Abstract
The aim of this study was to analyze the effects of intense exercise on brain redox status, associated with antioxidant supplementation of N-acetylcysteine (NAC), deferoxamine (DFX) or a combination of both. Seventy-two C57BL-6 adult male mice were randomly assigned to 8 groups: control, NAC, DFX, NAC plus DFX, exercise, exercise with NAC, exercise with DFX, and exercise with NAC plus DFX. They were given antioxidant supplementation, exercise training on a treadmill for 12 weeks, and sacrificed 48 h after the last exercise session. Training significantly increased (P < 0.05) soleus citrate synthase (CS) activity when compared to control. Blood lactate levels classified the exercise as intense. Exercise significantly increased (P < 0.05) oxidation of biomolecules and superoxide dismutase activity in striatum and hippocampus. Training significantly increased (P < 0.05) catalase activity in striatum. NAC and DFX supplementation significantly protected (P < 0.05) against oxidative damage. These results indicate intense exercise as oxidant and NAC and DFX as antioxidant to the hippocampus and the striatum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Improvements in the brain redox status are well influenced by physical activity experiences [1]. Generally, these exercise models do not discriminate the effort intensity. However, there is a relation between the production of Reactive Oxygen Species (ROS) and exercise intensity [2, 3]. Exercise produces high ROS levels when it is exhaustive [4]. If the ROS production overwhelms the antioxidant system, we have oxidative stress affecting lipids, proteins, and nucleic acids [5]. Previous studies suggest that exercise-induced lipoperoxidation is greater in maximal oxygen consumption (i.e., VO2max) [6].
Data from our group indicate that intense exercise induced oxidative damage also in the brain [7], and memory impairment [8]. Brain is considered abnormally sensitive to oxidative damage because it has low glutathione levels and high iron levels [5]. Iron is an essential trace element for cell function. However, the very property that enables iron to participate in oxygen metabolism explains its potential damaging effects: if not handled properly by the cell, iron interacts with molecular oxygen, generating ROS through the Fenton reaction [9]. Evidences have shown increased iron accumulation in the striatum of Parkinson disease (PD) [10]. Mounting evidence indicates that hippocampal neurons from Alzheimer disease (AD) brains are subject to a high oxidative load [11].
N-Acetylcysteine (NAC), a precursor for glutathione synthesis, has been described as an antioxidant and free radical scavenger in neurological disorders [12]. Deferoxamine (DFX) is the most potent iron chelator with antioxidant properties in neurodegeneration and apoptosis [13]. There is the hypothesis that the use of NAC alone may have limitations and present pro-oxidant effects, due to the facility with which it interacts with iron [14]. Given this, the use of DFX may improve response to the administration of NAC [15]. The isolated administration of NAC may contribute to the production and release of other oxidative mediators, probably due to the facility with which it interacts with iron [14], thus, the use of DFX can contribute to assuage these effects.
Since it is easy to adjust exercise intensity to physical and health conditions [8], it is essential to know the effects of intense exercise on oxidative parameters, because it may have major consequences on memory, learning, and work skills. Therefore, we investigated the oxidative stress biomarkers and catalase and superoxide dismutase activities in the striatum and the hippocampus after intense treadmill-running training. We focused our findings on the hippocampus and the striatum because they are the main regions of oxidative damage in the two most prevalent neurodegenerative diseases, AD and PD, respectively.
Experimental procedure
Animals
Seventy-two male C57BL-6 mice, weighing 30–35 g, 3–4 month-old, were used and cared for according to the European Communities Council Directive of 24 November 1986 (86/609/EEC). Food (Nuvilab CR1, Nuvital Nutrientes S/A, Brazil) and water were available ad libitum. The room was kept at 70% humidity/20 ± 2°C on a 12 h light/dark cycle with lights on at 06.00 h. The mice were periodically checked to verify their pathogen-free condition.
The mice were randomly assigned to 8 groups (n = 9 each group) called: untrained and unsupplemented control group (C); untrained, with NAC supplementation (NAC); untrained, with DFX supplementation (DFX); untrained, with NAC plus DFX supplementation (NAC + DFX); trained and unsupplemented (Ex); trained, with NAC supplementation (Ex + NAC); trained, with DFX supplementation (Ex + DFX); and trained, with NAC plus DFX supplementation (Ex + NAC + DFX).
