a series of complex biochemical events that are controlled by an evolutionarily conserved program. Dysregulation of apoptotic mechanisms plays important role in the pathogenesis and progression of various cancers and also poor responses of tumors to therapeutic interventions. Highly invasive cancer cells are protected from apoptosis by upregulation of various anti-apoptotic molecules including B cell lymphoma-2 (Bcl-2) protein.
Taxol (also known as paclitaxel) is a novel chemotherapeutic drug, which is widely used in various cancers including glioblastoma [30]. Taxol strongly binds to the β-subunit of tubulin, the building block of microtubules, to affect microtubule dynamics in the highly replicating cancer cells [7]. The dynamic instability of microtubules affects the positioning of chromosomes during replication and finally inhibits cell division. Taxol also induces apoptosis in a wide spectrum of cancer cells by caspase-dependent and caspase-independent mechanisms [10, 22, 24]. Taxol mediated chemotherapy may be an effective treatment option for aggressive brain tumors because it inhibits cell division by preventing microtubular restructuring to induce cell death. However, taxol at a high dose also inhibits normal cell division and causes undesirable side effects in patients. Use of taxol at a low dose, which inhibits only the fast dividing tumor cells, would be highly desirable to cause less toxicity to healthy normal cells.
Taxol induces death signal in mitochondria through Bax, which modulates permeability of the mitochondrial membrane [25]. Taxol also exerts cellular stress on endoplasmic reticulum, which in turn increases intracellular free [Ca2+] [1]. Increase in intracellular free [Ca2+] upregulates m-calpain, which in turn may cleave Bid to generate truncated Bid (tBid) [12]. Active subunits of both caspase-8 and caspase-3 also generate tBid [29]. This facilitates the mitochondrial release of cytochrome c that eventually associates with apoptotic protease activating factor-1 (Apaf-1) in the presence of ATP to induce Apaf-1 oligomerization, which then binds procaspase-9 and forms an ‘apoptosome’ containing Apaf-1, cytochrome c, and procaspase-9 [33]. This complex initiates auto cleavage at Asp315 of procaspase-9 (47 kDa) to generate a large active caspase-9 fragment (37 kDa) and small fragment (10 kDa) [33]. Procaspase-9 and procaspase-3 are also cleaved into active subunits by calpain [20]. Active caspase-9 processes other caspases, including caspase-3 and caspase-7 leading to apoptosis [28]. Active caspase 3 then cleaves DNA fragmentation factor-45 (DFF45) and PARP in course of apoptotic execution of the cells.
RNA interference (RNAi) through small interfering RNA (siRNA) is a powerful tool to knockdown the transcriptional expression and thereby the protein expression of the specific gene [5, 8]. The siRNA mediated gene knockdown, post-transcriptional gene silencing initiated by a synthetic double stranded RNA (dsRNA) molecule, is a highly sequence-specific process. The anti-apoptotic molecule Bcl-2 is upregulated in highly invasive brain tumor cells in order to protect them from apoptosis. Overexpression of Bcl-2 provides a survival advantage to cancer cells in response to a wide range of apoptotic stimuli through inhibition of mitochondrial cytochrome c release [15, 17]. Furthermore, Bcl-2 protects cells against taxol mediated apoptosis by inducing multi-nucleation [21]. Introduction of Bcl-2 siRNA into the cells triggers degradation of endogenous Bcl-2 mRNA so as to down regulate the cognate protein level.
The aim of our current study was to knockdown Bcl-2 level using gene-specific siRNA during a low dose taxol treatment to increase apoptosis in a relatively efficient manner in human glioblastoma cells. We used two highly invasive human glioblastoma U138MG and U251MG cell lines to test our therapeutic strategy.
Materials and Methods
Cell Cultures and Treatments
Human glioblastoma U138MG cell line was purchased from American Type Culture Collection (ATCC) (Manassas, VA). Another human glioblastoma U251MG cell line was procured from National Cancer Institute (Bethesda, MD). For experiments, U138MG and U251MG cells were propagated in DMEM and RPMI-1640 media (Mediatech, Herndon, VA), respectively, supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA) and selected antibiotics in an incubator containing 100% humidity and 5% CO2 at 37°C. Taxol (paclitaxel, 6 mg/ml) was procured from Bristol-Myers Squibb (Princeton, NJ). It was diluted to make 1 mM solution with dimethyl sulfoxide (DMSO) and protected from light. Taxol was further diluted in serum-free media. The human-specific Bcl-2 siRNA kit was procured from Cell Signaling Technology (Danvers, MA). The transfection reagent, designed specifically for highly efficient siRNA delivery into mammalian cells, was supplied by Mirus Bio Corporation (Madison, WI). The transfection efficiency was monitored using a fluorescent conjugated non-targeted control siRNA duplex (Cell Signaling Technology, Danvers, MA). Cells were treated with a final concentration of 100 nM taxol alone or 100 nM Bcl-2 siRNA alone or both together in culture media containing 1% FBS. The concentration of 100 nM taxol was selected after a dose response study for induction of apoptosis, as analyzed by the Terminal deoxynucleotidyl Transferase mediated dUTP Nick-End Labeling (TUNEL) assay. The amount of Bcl-2 siRNA was selected as recommended by the manufacturer, which results in optimum silencing of cognate mRNA. Also, both U138MG and U251MG cells were transfected with a non-targeted negative control siRNA duplex (Cell Signaling Technology, Danvers, MA) and analyzed for apoptosis using TUNEL assay. After 24 h, the low serum medium was replaced with the regular serum-containing medium and the cells were incubated for another 48 h. After treatments, cells were scraped and cell lysates were prepared as described below. Western blotting was performed using lysates for Bcl-2 protein to make sure that the Bcl-2 protein was down regulated after treatment with Bcl-2 siRNA.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) for Examining Level of Bcl-2 mRNA
The RT-PCR experiment was carried out to ensure the down regulation of Bcl-2 mRNA expression after treatment with the cognate siRNA. Both U138MG and U251MG cells were cultured in 6-well chambers (Corning Life Sciences, Corning, NY) up to 80% confluency and treated with 100 nM taxol alone or transfected with 100 nM Bcl-2 siRNA alone or treated with both agents together. Total cellular RNA was isolated using the Aurum kit (Bio-Rad Laboratories, Hercules, CA). Gene-specific primers were designed using Beacon Designer software (Premier Biosoft International, Palo Alto, CA). We used the primer sequences for Bcl-2 gene (NM_000657; forward 5′-GTG TGG AGA GCG TCA ACC-3′, reverse 5′-TAC CCA GCC TCC GTT ATC C-3′) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (NM_002046; forward 5′-CCA CCC ATG GCA AAT TCC-3′, reverse 5′-CAG GAG GCA TTG CTG ATG AT-3′). The primers were transcribed with 300 ng of total RNA using a one-step RT-PCR kit (Invitrogen, Carlsbad, CA) in a Mastercycler gradient (Eppendorf, Westbury, NY) under the following reaction conditions: cDNA synthesis (50°C for 30 min), inactivation of reverse transcriptase (94°C for 2 min), PCR amplification of target message for 35 cycles (denaturing at 94°C for 20 s, annealing at 58°C for 30 s, and chain extension at 72°C for 45 s; and a final chain extension at 72°C for 10 min). The amplified RT-PCR products were separated on 1.5% agarose gels containing ethidium bromide (1 μg/ml) and visualized using a UV transilluminator (Alpha Innotech Corporation, San Leandro, CA). The expression of the housekeeping gene GAPDH was used as an internal standard.
