Abstract
Vitamin A is known to regulate some central nervous system (CNS)-associated functions. Vitamin A at high doses has been demonstrated to be beneficial in the treatment of some diseases, for instance acute promyelocytic leukemia. However, vitamin A and its naturally occurring metabolites (retinoids) are known to alter neuronal function, inducing behavioral disorders. Here we provide an evidence to indicate that vitamin A supplementation, at both therapeutic and excessive doses, induces oxidative stress in the rat substantia nigra. Vitamin A supplementation induced lipid peroxidation, protein carbonylation, and oxidation of protein thiol groups, as well as change in catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) activity. Surprisingly, locomotory and exploratory activity of rats were decreased after acute and chronic vitamin A supplementation. Therefore, we may conclude from our results that vitamin A supplementation is prooxidant to the rat substantia nigra and effective in altering behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin A (also referred as retinol) is essential to both developing and adult central nervous system (CNS), immune system, epithelial proliferation and vision [1–3], among others. Vitamin A and retinoids modulate dopamine pathways and participate in locomotory behavior [4] and to activate D2 dopamine receptor promoter [5]. Additionally, retinoids regulate the dopamine-dependent signal transduction in dopaminergic cells, suggesting an important role of vitamin A upon these cells [6].
However, an increasing body of evidence suggests that vitamin A may induce neurotoxic effects in humans. Excessive vitamin A intake either acutely or chronically has been suggested to induce intra-cranial hypertension, headache, and irritability in adult humans [7, 8]. In addition, even the intake of low vitamin A doses during pregnancy has been associated to congenital malformations in the CNS [9]. The treatment with retinoids at therapeutic doses also has been demonstrated to induce cognitive disturbances, for instance depression, in mice [10], and humans [11].
Oxidative stress is a condition in which reactive molecules such as superoxide (O −•2 ), peroxynitrite (ONOO−), hydroxyl (OH•), and other radicals are produced in excess. It is a chemical alteration of the cellular environment imposed by exogenous chemicals (e.g., agrochemicals or pollutants), or resulted from an intrinsic alteration, such as that in antioxidant defenses [12]. Undoubtedly, reactive oxygen species (ROS) and reactive nitrogen species (RNS) participate in so many neurodegenerative diseases including, Parkinson’s disease (PD), Alzheimer’s disease (AD), and amyotrophic lateral sclerosis [12, 13].
Vitamin A may be either anti [14] or prooxidant (see below), depending on dosage and cellular condition. Our group has demonstrated the prooxidant effects of retinol treatment upon cultured Sertoli cells, such as induction of lipid peroxidation and protein carbonylation, and alteration in the activity of antioxidant enzymes [15–19]. Furthermore, we found increased levels of oxidative stress in rat liver mitochondria exposed to retinol [20]. Interestingly, retinol induced activation of the Src/MEK/MAPK/CREB pathway through an oxidative-dependent manner [21].
Substantia nigra is particularly vulnerable to oxidative stress given the presence of some intrinsic prooxidant factors. Dopamine degradation, for instance, is a source of hydrogen peroxide (H2O2) via monoamine oxidase (MAO) action [22]. Moreover, substantia nigra presents a high iron and copper content, which facilitate the formation of hydroxyl (OH•) radical after reacting with H2O2 [12].
Oral vitamin A is therapeutically used at high doses to treat acute myeloid leukemia [see 23 for review]. In addition, the excessive intake of vitamin A as supplemented foods is a current concern regarding the consequences that may result from this procedure [24]. Then, based on previously reports indicating a prooxidant role of vitamin A in different experimental models, we decided to investigate the effects of acute and chronic vitamin A supplementation at either therapeutic (1,000 or 2,500 IU/kg) or excessive (4,500 or 9,000 IU/kg) doses on the redox status of rat substantia nigra, and on locomotory and exploratory activity of rats in an open field.