Exercise protocol, supplementation and sacrifice
All groups were habituated on a nine-channel motor-drive treadmill with the speed of 10 m/min for 10 min/day during 1 week to reduce their stress to the new environment. The mice did not receive any stimuli to run. The intermittent and continuous exercise groups performed an incremental running program to obtain progressive levels of intensity during 12 weeks for 5 days/week and for a total period of 40 days (Table 1). The untrained control animals were put on the switched-off treadmill during the same 12 weeks as the exercise-trained groups.
The antioxidant supplementation was performed before the running sessions. NAC (Zambon Group S.p.A., Brazil) was administered in 3 doses/week (20 mg NAC/kg weight body) dissolved in 30 μl saline solution 0.9% (s.c). DFX (Novartis Pharma AG, Brazil) was administered in 1 dose/week (20 mg DFX/kg weight body) dissolved in 10 μl saline solution 0.9% (s.c).
The exercise training protocol was stopped 48 h before sacrifice. The mice were sacrificed by cervical dislocation. The soleus muscle and the brain were immediately removed and freed from blood; and the striatum and hippocampus were dissected on ice, stored and frozen at −80°C until analysis.
Physical exercise intensity
Blood lactate (BL) level was defined after the last session of exercise from 50 μl of tail capillary blood, using a commercial kit according to the manufacturer’s instructions (Roche, Germany). The blood sample was put onto a glass fiber fleece where the erythrocytes were retained. BL was determined by reflectance photometry at a wave length of 657 nm via colorimetric lactate-oxidase mediator reaction.
Muscle oxidative capacity
The soleus was weighed and homogenized with a glass homogenizer on ice in 100 mM Tris-HCl at a constant weight-to-volume ratio. Sample homogenate was then added to a reaction mix of 100 mM Tris–HCl, 1.0 mM dithio-bis (2-nitrobenzoic acid), and 3.9 mM acetyl coenzyme A. After addition of 1.0 mM oxaloacetate, absorbance at 412 nm was recorded for a 2-min period. Mean absorbance change per minute was recorded for each sample, and citrate synthase (CS) activity in millimole per minute per gram was then calculated by using an extinction coefficient of 13.600 [16].
Lipid peroxidation assay
The 2-thiobarbituric Acid Reactive Species (TBARS) levels were measured by method of Draper and Hadley [17] and expressed like Malondialdehyde (MDA) equivalent. Briefly, striatum and hippocampus were mixed with 1 ml 10% trichloroacetic acid and 1 ml of 0.67% thiobarbituric acid, subsequently; they were heated in a boiling water bath for 15 min. The TBARS were determined by measuring absorbance at 535 nm and the results are given as nmol MDA/mg protein.
Carbonyl assay
The protein concentration of the soluble protein fractions was determined according to Levine et al. [18]. The protein carbonyl content was measured by first forming labeled protein hydrazone derivatives using 2,4-dinitrophenylhydrazide (DNPH). These derivates were sequentially extracted with 10% (vol/vol) trichloroacetic acid followed by treatment with ethanol/ethylacetate, 1:1 (vol/vol) and reextraction with 10% trichloroacetic acid. The resulting precipitate was dissolved in 6 M urea hydrochloride. The difference spectrum between a 2.4-dinitrophenylhydrazide-protein blank was used to calculate nmol of 2.4-dinitrophenylhydrazide incorporated per mg of protein. Results are reported for each sample read at 370 nm in a spectrophotometer.
Catalase (CAT) activity assay
In order to determine CAT activity, striatum and hippocampus were sonicated in a 50 mM phosphate buffer and the resulting suspension was centrifuged at 3,000g for 10 min. The supernatant was used for enzyme assay. CAT activity was measured by the rate of decrease in hydrogen peroxide absorbance at 240 nm [19]. Enzyme activity was expressed as U/mg protein.
Superoxide dismutase (SOD) activity assay
SOD activity of striatum and hippocampus was determined according method of Bannister and Calabrese [20]. The enzymatic activity estimation occurs by adrenaline auto-oxidation inhibition read at 480 nm in a spectrophotometer. Enzyme activity was expressed as U/mg protein.
Measurement of protein
Protein concentration was estimated by the method of Lowry et al. [21], using bovine serum albumin as standard.