Double Immunofluorescent Stainings for Calpain and Active Caspase-3
Parental U138MG and U251MG cells were cultured on the 4-well chamber slides (Lab-Tek, Nalge Nunc International, Rochester, NY) at a density of 1 × 104 cells per well. After 24 h, cells were treated with 100 nM taxol alone or 100 nM Bcl-2 siRNA alone or combination of both agents. All treatments were terminated at 72 h and the cells were fixed in 95% ethanol (cold) for 15 min. Cells were then washed twice in PBS and blocked with 2% goat and 2% donkey sera in PBS (1:1, v/v) for 1 h. The cells were then treated with 1:100 diluted (in blocking solution) rabbit polyclonal anti-human m-calpain (raised and purified in our laboratory) and 1:100 diluted (in blocking agent) goat polyclonal anti-human active caspase-3 (Santa Cruz Biotechnology, Santa Cruz, CA) primary IgG antibodies simultaneously and incubated overnight at 4°C. The cells were then washed three times in PBS and incubated with fluorescein isothiocyanate (FITC) conjugated goat anti-rabbit (Biomeda, Foster City, CA) and Texas Red conjugated donkey anti-goat (Biomeda, Foster City, CA) secondary IgG antibodies (diluted in the blocking solution) at room temperature for 1 h. The cells were further washed in PBS, mounted with Vectashield (Vector Laboratories, Burlingame, CA), and examined under a fluorescent microscope (Olympus BH2, Tokyo, Japan) using green and dsRed filters for examining the expression of calpain and active caspase-3, respectively. The fluorescent photographs were then merged electronically using Photoshop software (Adobe Systems. Seattle, WA). For demonstration, the double immunofluorescent staining data were compiled only for U251MG cells.
TUNEL Staining
The fluorometric TUNEL staining is a widely used for the detection and quantitation of apoptotic cells within a cell population. The parental U138MG and U251MG cells were cultured on the 4-well chamber slides (Lab-Tek, Nalge Nunc International, Rochester, NY) at a density of 5 × 103 cells per well. After 24 h, the cells were treated with 100 nM taxol alone or 100 nM Bcl-2 siRNA alone or both together. The treatments were terminated at 72 h and the cells were fixed in 95% cold ethanol for 15 min. TUNEL staining was performed for detection of apoptotic cells using the fluorometric TUNEL staining kit (Promega Corporation, Madison, WI). In brief, the fixed cells were washed twice in PBS and permeabilized with 0.2% Triton X-100 for 5 min. The cells were further washed twice in PBS, equilibrated with the equilibration buffer (supplied with the kit) and incubated with 50 μl of recombinant TdT- fluorescein-12-dUTP cocktail for 1 h at 37°C in a humidified chamber. The reaction was terminated and slides were washed 3 times in PBS and mounted with anti-fading mount gel (Biomeda, Foster City, CA). Slides were allowed to dry in the dark before being observed under a fluorescent microscope (Olympus BH2, Tokyo, Japan) and photographed. Fluorescent apoptotic cells were quantitatively evaluated (10 randomly selected microscopic fields per sample) using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). For demonstration, TUNEL staining data were compiled only for U251MG cells.