Experimental procedure
Animals
Adult male Wistar rats (Rattus norvegicus) (90 days old; 300 ± 20 g body weight) were obtained from our own breeding colony. They were caged in groups of five with free access to food and water and were maintained on a 12-h light–dark cycle (lights on 7:00 a.m.), at a temperature-controlled colony room (23 ± 1°C). All behavioral testing was conducted during the light phase. These conditions were maintained constant throughout the experiments. All experimental procedures were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and the Brazilian Society for Neuroscience and Behavior recommendations for animal care.
Drugs and reagents
Arovit® (retinol palmitate, a water-soluble form of vitamin A) was purchased from Roche, Sao Paulo, SP, Brazil. All other chemicals were purchased from Sigma, St. Louis, MO, USA.
Drug administration
The animals were treated once a day during three different periods: 3, 7, or 28 days (i.e., acute and chronic vitamin A-supplementation effects were analyzed). All treatments were carried out at night (i.e., when animals usually are more active and take a greater amount of food) in order to ensure maximum vitamin A absorption, since this vitamin is better absorbed during or after a meal. The animals were gavaged with vehicle (0.15 M NaCl), 1,000, 2,500, 4,500, or 9,000 IU/kg of retinol palmitate, orally, in a maximum volume of 0.8 mL during each period of interest. Adequate measures were taken to minimize pain or discomfort.
Preparations of the samples
The animals were sacrificed by decapitation at 24 h after the last vitamin A administration. The substantia nigra was dissected out immediately after the rat was sacrificed and stored at −70°C for posterior analyses. Substantia nigra was homogenized in ice-cold 0.1 M phosphate buffer (pH 7.4) using a Potter–Elvehjem-type glass homogenizer. The homogenates were centrifuged 700g for 5 min to remove cellular debris. Supernatants were used to all the biochemical assays described herein. Results were normalized by the protein content using bovine serum albumin as standard [25].
Thiobarbituric acid reactive species (TBARS)
As an index of lipid peroxidation, we used the formation of TBARS during an acid-heating reaction, which is widely adopted as a method for measurement of lipid redox state, as previously described [26]. Briefly, the samples were mixed with 0.6 mL of 10% trichloroacetic acid (TCA) and 0.5 mL of 0.67% thiobarbituric acid, and then heated in a boiling water bath for 25 min. TBARS were determined by the absorbance in a spectrophotometer at 532 nm. Results are expressed as nmol TBARS/mg protein.
Measurement of protein carbonyls
The oxidative damage to proteins was measured by the quantification of carbonyl groups based on the reaction with dinitrophenylhidrazine (DNPH) as previously described [27]. Briefly, proteins were precipitated by the addition of 20% TCA and redissolved in DNPH and the absorbance read in a spectrophotometer at 370 nm. Results are expressed as nmol carbonyl/mg protein.
Measurement of protein and non-protein thiol content
Other form to analyze oxidative alterations in proteins is to measure the level of protein thiol content. Briefly, sample was diluted in SDS 0.1% and 0.01 M 5,5′’dithionitrobis 2-nitrobenzoic acid (DTNB) in ethanol was added and the intense yellow color was developed and read in a spectrophotometer at 412 nm after 20 min [28]. Free sulfhydryl (–SH) content was estimated in supernatants of 20% TCA precipitated homogenates by the same method. Results are expressed as μmol SH/mg protein.
Antioxidant enzyme activity estimations
Catalase (CAT) activity was assayed by measuring the rate of decrease in H2O2 absorbance in a spectrophotometer at 240 nm [29]. The results of CAT activity are expressed as U CAT/mg protein. Superoxide dismutase (SOD) activity was assessed by quantifying the inhibition of superoxide-dependent adrenaline auto-oxidation in a spectrophotometer at 480 nm, as previously described [30]. The results of SOD are expressed as U SOD/mg protein. Glutathione peroxidase (GPx) activity was determined by measuring the rate of NAD(P)H oxidation in a spectrophotometer at 340 nm, as previously described [31]. The results of GPx are expressed as mM NADPH consumed/min/mg protein. A ratio between SOD and CAT activity (SOD/CAT) was applied to better understand the effect of vitamin A-supplementation upon these two oxidant-detoxifying enzymes that work in sequence converting superoxide anion to water. An imbalance between their activity is thought to facilitate oxidative-dependent alterations in the cellular environment, which may culminates in oxidative stress.