Statistical analyses
Means ± S.E.M were calculated, and multiple comparisons were performed by using one-way ANOVA with Tukey post hoc tests. A P value <0.05 was considered significant. The software used for data analysis was Statistical Package for the Social Sciences (SPSS) version 12.0 for Microsoft Windows.
Results
Animals showed adaptations to exhaustive exercise program
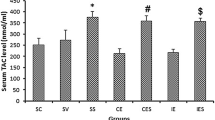
Anaerobic threshold is a term that refers to the oxygen consumption during high-intensity exercise above which the rate of lactate production exceeds the rate of lactate removal, thus causing increased BL levels in tissues [22]. The unsupplemented control group ran only the last session of exercise to achieve a BL level of untrained animals. Our results showed BL levels of 6.9 ± 0.7 mmol/l in the unsupplemented control group and 4.2 ± 0.4 mmol/l in the continuous exercise group in the final stage of the last day of exercise. The data indicated a significantly higher BL content (P < 0.05) in untrained animals than in the exercise group, an evidence of exercise-induced muscle adaptation. These BL levels after a treadmill-running session classified the exercise as being of high intensity [7]. In rodents, high exercise intensity results in BL concentration of approximately 4.2 mmol/l [23].
Several studies have been carried out to determine the influence of exercise training on mitochondrial enzyme adaptation in skeletal muscle of rats [2]. The CS activity in the soleus muscle in the exercise group (0.589 ± 0.010 U CS/mg protein) was significantly higher (P < 0.05) than in the untrained control group (0.327 ± 0.042 U CS/mg protein). These results indicate that the treadmill-training program used was sufficient to increase oxidative metabolism in skeletal muscle of mice.
Intense exercise increased lipid and protein oxidation levels in the striatum and the hippocampus
The mitochondrial work constantly supplies energy for neuronal processes by using oxidative phosphorylation. A normal by-product of mitochondrial respiration is the formation of free radicals. When an imbalance occurs between the production of free radicals and the ability of cells to guard against them, it is commonly referred to as oxidative stress [24].
We wanted to examine the possibility that intense exercise can limit the amount of oxidative stress occurring in the striatum and the hippocampus. Therefore, we assessed lipid and protein oxidation levels as markers of oxidative stress following exercise [7].
The NAC, DFX and NAC plus DFX supplemented and untrained mice have not significantly differences (P < 0.05) in lipid and protein oxidation levels in the striatum and the hippocampus from unsupplemented and untrained control group, meaning unchanged basal oxidative parameters (Figs. 1 and 2).
Lipoperoxidation in the striatum and the hippocampus of mice induced by 12 weeks of intense treadmill program was protected with antioxidant supplementation. Values are mean ± S.E.M for nine animals per group. Ex—Exercise, NAC—N-Acetylcysteine, DFX—Deferoxamine. *P < 0.05 vs. control, # P < 0.05 vs. exercise, ANOVA, Tukey’s post-hoc test
Striatal carbonyl levels increased after intense exercise training, but not in the hippocampus. NAC or DFX antioxidant supplementation decreased carbonylation in the striatum and the hippocampus, but the association of both had protective effects only in the striatum. Values are mean ± S.E.M for nine animals per group. Ex—exercise, NAC—N-Acetylcysteine, DFX—Deferoxamine. *P < 0.05 vs. control, # P < 0.05 vs. exercise, ANOVA, Tukey’s post-hoc test
Hippocampal Thiobarbituric Acid Reactive Species (TBARS) levels were significantly higher (P < 0.05) in the exercise group (522.8 ± 106.8 nmol MDA/mg protein) than in the control group (227.2 ± 17.9 nmol MDA/mg protein). The groups of exercise associated with antioxidant supplementation of NAC (276.5 ± 22.7 nmol MDA/mg protein), DFX (278.4 ± 22.9 nmol MDA/mg protein) and NAC plus DFX (183.4 ± 14.4 nmol MDA/mg protein) showed a significant decrease (P < 0.05) in hippocampal TBARS levels when compared to unsupplemented trained animals (Fig. 1).
TBARS levels were found to be significantly elevated (P < 0.05) in the striatum of trained groups (460.2 ± 85.5 nmol MDA/mg protein) in relation to the untrained control group (236.7 ± 12.4 nmol MDA/mg protein). However, the association of intense exercise with antioxidants NAC (221.8 ± 31.7 nmol MDA/mg protein), DFX (285.2 ± 38.0 nmol MDA/mg protein) and NAC plus DFX (275.6 ± 6.7 nmol MDA/mg protein) significantly decreased (P < 0.05) striatal TBARS levels when compared to the trained but not supplemented group (Fig. 1).