Determination of Intracellular Free [Ca2+] Using Fura-2 Assay
The levels of intracellular free [Ca2+] were measured in U138MG and U251MG cells after treatment with taxol or Bcl-2 siRNA or both together. Fura-2 is a ratiometric fluorescent dye, which binds to intracellular free Ca2+. Fura-2 is excited at 340 nm (when bound to Ca2+) or 380 nm (when not bound to Ca2+) and the ratio of the emissions at these wavelengths can be used to calculate intracellular free [Ca2+]. Both U138MG and U251MG parental cells were cultured in 6-well plates (Corning Life Sciences, Corning, NY) at a density of 2 × 105 cells per well. After 24 h, the cells were treated with 100 nM taxol alone or 100 nM Bcl-2 siRNA alone or combination of both in media containing 1% FBS. The cells were harvested using TrypLE Express (Invitrogen, Carlsbad, CA) at 72 h after treatments and washed once with Locke’s buffer (154 mM NaCl, 5.6 mM KCl, 3.4 mM NaHCO3, 1.2 mM MgCl2, 5.6 mM glucose, 5 mM HEPES, pH 7.4). The cells were suspended in 0.5 ml of Locke’s buffer and counted. The volume of the cell preparations was adjusted to have 1 × 106 cells/ml. Fura-2 AM (50 μg, Molecular Probes, Eugene, OR) was dissolved in 50 μl of DMSO and loaded in cells at 5 μM. The cells were incubated at 37°C with constant rotation for about 45 min in the dark. The tubes were transferred to ice and centrifuged at 2000 rpm for 5 min at 4°C. The cells were washed twice with Locke’s buffer at 4°C. Finally, the cells were suspended in ice-cold Locke’s buffer at 1.5 × 106 cells/ml. In triplicate, exactly 200 μl of the cell preparation (3 × 105 cells) was transferred to a 96-well plate. The fluorescence intensity was measured on a SpectraMax Gemini XPS microplate spectrofluorometer (Molecular Devices, Sunnyvale, CA) with dual excitations at 340 nm and 380 nm and emission at 510 nm at 37°C. The plate was removed from the reader, and 25 μl of 250 μM digitonin (Sigma, St. Louis, MO) was added and read again to determine the Rmax. The plate was again removed and 25 μl of 50 mM ethylene glycol tetraacetic acid (EGTA, Sigma, St. Louis, MO) was added to a final concentration of 5 mM. The plate was again read to determine the Rmin. The intracellular free [Ca2+] is calculated as described previously [3] using the following equation: [Ca2+] = K d Q(R−R min)/(R max−R), where R represents the fluorescence intensity Fλ1/Fλ2 (λ1 = 340 nm and λ2 = 380 nm are the fluorescence detection wavelengths for the ion-bound and ion-free fura-2, respectively). R max and R min are ratios of maximum and minimum fluorescences obtained after treatment with digitonin and EGTA, respectively. Q is the ratio of Fmax/Fmin at λ2 (380 nm). The dissociation constant (K d ) of fluorescent Ca2+ was determined using a calcium calibration buffer kit with magnesium (Molecular Probes, Eugene, OR). For demonstration, intracellular free [Ca2+] data were compiled only for U251MG cells.
Fluorometric Assay for Calpain Activity
Fluorometric assay was carried out to measure the enzymatic activity of total calpain after treating U138MG and U251MG cells with taxol alone or Bcl-2 siRNA or both together. The assay is based on the cleavage of fluorogenic substrate by calpain around the scissile amide bond and the release of the flurophore, resulting in increased fluorescence. Both U138MG and U251MG cells were cultured in 6-well plates (Corning Life Sciences, Corning, NY) at a density of 2 × 105 cells per well. After 24 h, the cells were treated with 100 nM taxol alone or 100 nM Bcl-2 siRNA alone or combination of both agents in culture media containing 1% FBS. The cells were collected at 72 h after the treatments and cell lysates were prepared. Protein concentrations of the cell lysates were determined using Coomassie plus protein assay reagents (Pierce Biotechnology, Rockford, IL). Calpain activity was assayed using a fluorometric assay kit (Calbiochem, EMD Biosciences, San Diego, CA). The final fluorescence was measured on a spectrofluorometer (Molecular Devices, Sunnyvale, CA) at an excitation of 320 nm and emission at 480 nm. The assay was carried out using samples in triplicate and the results were presented as percent change in total calpain activity, compared with controls. For demonstration, calpain activity data were compiled only for U251MG cells.
Fluorometric Assay for Caspase-3 Activity
The fluorometric caspase-3 activity assay was performed using a kit (Sigma, St. Louis, MO). This fluorometric assay is based on the hydrolysis of the peptide acetyl-Asp-Glu-Val-Asp-7-amido-4-methylcoumarin (Ac-DEVD-AMC) by caspase-3, resulting in release of the fluorescent AMC moiety. The cells were cultured and treated as described above. The cells were harvested using TrypLE Express (Invitrogen, Carlsbad, CA) and washed once with PBS. The cell pellets were suspended in 1xlysis buffer (supplied with the kit) at a concentration of 500 μl per 107 cells, incubated on ice for 20 min and centrifuged at 14,000g for 15 min at 4°C. Then, 5 μl supernatant was added to a microplate well followed by 200 μl of reaction mixture (5 μl of 10 mM Ac-DEVD-AMC + 3 ml of 1× assay buffer). The contents were mixed gently and incubated at room temperature in dark for 1 h. The final fluorescence was measured in a spectrofluorometer (Molecular Devices, Sunnyvale, CA) with an excitation at 360 nm and emission at 460 nm. The assay was carried out using four independent samples in triplicate. The results were presented as percent change in caspase-3 activity, compared with controls. For demonstration, caspase-3 activity data were compiled only for U251MG cells.
Western Blotting to Examine Calpain and Caspase Mediated Apoptotic Signaling Pathways
Preparation of Cell Lysate
Parental U138MG and U251MG cells were cultured in 100 mm plates (Corning Life Sciences, Corning, NY) at a density of 1 × 106 cells per plate. After 24 h, the cells were treated with 100 nM taxol alone or transfected with 100 nM Bcl-2 siRNA alone or combination of both treatments in media containing 1% FBS. All the cultures were terminated at 72 h after the treatments. The cells were washed twice with ice-cold PBS and scraped with 1 ml of freshly prepared radio-immunoprecipitation assay (RIPA) buffer with protease inhibitors (50 mM Tris–HCl, pH 7.4, containing 1% Nonidet P-40, 150 mM NaCl, 1 mM sodium orthovanadate (activated), 1 mM sodium fluoride, 1 mM PMSF, 1 mM EDTA, 5 μg/ml aprotinin, and 5 μg/ml pepstatin). Cells were centrifuged at 12,000 rpm for 10 min at 4°C in an Eppendorf centrifuge. The supernatant was discarded and the cell pellet was suspended in RIPA buffer with protease inhibitors according to the size of the pellet and sonicated gently in a micro-ultrasonic cell disruptor (Kontes, Vineland, NJ). Cell lysates were centrifuged at 14,000 rpm for 10 min at 4°C and the supernatants were collected. The protein concentration in the supernatant was determined using Coomassie plus protein assay reagents (Pierce Biotechnology, Rockford, IL). The samples were stored at −20°C until used. Mitochondria were isolated from the total cell lysate using the mitochondria isolation kit for cultured cells (Pierce Biotechnology, Rockford, IL), and the cytosolic fraction was used to determine the released cytochrome c levels. The nuclear fraction was extracted from the cultured cells using the nuclear extraction kit (Panomics, Fremont, CA) and the Western blotting was performed for the nuclear protein poly (ADP-ribose) polymerase (PARP) using the nuclear fraction.