Intense training associated with supplementation of NAC (1.5 ± 0.4 nmol carbonyl/mg protein) or DFX (2.2 ± 0.8 nmol carbonyl/mg protein) produced lower carbonyl content in the hippocampus than that found in unsupplemented trained animals (9.4 ± 2.6 nmol carbonyl/mg protein). However, the association between NAC and DFX (6.9 ± 1.2 nmol carbonyl/mg protein) did not exert a protective effect in oxidized protein in the hippocampus (Fig. 2).
The unsupplemented exercise group (12.0 ± 3.1 nmol carbonyl/mg protein) showed a significant increase (P < 0.05) in carbonyl levels in the striatum when compared to the control group (2.8 ± 1.0 nmol carbonyl/mg protein) (Fig. 2). The content of oxidized protein in mice striatum was significantly lower (P < 0.05) in the exercise group when supplemented with NAC (1.3 ± 0.2 nmol carbonyl/mg protein), DFX (2.1 ± 0.4 nmol carbonyl/mg protein), and NAC plus DFX (2.0 ± 0.4 nmol carbonyl/mg protein) when compared to the unsupplemented exercised group (Fig. 2).
Intense exercise increased SOD and CAT activity in the striatum and the hippocampus
Free radicals, such as superoxide, hydroxyl free radicals, and hydrogen peroxide, are produced because of physiological metabolic reactions in the central nervous system. Superoxide radicals are converted into hydrogen peroxide by SOD enzyme and hydrogen peroxide is converted into water by CAT enzyme [25].
The antioxidant enzyme activities in the striatum and the hippocampus after NAC, DFX and NAC plus DFX supplementation of untrained mice have not significantly differences (P < 0.05) from unsupplemented and untrained control group, meaning unchanged basal oxidative parameters in Figs. 3 and 4.
Treadmill training enhanced SOD activity in the striatum and the hippocampus of mice. Since the antioxidant supplementation was protective, the supplementation groups showed values equal to control. Values are mean ± S.E.M for nine animals per group. Ex—exercise, NAC—N-Acetylcysteine, DFX—Deferoxamine. *P < 0.05 vs. control, # P < 0.05 vs. exercise, ANOVA, Tukey’s post-hoc test
Catalase activity in the striatum of mice increased after the treadmill program and with DFX supplementation. NAC and NAC plus DFX did not have effect on the activity of striatal catalase. NAC and DFX supplementation increased NAC activity in the hippocampus after the exercise program. Values are mean ± S.E.M for nine animals per group. Ex—exercise, NAC—N-Acetylcysteine, DFX—Deferoxamine. *P < 0.05 vs. control, # P < 0.05 vs. exercise, ANOVA, Tukey’s post-hoc test
Our results indicated that the treadmill-training program used was sufficient to significantly improve (P < 0.05) the SOD activity in the hippocampus of trained mice (2.81 ± 0.49 U SOD/mg protein) when compared to the untrained control group (0.83 ± 0.19 U SOD/mg protein). The supplementation of NAC (0.56 ± 0.07 U SOD/mg protein), DFX (0.56 ± 0.04 U SOD/mg protein) or NAC plus DFX (0.96 ± 0.07 U SOD/mg protein) significantly decreased (P < 0.05) hippocampal SOD activity in relation to the unsupplemented trained group (2.81 ± 0.49 U SOD/mg protein) (Fig. 3).
SOD activity was significant higher (P < 0.05) in the trained group (2.61 ± 0.09 U SOD/mg protein) than in the untrained control group (1.01 ± 0.08 U SOD/mg protein). In the trained mice, NAC (0.76 ± 0.16 U SOD/mg protein), DFX (0.60 ± 0.08 U SOD/mg protein), and NAC plus DFX (0.86 ± 0.08 U SOD/mg protein) resulted in a significant decrease (P < 0.05) of striatal SOD activity when compared to the unsupplemented trained group (Fig. 3).