Western Blotting
Protein concentrations ranging from 5 to 100 μg were used for Western blotting of various molecules involved in the calpain and caspase mediated apoptotic signaling pathways. The protein samples were mixed with 6× loading buffer containing 600 mM dithiothreitol (DTT) to obtain a final concentration 100 mM DTT. The samples were kept in a boiling water bath for 3 min, instantly cooled in ice-water and loaded onto precast 4–20% polyacrylamide gradient gels (Bio-Rad Laboratories, Hercules, CA). A 5% polyacrylamide gel was used for resolving α-fodrin. The resolved proteins were electroblotted to an activated polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA). After transfer, the non-specific binding sites were blocked with 5% non-fat dry milk for 30 min at room temperature. The membranes were then incubated overnight at 4°C on a rocker with either polyclonal or monoclonal human specific antibodies for various protein molecules. All antibodies were diluted at either 1:1000 or as per supplier’s instructions in 5% non-fat dry milk. The antibodies for caspases-8, caspase-6 and caspase-3, Bax, Bak, Bid, and cytochrome c oxidase 4 (COX4) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); antibodies for Bcl-2, Apaf-1, caspase-7, cleaved lamin A and PARP were procured from Cell Signaling Technology (Danvers, MA); antibody for α-fodrin was obtained from Biomol International (Plymouth Meeting, PA); DNA fragmentation factor-40 (DFF40) antibody was purchased from Chemicon International (Temecula, CA); caspase-9 and cytochrome c antibodies were from BD Biosciences (San Jose, CA) and the m-calpain antibody was raised and purified in our laboratory. After incubation, the membranes were washed three times in 0.05% Tween-20 and treated with either 1:500 or 1:2000 (depending on dilution of primary antibody) anti-rabbit or anti-mouse horseradish peroxidase (HRP)-conjugated secondary IgG antibody (Biomeda, Foster City, CA) at room temperature for 1–2 h. The membranes were then washed three times in 0.05% Tween-20 and treated with enhanced chemiluminescence (ECL) reagents (Amersham Biosciences, Buckinghamshire, UK). Finally, the membranes were exposed to autoradiography film (BioMax XAR, Kodak, New Haven, CT) and developed. The intensity of the bands on the film was adjusted appropriately with exposure for different times. The membranes were reprobed using Western reprobe buffer (Gbiosciences, St. Louis, MO) and Western blotting was performed using mouse monoclonal GAPDH antibody (Novus Biologicals, Littleton, CO) to demonstrate that equal amount of protein was loaded in each lane. In case of cytochrome c, the membranes were reprobed for COX4 to monitor equal loading of mitochondrial fraction.
Statistical Analysis
Arithmetic mean and standard deviation (SD) were calculated using one-way analysis of variance (ANOVA). The control mean values were compared with taxol alone or Bcl-2 siRNA alone mean values using the least significant difference method. The taxol alone or Bcl-2 siRNA alone mean values were also compared with the combination treatment values. The difference between two values was considered statistically significant at P < 0.05.
Results
Dose-response Studies and Effect of Non-targeted siRNA Duplex in Glioblastoma Cells
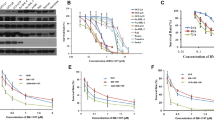
The dose-response studies were conducted using taxol alone and in combination with Bcl-2 siRNA to monitor the extent of apoptosis in glioblastoma cells, as examined by TUNEL staining (Fig. 1). We found that 100 nM taxol was optimum to induce apoptosis without necrosis in U138MG cells (Fig. 1, panel a) as well as in U251MG cells (Fig. 1, panel b). The treatment with the combination of 100 nM taxol and 100 nM Bcl-2 siRNA resulted in around 60% apoptosis in both U138MG cells (Fig. 1, panel c) and U251MG cells (Fig. 1, panel d). Therefore, we decided to use 100 nM taxol and 100 nM Bcl-2 siRNA in combination for further studies. Transfections with a non-targeted siRNA duplex as negative control in both cell lines did not result in any significant increase in apoptosis, based on TUNEL staining.
Dose-response studies for determination of amounts of apoptosis in U138MG and U251MG cells. (a and c) Treatment of cells with 25, 50, and 100 nM taxol and then TUNEL staining for determination of amounts of apoptotic cells (*P < 0.001, mean ± SD, n = 4). (b and d) Treatment of cells with combination of 25, 50, or 100 nM taxol and Bcl-2 siRNA and then TUNEL staining for determination of amounts of apoptotic cells (*P < 0.001, mean ± SD, n = 4)
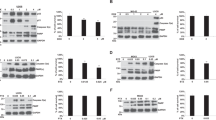
siRNA Down Regulated Bcl-2 at mRNA and Protein Levels in Glioblastoma Cells
The anti-apoptotic molecule Bcl-2 is frequently upregulated in glioblastoma cells in order to block mitichondrial release of cytochrome c into the cytosol and thus protect the tumor cells from apoptosis. We examined the consequences of our treatments on Bcl-2 expression at mRNA and protein levels in glioblastoma U138MG and U251MG cells (Fig. 2). Our RT-PCR experiments (Fig. 2, panel a) demonstrated almost 80% knockdown of Bcl-2 mRNA expression in cells after transfection with the cognate siRNA. Western blotting (Fig. 2, panel b) demonstrated that transfection of Bcl-2 siRNA resulted in about 80% down regulation of Bcl-2 protein expression in both cell lines. There was no significant decrease in Bcl-2 expression in either cell line treated with taxol alone. Transfections with a non-targeted siRNA duplex as negative control did not result in any significant alteration in Bcl-2 expression at mRNA and protein levels in both U138MG and U251MG cells. We decided to discontinue the use of non-targeted siRNA duplex as negative control in the subsequent experiments.