Intense exercise (9.1 ± 1.5 U CAT/mg protein) significantly increased (P < 0.05) striatal CAT activity when compared to the levels observed in the control group (2.9 ± 0.6 U CAT/mg protein) (Fig. 4). NAC supplementation alone (3.8 ± 0.3 U CAT/mg protein) or associated with DFX (4.3 ± 0.1 U CAT/mg protein) produced significantly lower (P < 0.05) striatal CAT activity than that observed in the unsupplemented trained group (9.1 ± 1.5 U CAT/mg protein) (Fig. 4).
The exercise group (2.8 ± 0.4 U CAT/mg protein) and the associations of NAC plus DFX supplementations (2.3 ± 0.2 U CAT/mg protein) did not show significant changes (P < 0.05) in the activity of hippocampal CAT when compared to the control group (1.6 ± 0.03 U CAT/mg protein). The supplementation of NAC (8.6 ± 0.2 U CAT/mg protein) or DFX (7.4 ± 0.5 U CAT/mg protein) significantly improved (P < 0.05) CAT activity in the hippocampus of trained mice in relation to the unsupplemented control (1.6 ± 0.03 U CAT/mg protein) (Fig. 4).
Discussion
The CNS has a high oxidative capacity and high oxygen consumption [26]. The antioxidant capacity of the brain is limited by a high content of easily oxidizable fatty acids [23] and free iron [27], and low levels of antioxidants enzymes and substrates, respectively: catalase and superoxide dismutase [28] and glutathione [29].
The beneficial effects of endurance training on the redox status of the nervous system of rodents have been reported in swimming [30], treadmill training [31, 32], and running-wheel training [33] as well. On the other hand, there are also studies where swimming [34] and treadmill [23] training have been found not to affect the antioxidant defenses of the brain. Rats that performed severe and overtraining swimming did not show brain oxidative stress [35].
It is well known that different forms of exercise result in different levels of tissue stress [36]. Generally, endurance training consisted of exercise of low intensity and long duration [37]. Treadmill forces the animal to run according to the exercise demands: time, duration, and intensity [36]. Somani et al. [32] imposed usual endurance running training and did not found brain oxidative stress. Earlier, we have demonstrated that intense treadmill exercise induced mitochondrial dysfunction in the frontal cortex of mice [7]. We used the endurance running training of high intensity and found oxidative stress in the striatum and the hippocampus (Figs. 1 and 2).
Our data also suggest that the striatum is more responsive to exercise-induced oxidative damage than the hippocampus in high intensity exercise. Catecholaminergic striatal neurons containing neuromelanin, an autoxidation by-product of catecholamines, are more vulnerable to oxidative stress than non-melanized catecholaminergic neurons [38]. Iron, an element that exacerbates the production of free radicals in catecholaminergic neurons, is increased in the striatum of PD [39].
NAC, a precursor of the potent antioxidant glutathione and a free radical scavenger leading to a reduced level of O •2 and H2O2, has been shown to reduce oxidative stress [40]. Oxidative stress could be minimized by maintaining reduced-glutathione levels with the administration of cysteine precursors such as NAC [40]. However, there are reports showing that NAC had no effect on the peroxidation of lipid membranes [41]. The likely causes of NAC’s inability to decrease lipid peroxidation were the amount of free iron available to oxidize the sulfhydryl group of NAC, inadequate dose and method of NAC administration, and the presence of other possible pathways to lipid peroxidation. In addition, iron is the most abundant transition metal in the body and has a unique distribution in the brain where the striatum is one of the largest areas. Increased iron levels in the brain may play an important role in the oxidative-stress reaction [27]. Our study showed that injection with NAC, DFX, and NAC plus DFX protected against the exercise-induced oxidative damage in the striatum and the hippocampus. Interestingly, NAC plus DFX did not exert a protective effect on oxidized protein levels in the hippocampus, but the reason remains unclear.
Our results have also demonstrated increased SOD activity due to exercise training in the striatum and the hippocampus, possibly by enzyme induction from exercise (Fig. 3). The oxidative stress seems to originate predominantly by the production of superoxide radicals, which are scavenged by SOD [32]. O •2 dismuted by SOD to H2O2 is then converted by CAT or glutathione peroxidase (GPx) into H2O2 and O2. With physical training, CAT activity increased in the striatum and decreased when associated with NAC supplementation. We believe that this occurred because the striatal neurons are surrounded by a low density of cells containing GPx and are more susceptible to oxidative damage [38]. Therefore, intense exercise induced ROS production followed by an increase in SOD and CAT activity, and NAC administration decreased the exercise-induced oxidative damage in the striatum. With NAC and DFX supplementation, trained mice showed an increased CAT activity in the hippocampus. NAC and DFX supplementation generally improved CAT activity in oxidative events such as brain ageing [42] and neurodegeneration [43, 44].