Examination of levels of Bcl-2 expression in U138MG and U251MG cells after the treatments. (a) RT-PCR for levels of Bcl-2 mRNA expression in the cells after treatment with 100 nM taxol or 100 nM Bcl-2 siRNA or both. The level of GAPDH mRNA expression was used as an internal control. (b) Western blotting for levels of Bcl-2 protein expression in the cells after treatment with 100 nM taxol or 100 nM Bcl-2 siRNA or both. The blots were reprobed for GAPDH to monitor loading of equal amounts of protein in all lanes
Double Immunofluorescent Staining Revealed Increases in Expression of Calpain and Caspase-3 in Course of Apoptosis
In order to demonstrate the increased expression of calpain and active caspase-3 in apoptosis, we used double immunofluorescent staining and TUNEL assay (Fig. 3). Use of an antibody specific for active caspase-3 eliminated the possibility of any cross-reaction with the inactive molecule. Double immunofluorescent staining demonstrated progressive increases in the expression of calpain and active caspase-3 after treatment of U251MG cells with taxol, Bcl-2 siRNA, or both agents together (Fig. 3, panel a). The staining was highly conspicuous after treatment of cells with the combination of taxol and Bcl-2 siRNA. The staining for calpain or active caspase-3 was not quite visible in the control cells. A major hallmark of apoptotic death is nuclear DNA fragmentation. We performed fluorescent TUNEL staining in order to determine the number of apoptotic cells after treatment with taxol, Bcl-2 siRNA, or both agents together (Fig. 3, panel b). The TUNEL staining was almost absent in control cells. Quantitative evaluation of the TUNEL stained cells using Image-Pro plus software revealed production of 25%, 29%, and 58% apoptotic cells after treatments with taxol, Bcl-2 siRNA, and combination of both, respectively (Fig. 3, panel c). The mean values of TUNEL stained cells in treatment with combination of taxol and Bcl-2 siRNA were significantly different (P < 0.001) when compared with mean values in treatment with taxol or Bcl-2 siRNA.
Double immunofluoresent staining and TUNEL staining to determine occurrence of apoptosis in glioblastoma U251MG cells. (a) Double immunofluorescent staining to examine the expression of m-calpain and active caspase-3. After treatments, cells were incubated with rabbit polyclonal m-calpain and goat polyclonal active caspase-3 primary antibodies simultaneously. Then, cells were incubated with FITC conjugated anti-rabbit and Texas red conjugated anti-goat secondary antibodies for detection of m-calpain and active caspase-3, respectively. Merged microphotographs demonstrated the simultaneous expression of m-calpain and active caspase-3 in apoptotic cells. (b) Fluorescent TUNEL staining for detection of apoptotic cells after the treatments. The treatment with combination of taxol and Bcl-2 siRNA resulted in more apoptotic death than either treatment alone. (c) Quantitation of TUNEL-positive cells using ImagePro-Plus software. Data were presented from 4 independent experiments in duplicate (*P < 0.001 when compared with the control mean values and # P < 0.001 when compared with taxol or Bcl-2 siRNA mean values)
Increases in Intracellular Free [Ca2+] and Activities of Calpain and Caspase-3
Fura-2 assay was used to determine the amounts of intracellular free [Ca2+] and spectrofluorometric assays to measure the activities of calpain and caspase-3 after the treatments (Fig. 4). Fura-2 AM is cell permeable whereas fura-2 is not. Intracellular esterases convert fura 2-AM to active fura 2, which then binds to the intracellular free Ca2+. We noticed significant increases in intracellular free [Ca2+] after treatment of U251MG cells with taxol, Bcl-2 siRNA, or combination of taxol and Bcl-2 siRNA (Fig. 4, panel a). The percent increase in intracellular free [Ca2+] after treatment with combination of taxol and Bcl-2 siRNA was significantly different (P < 0.001) from the mean value of either treatment alone. An increase in the intracellular free [Ca2+] could upregulate activity of the Ca2+-dependant protease calpain, which in turn could promote proteolysis leading to apoptotic death.
Determination of intracellular free [Ca2+] and activities of calpain and caspase-3 in U251MG cells after the treatments. The data were represented as percent changes (mean ± SD, n = 6, *P < 0.001 when compared with controls and # P < 0.001 when compared with taxol or Bcl-2 siRNA mean values). (a) Fura-2 assay for determining intracellular free [Ca2+]. (b) Fluorometric assay for total calpain activity. (c) Fluorometric assay for total caspase-3 activity
We also measured the activities of total calpain (Fig. 4, panel b) and caspase-3 (Fig. 4, panel c) using the sensitive spectrofluorometric assays after treating the U251MG cells with taxol, Bcl-2 siRNA, or both agents together. The total calpain activity was significantly (P < 0.001) higher in treatment with combination of taxol and Bcl-2 siRNA than either treatment alone (Fig. 4, panel b). An increase in the activity of calpain can cause cleavage of α-fodrin as well as cleavage of Bid to tBid, which promotes mitochondrial release of cytochrome c into the cytosol. Moreover, we observed a significant increase (P < 0.001) in the total activity of caspase-3 in U251MG cells treated with taxol and Bcl-2 siRNA together, compared with cells treated with either agent alone (Fig. 4, panel c). Caspase-3 is directly or indirectly responsible for cleavage of cellular key proteins, including the nuclear proteins PARP and DFF45, for apoptosis.