In conclusion, our findings suggest that physical training of high intensity and long duration induce brain lipoperoxidation in the striatum and the hippocampus, and striatal protein oxidation in adult mice. NAC and DFX administration might be effective on striatal and hippocampal oxidative damage and antioxidant enzyme activity in this training shape. Further studies are certainly needed to define the potential benefits of NAC/DFX supplementation and to determine the correct dose and timing for maximum effectiveness.
References
Vaynman S, Ying Z, Wu A et al (2006) Coupling energy metabolism with a mechanism to support brain-derived neurotrophic factor-mediated synaptic plasticity. Neurosci 139:1221–1234
Pinho RA, Andrades ME, Oliveira MR et al (2006) Imbalance in SOD/CAT activities in rats skeletal muscles submitted to treadmill training exercise. Cell Biol Intern 10:848–853
Sureda A, Tauler P, Aguilo A et al (2005) Relation between oxidative stress markers and antioxidant endogenous defences during exhaustive exercise. Free Radic Res 12:1317–1324
Tauler P, Sureda A, Cases N et al (2006) Increased lymphocyte antioxidant defences in response to exhaustive exercise do not prevent oxidative damage. J Nutr Biochem 10:665–671
Droge W, Schipper HM (2007) Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell 6:361–370
Antunes-Neto JM, Toyama MH, Carneiro EM et al (2006) Circulating leukocyte heat shock protein 70 (HSP70) and oxidative stress markers in rats after a bout of exhaustive exercise. Stress 2:107–115
Aguiar AS, Tuon T, Pinho CA et al (2007) Intense exercise induces mitochondrial dysfunction in mice brain. Neurochem Res (in press)
Rosa EF, Takahashi S, Aboulafia J et al (2007) Oxidative stress induced by intense and exhaustive exercise impairs murine cognitive function. J Neurophysiol (in press)
Hidalgo C, Nunez MT (2007) Calcium, iron and neuronal function. IUBMB Life 4:280–285
Oakley AE, Collingwood JF, Dobson J et al (2007) Individual dopaminergic neurons show raised iron levels in Parkinson disease. Neurol 21:1820–1825
Zhu X, Su B, Wang X et al (2007) Causes of oxidative stress in Alzheimer disease. Cell Mol Life Sci 64:2202–2210
Arakawa M, Ushimaru N, Osada N et al (2006) N-acetylcysteine selectively protects cerebellar granule cells from 4-hydroxynonenal-induced cell death. Neurosci Res 3:255–263
Freret T, Valable S, Chazalviel L et al (2006) Delayed administration of deferoxamine reduces brain damage and promotes functional recovery after transient focal cerebral ischemia in the rat. Eur J Neurosci 7:1757–1765
Ritter C, Andrades ME, Reinke A et al (2004) Treatment with n-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med 32:342–349
Pinho RA, Silveira PCL, Silva LA et al (2005) N-Acetylcysteine and deferoxamine reduce pulmonary oxidative stress and inflammation in rats after coal dust exposure. Environmental Res 99:355–360
Alp PR, Newsholme EA, Zammit VA (1976) Activities of citrate synthase and NAD+-linked and NADP+-linked isocitrate dehydrogenase in muscle from vertebrates and invertebrates. Biochem J 154:689–700
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Meth Enzymol 186:421–431
Levine RL, Garland D, Oliver CN et al (1990) Determination of carbonyl content in oxidatively modified proteins. Meth Enzymol 186:464–478
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126
Bannister JV, Calabrese L (1987) Assays for SOD. Meth Biochem Anal 32:279–312
Lowry OH, Rosebough NG, Farr AL et al (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Voltarelli FA, Gobatto CA, Mello MAR (2002) Determination of anaerobic threshold in rats using the lactate minimum test. Braz J Med Biol Res 35:1389–1394
Özkaya YG, Agar A, Yargicoglu P et al (2002) The effect of exercise on brain antioxidant status of diabetic rats. Diabetes Metab 5:377–384