Upregulation of Calpain, Cleavage of Bid to tBid, and Mitochondrial Release of Cytochrome c
We performed Western analysis to determine upregulation of calpain expression and cleavage of Bid to tBid leading to mitochondrial release of cytochrome c into the cytosol in both U138MG and U251MG cells after the treatments (Fig. 5). Western blots for GAPDH expression demonstrated equal loading of cytosolic protein in all the samples (Fig. 5, panel a). In case of mitochondrial cytochrome c, the blots were reprobed for COX4 expression as an internal control in mitochondrial fractions (Fig. 5, panel a). Quantitation of the band intensities on the Western blots showed that transfection with Bcl-2 siRNA alone did not result in any significant increase in m-calpain levels (Fig. 5, panel b). There were no significant changes in the levels of Apaf-1 and Bak in the cells after transfection with Bcl-2 siRNA alone (data not shown). However, Bax and tBid levels were increased significantly in the cells treated with taxol alone, Bcl-2 siRNA, or both agents together (Fig. 5, panel b). Levels of cytosolic cytochrome c were significantly increased (P < 0.001) in cells after transfection with Bcl-2 siRNA and also after the treatment with combination of taxol and Bcl-2 siRNA (Fig. 5, panel b).
Western blotting for determining levels of m-calpain and other molecules leading to mitochondrial release of cytochrome c for apoptosis in U138MG and U251MG cells after the treatments. (a) Representative Western blots showing levels of m-calpain, Apaf-1, Bax, Bak, tBid, and cytochrome c (mitochondrial and cytosolic fractions). Mitochondria were isolated from the total cell lysate and the cytochrome c levels were examined in both mitochondrial and cytosolic fractions. Western blots were reprobed for COX4 and GAPDH to demonstrate that equal amounts of mitochondrial and cytosololic, respectively, proteins were loaded in all lanes. (b) Quantitative evaluation of the percent change in levels of m-calpain, Bax, tBid, and cytochrome c (cytosolic) in the cells. The Western blots were quantified using Image-J software. Data are representative of 3 independent experiments (*P < 0.001 when compared with controls and # P < 0.001 when compared with taxol or Bcl-2 siRNA mean values)
Increased Activity of Caspases and Apoptosis Executioner Molecules
An association of cytosolic cytochrome c with procaspase-9 and Apaf-1 forms apoptosome for generation of active caspase-9 that then initiates the intrinsic caspase pathway of apoptosis. We performed Western analysis to determine any increases in the cleaved (active) forms of caspases after treatment of cells with taxol alone, Bcl-2 siRNA alone, or both agents together (Fig. 6). The increases in cleaved caspases were highly conspicuous after treatment of cells with the combination of taxol and Bcl-2 siRNA (Fig. 6, panel a). Quantitation showed significant increases in cleaved caspase-3 in the cells after the treatments (Fig. 6, panel b). Notably, treatment with combination of taxol and Bcl-2 siRNA produced significantly more cleaved caspase-3 than either treatment alone (Fig. 6, panel b). Human DFF40 (usually sequestered with its molecular chaperone DFF45 in normal cells) is released in connection with caspase-3 activation in apoptotic cells [32]. The cleavages of lamin A and α-fodrin by activated caspase-6 and caspase-3, respectively, result in cellular instability and cell death [2, 27]. The 116 kDa PARP is a nuclear polymerase involved in DNA repair predominantly in response to environmental stress [23]. The cleavage of 116 kDa PARP to 89 kDa PARP by caspase-3 is considered to be a marker in cells undergoing apoptosis. In our study, we revealed significant increases in DFF40 and also the cleaved forms of lamin A, α-fodrin, and PARP after treatment with taxol, Bcl-2 siRNA, or both agents together (Fig. 6, panel b). The cleavage values obtained from treatment with combination of taxol and Bcl-2 siRNA were significantly higher than those obtained from either treatment alone (Fig. 6, panel b).
Western blotting for levels of cleaved (active) caspases and degradation of specific substrates in U138MG and U251MG cells after the treatments. (a) Representative Western blots showing levels of active caspase-9, caspase-8, caspase-7, caspase-6 and caspase-3, DFF40, cleaved lamin A, cleaved α-fodrin, and cleaved PARP in the cells. The cleaved PARP was determined in the nuclear fractions of the cells. The Western blots were reprobed for levels of GAPDH to confirm that equal amounts of protein were loaded in all lanes. (b) Quantitative evaluation of the percent change in levels of active caspase 3, DFF40, lamin A, α-fodrin, and PARP. The Western blots were quantified using Image-J software. Data were representative of 3 independent experiments (*P < 0.001 when compared with controls and # P < 0.001 when compared with taxol or Bcl-2 siRNA mean values)
Based on our results, we proposed a schematic model to demonstrate the molecular events leading to activation of calpain and caspases for apoptosis in human glioblastoma cells following treatment with combination of taxol and Bcl-2 siRNA (Fig. 7).
Schematic presentation of molecular events leading to increased activities of calpain and caspases for apoptotic death in glioblastoma cells after treatment with combination of taxol and Bcl-2 siRNA. Taxol triggers death signals which cause endoplasmic reticulum (ER) stress and upregulation of Bax. Alternatively, taxol binds to microtubules which in turn results in ER stress and increase in intracellular free [Ca2+] for upregulation of calpain. Calpain contributed to apoptosis through cleavage of Bid, α-fodrin, and procaspases. Bcl-2 siRNA down regulated the anti-apoptotic molecule Bcl-2 and accelerated the mitochondrial release of cytochrome c into the cytosol. The association of cytosolic cytochrome c with procaspase-9 and Apaf-1 processed procaspase-9 to its active form, which then initiated the intrinsic pathway of apoptosis. Calpain, caspase-9, and caspase-8 cleaved procaspase-3 to its active form. The active caspase-3 in turn cleaved α-fodrin, DFF45, and PARP leading to nuclear DNA fragmentation and apoptosis
Discussion
We previously used different strategies for induction of apoptosis in glioblastoma cells [9, 11]. However, this is the first study showing that combination of taxol and Bcl-2 siRNA triggers molecular mechanisms involving calpain and caspase activities for induction of apoptosis in two highly invasive glioblastoma cell lines. Double immunofluorescent stainings for m-calpain and active caspase-3 and the TUNEL staining demonstrated increases in apoptosis after treatment with taxol alone, Bcl-2 siRNA alone, and both agents together. Fura-2 assay showed increases in intracellular free [Ca2+], which upregulated m-calpain. Further, fluorometric assays exhibited increases in activities of calpain and caspase-3. Western blotting showed increases in levels of Bax, tBid, and also active caspases to promote apoptosis. We also showed increases in DFF40 and cleaved forms of lamin A, α-fodrin, and PARP in course of apoptosis. This study showed that combination of low doses of taxol and Bcl-2 siRNA more effectively induced the calpain and caspase mediated death signaling pathways than either agent alone for apoptosis in human glioblastoma cells.
Taxol binds to the β-subunit of tubulin and blocks the microtubules’ normal functions and mitosis. However, high doses of taxol also inhibit cell division in normal cells. Reportedly, high dose of taxol has several other side effects. Glioblastoma cells avoid apoptosis due to upregulation of the anti-apoptotic Bcl-2 protein. In this study, we presented data to show that taxol is effective at as low as 100 nM when combined with Bcl-2 siRNA for induction of apoptosis. Therefore, an amalgamation of a conventional chemotherapeutic agent (taxol) with an emerging gene therapeutic strategy (Bcl-2 siRNA) can be a promising approach for treatment of the most malignant brain tumor.
Calpain is a Ca2+ dependent ubiquitous protease with prospective involvement in apoptosis [12]. Calpain cleaves procaspase-3 and procaspase-12 to produce their active subunits [14, 18]. Calpain may also cleave PARP and α-fodrin [14, 19]. Furthermore, calpain truncates Bid to active tBid, which translocates to mitochondria, where it is involved in oligomerization of Bak and/or Bax leading to cytochrome c release [12]. We observed a significant increase in the activity of calpain by a fluorometric assay, immunofluorescent staining, and Western blotting especially after treatment of glioblastoma cells with combination of taxol and Bcl-2 siRNA.
Caspases play significant roles as mediators of apoptosis in mammalian cells. They are divided into initiator and effector (executioner) caspases depending on their site of action. In the present study, we observed significantly increases in levels of active forms of both initiator and effector caspases (caspase-9, caspase-8, caspase-7, caspase-6, and caspase-3) after treatment with taxol, Bcl-2 siRNA, and both agents in combination. Treatment with combination taxol and Bcl-2 siRNA appeared to be more effective than either treatment alone.
Lamins and fodrins are nuclear membrane and cytoskeletal structural components, respectively, which are important in maintaining normal cellular structure. During apoptosis, the cleavage of 70 kDa lamin A by active capsase-6 to a large 45 kDa fragment and a small 25 kDa fragment serves as a marker for caspase-6 activation in the cells. Cleavage of α-fodrin is a marker for an increase in active caspase-3. It is widely assumed that the caspase-specific cleavage of lamins and fodrins is responsible for the various hallmarks of apoptosis such as nuclear fragmentation, cytoplasmic membrane blebbing, and DNA fragmentation [13, 31]. Human DFF45 and its mouse homologue inhibitor of caspase-3-activated DNase (ICAD) serve as chaperones for DFF40 or caspase-3-activated DNase (CAD) during synthesis [6]. Upon cleavage of DFF45 by active caspase 3, DFF40 is activated and released to move to the nucleus to cause DNA fragmentation, which is the hallmark of apoptosis. PARP is a highly conserved nuclear enzyme present in higher eukaryotes. PARP is activated at an intermediate stage of apoptosis and is then cleaved and inactivated at a late stage by apoptotic proteases, namely caspase-3 and caspase-7 [4]. In the present study, we observed significant increases in DFF40 and cleaved forms of lamin A, α-fodrin, and PARP after treatment with taxol, Bcl-2 siRNA, and especially both agents in combination.
In conclusion, our study demonstrated that treatment with a low dose of taxol and knockdown of Bcl-2 expression could be a highly effective strategy to induce proteolytic activities of calpain and caspases for increasing apoptosis in human glioblastoma cells.