Ebadi M, Leuschen MP, el Refaey H et al (1996) The antioxidant properties of zinc and metallothionein. Neurochem Int 2:159–166
Acikgoz O, Aksu I, Topcu A et al (2006) Acute exhaustive exercise does not alter lipid peroxidation and antioxidant enzyme activities in rat hippocampus, prefrontal cortex and striatum. Neurosc Lett 406:148–151
Calabrese V, Lodi TR, Tonon C et al (2005) Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich’s ataxia. J Neurol Sci 1–2:145–162
Fauchex BA, Martin ME, Beaumont C et al (2003) Neuromelanin associated redox-active iron is increased in the substantia nigra of patients with Parkinson’s Disease. J Neurochem 5:1142–1148
Floyd RA (1999) Antioxidants, oxidative stress, neurological disorders. Exp Biol Med 222:236–245
Sen CK, Packer L (2000) Thiol homeostasis and supplements in physical exercise. Am J Nutr 72:653–669
Jolitha AB, Subramanyam MVV, Asha Devi S (2006) Modification by vitamin E and exercise of oxidative stress in regions of aging rat brain: Studies on superoxide dismutase isoenzymes and protein oxidation status. Exp Geront 41:753–763
Cosķun S, Gonul B, Guzel NA et al (2005) The effects of vitamin C supplementation on oxidative stress and antioxidant content in the brains of chronically exercised rats. Mol Cell Biochem 1–2:135–138
Somani SM, Ravi R, Rybak LP (1995) Effect of exercise training on antioxidant system in brain regions of rat. Pharmacol Biochem Behav 4:635–639
Suzuki M, Katamine S, Tatsumi S (1983) Exercise-induced enhancement of lipid peroxide metabolism in tissues and their transference into the brain in rat. J Nutr Sci Vitaminol 2:141–151
Radák Z, Toldy A, Szabo Z et al (2006) The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem Int 4:387–392
Ogonovszky H, Berkes I, Kumagai S et al (2005) The effects of moderate-, strenuous- and over-training on oxidative stress markers, DNA repair, and memory, in rat brain. Neurochem Int 46:635–640
Arida RM, Scorza CA, Silva AV et al (2004) Differential effects of spontaneous versus forced exercise in rats on the staining of parvalbumin-positive neurons in the hippocampal formation. Neurosci Lett 364:135–138
Meyer T, Auracher M, Heeg K et al (2007) Effectiveness of low-intensity endurance training. Int J Sports Med 1:33–39
Hirsch EC (1994) Biochemistry of Parkinson’s disease with special reference to the dopaminergic systems. Mol Neurobiol 9:135–142
Hirsch EC (1993) Does oxidative stress participate in nerve cell death in Parkinson’s disease? Eur Neurol 33:52–59
Zhang QG, Tian H, Li HC et al (2006) Antioxidant N-acetylcysteine inhibits the activation of JNK3 mediated by the GluR6-PSD95-MLK3 signaling module during cerebral ischemia in rat hippocampus. Neurosci Lett 408:159–164
Kaynar MY, Erdincler P, Tadayyon E et al (1998) Effect of nimodipine and N-acetylcysteine on lipid peroxidation after experimental spinal cord injury. Neuro-surg Rev 21:260–264
Kanwar SS, Nehru B (2007) Modulatory effects of N-acetylcysteine on cerebral cortex and cerebellum regions of ageing rat brain. Nutr Hosp 22:95–100
Hicdonmez T, Kanter M, Tiryaki M et al (2006) Neuroprotective effects of N-acetylcysteine on experimental closed head trauma in rats. Neurochem Res 31:473–481
Liddell JR, Hoepken HH, Crack PJ et al (2006) Glutathione peroxidase 1 and glutathione are required to protect mouse astrocytes from iron-mediated hydrogen peroxide toxicity. J Neurosci Res 84:578–586
Acknowledgments
This research was supported by grants from CNPq/MCT (Brazil), CAPES/MEC (Brazil), and UNESC (Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguiar, A.S., Tuon, T., Soares, F.S. et al. The Effect of n-acetylcysteine and Deferoxamine on Exercise-induced Oxidative Damage in Striatum and Hippocampus of Mice. Neurochem Res 33, 729–736 (2008). https://doi.org/10.1007/s11064-007-9485-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9485-8