References
Boehmerle W, Splittgerber U, Lazarus MB, McKenzie KM, Johnston DG, Austin DJ, Ehrlich BE (2006) Paclitaxel induces calcium oscillations via an inositol-1,4,5-trisphosphate receptor and neuronal calcium sensor 1-dependent mechanism. Proc Natl Acad Sci USA 103:18356–18361
Cryns VL, Bergeron L, Zhu H, Li H, Yuan J (1996) Specific cleavage of α-fodrin during Fas- and tumor necrosis factor-induced apoptosis is mediated by an interleukin-1β-converting enzyme/Ced-3 protease distinct from the poly(ADP-ribose) polymerase protease. J Biol Chem 271:31277–31282
Das A, Banik NL, Ray SK (2006) Mechanism of apoptosis with the involvement of calpain and caspase cascades in human malignant neuroblastoma SH-SY5Y cells exposed to flavonoids. Int J Cancer 119:2575–2585
Decker P, Muller S (2002) Modulating poly(ADP-ribose) polymerase activity: potential for the prevention and therapy of pathogenic situations involving DNA damage and oxidative stress. Curr Pharm Biotechnol 3:275–283
Devi GR (2006) siRNA-based approaches in cancer therapy. Cancer Gene Ther 13:819–829
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391:43–50
Ganesh T, Yang C, Norris A, Glass T, Bane S, Ravindra R, Banerjee A, Metaferia B, Thomas SL, Giannakakou P, Alcaraz AA, Lakdawala AS, Snyder JP, Kingston DG (2007) Evaluation of the tubulin-bound paclitaxel conformation: synthesis, biology, and SAR studies of C-4 to C-3′ bridged paclitaxel analogues. J Med Chem 50:713–725
George J, Tsutsumi M (2007) siRNA-mediated knockdown of connective tissue growth factor prevents N-nitrosodimethylamine-induced hepatic fibrosis in rats. Gene Ther 14:790–803
George J, Gondi CS, Dinh DH, Gujrati M, Rao JS (2007) Restoration of tissue factor pathway inhibitor-2 in a human glioblastoma cell line triggers caspase-mediated pathway and apoptosis. Clin Cancer Res 13:3507–3517
Huisman C, Ferreira CG, Broker LE, Rodriguez JA, Smit EF, Postmus PE, Kruyt FA, Giaccone G (2002) Paclitaxel triggers cell death primarily via caspase-independent routes in the non-small cell lung cancer cell line NCI-H460. Clin Cancer Res 8:596–606
Karmakar S, Banik NL, Patel SJ, Ray SK (2006) Curcumin activated both receptor-mediated and mitochondria-mediated proteolytic pathways for apoptosis in human glioblastoma T98G cells. Neurosci Lett 407:53–58
Mandic A, Viktorsson K, Strandberg L, Heiden T, Hansson J, Linder S, Shoshan MC (2002) Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol 22:3003–3013
Martin SJ, O’Brien GA, Nishioka WK, McGahon AJ, Mahboubi A, Saido TC, Green DR (1995) Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J Biol Chem 270:6425–6428
McGinnis KM, Gnegy ME, Park YH, Mukerjee N, Wang KK (1999) Procaspase-3 and poly(ADP)ribose polymerase (PARP) are calpain substrates. Biochem Biophys Res Commun 263:94–99
Metrailler-Ruchonnet I, Pagano A, Carnesecchi S, Ody C, Donati Y, Barazzone Argiroffo C (2007) Bcl-2 protects against hyperoxia-induced apoptosis through inhibition of the mitochondria-dependent pathway. Free Radic Biol Med 42:1062–1074
Mineo JF, Bordron A, Baroncini M, Ramirez C, Maurage CA, Blond S, Dam-Hieu P (2007) Prognosis factors of survival time in patients with glioblastoma multiforme: a multivariate analysis of 340 patients. Acta Neurochir (Wien) 149:245–253
Murphy KM, Ranganathan V, Farnsworth ML, Kavallaris M, Lock RB (2000) Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Differ 7:102–111
Nakagawa T, Yuan J (2000) Cross-talk between two cysteine protease families: Activation of caspase-12 by calpain in apoptosis. J Cell Biol 150:887–894
Nath R, Raser KJ, Stafford D, Hajimohammadreza I, Posner A, Allen H Talanian RV, Yuen P, Gilbertsen RB, Wang KK (1996) Non-erythroid α-spectrin breakdown by calpain and interleukin-1β-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J 319:683–690
Neumar RW, Xu YA, Gada H, Guttmann RP, Siman R (2003) Cross-talk between calpain and caspase proteolytic systems during neuronal apoptosis. J Biol Chem 278:14162–14167
Nuydens R, Dispersyn G, Van Den Kieboom G, de Jong M, Connors R, Ramaekers F Borgers M, Geerts H (2000) Bcl-2 protects neuronal cells against taxol-induced apoptosis by inducing multi-nucleation. Apoptosis 5:335–343
Ofir R, Seidman R, Rabinski T, Krup M, Yavelsky V, Weinstein Y, Wolfson M (2002) Taxol-induced apoptosis in human SKOV3 ovarian and MCF7 breast carcinoma cells is caspase-3 and caspase-9 independent. Cell Death Differ 9:636–642
Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM (1998) Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis: lesson from an uncleavable mutant. J Biol Chem 273:33533–33539
Park SJ, Wu CH, Gordon JD, Zhong X, Emami A, Safa AR (2004) Taxol induces caspase-10-dependent apoptosis. J Biol Chem 279:51057–51067
Pasquier E, Carre M, Pourroy B, Camoin L, Rebai O, Briand C, Braguer D (2004) Antiangiogenic activity of paclitaxel is associated with its cytostatic effect, mediated by the initiation but not completion of a mitochondrial apoptotic signaling pathway. Mol Cancer Ther 3:1301–1310
Rao JS (2003) Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer 3:489–501
Rao L, Perez D, White E (1996) Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol 135:1441–1455
Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ (1999) Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol 144:281–292
Slee EA, Keogh SA, Martin SJ (2000) Cleavage of Bid during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ 7:556–565
Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT (1971) Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 93:2325–2327
Zheng TS, Schlosser SF, Dao T, Hingorani R, Crispe IN, Boyer JL, Flavell RA (1998) Caspase-3 controls both cytoplasmic and nuclear events associated with Fas-mediated apoptosis in vivo. Proc Natl Acad Sci USA 95:13618–13623
Zhou P, Lugovskoy AA, McCarty JS, Li P, Wagner G (2001) Solution structure of DFF40 and DFF45 N-terminal domain complex and mutual chaperone activity of DFF40 and DFF45. Proc Natl Acad Sci USA 98:6051–6055
Zou H, Li Y, Liu X, Wang X (1999) An Apaf-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem 274:11549–11556
Acknowledgements
This investigation was supported in part by the R01 grants (CA-91460 and NS-57811) from the National Institutes of Health (Bethesda, MD) to S.K.R.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue article in honor of Dr. George DeVries.
Rights and permissions
About this article
Cite this article
George, J., Banik, N.L. & Ray, S.K. Bcl-2 siRNA Augments Taxol Mediated Apoptotic Death in Human Glioblastoma U138MG and U251MG Cells. Neurochem Res 34, 66–78 (2009). https://doi.org/10.1007/s11064-008-9659-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9659-z