Behavioral task
The behavioral task occurred 15 h after the last treatment, and was performed between 14:00 and 16:00 hours (i.e., during the light phase). Briefly, the animals were gently placed in an open field and left free to explore it for 5 min in order to assess their locomotory and exploratory activity. The open field task was carried out in 60 × 40 cm2 open field surrounded by 50 cm high walls made of brown plywood with a frontal glass wall. The floor of the open field was divided into 12 equal rectangles by black lines. The number of crossings of the black lines and rearings was counted. In behavioral tasks, rats were used only once.
Statistical analysis
Biochemical and behavioral results are expressed as mean ± SD and p-values were considered significant when p < 0.05. Differences in each experimental group were determined by the one-way ANOVA. Comparison between means was carried out using the post hoc Tukey’s test.
Results
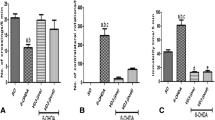
Vitamin A supplementation at 1,000 IU/kg increased lipid peroxidation in the substantia nigra of the rats that were treated for 3 days (Fig. 1a). However, we found a 3.6- to 4.0-fold increase in lipid peroxidation levels in the substantia nigra of the rats that were treated with vitamin A at any dose for 7 days. This effect was maintained in the rats that received vitamin A for 28 days (Fig. 1a). A 2.0- to 3.0-fold increase was found in the protein carbonylation levels in the substantia nigra of the rats that received vitamin A acutely or chronically (Fig. 1b). Vitamin A supplementation at any dose decreased (30–60%) the protein thiol content after all periods of exposition studied here (Fig. 1c). Vitamin A supplementation at 9,000 IU/kg for 3 days induced a decrease (42%) in the nigral non-protein thiol content (Fig. 1d). After 7 days of supplementation, 4,500 and 9,000 IU/kg vitamin A was able to decrease the nigral non-protein thiol content. After 28 days of supplementation, any dose tested decreased the non-protein thiol content in the rat substantia nigra (Fig. 1d).
Effects of acute and chronic vitamin A supplementation on lipid peroxidation (A), protein carbonylation (B), protein thiol content (C), and non-protein thiol content (D) the in adult rat substantia nigra. Data are mean ± SD of 7–12 animals per group performed in triplicate. Different from the respective control group a p < 0.05; b p < 0.01; c p < 0.002; *p < 0.0001, as determined by one-way ANOVA followed by Tukey’s test
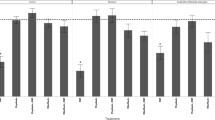
SOD activity did not change in the substantia nigra of the rats that were treated with vitamin A for 3 days (Fig. 2a). However, vitamin A at 2,500, 4,500, and 9,000 IU/kg for 7 days induced a decrease (40%) in the nigral SOD activity (Fig. 2a). Chronically, vitamin A supplementation at 2,500, 4,500, and 9,000 IU/kg increased SOD activity (Fig. 2a). Acute and chronic vitamin A supplementation decreased (1.2- to 2.8-fold) CAT activity (Fig. 2b). GPx activity (Fig. 2c) did not change after vitamin A supplementation. Finally, the SOD/CAT ratio was increased (1.3- to 6.0-fold) after acute or chronic vitamin A supplementation (Fig. 2d).
Effects of acute and chronic vitamin A supplementation on superoxide dismutase (A), catalase (B), and glutathione peroxidase (C) activity. The SOD/CAT ratio is shown in D. Data are mean ± SD of 7–12 animals per group performed in duplicate or triplicate. Different from the respective control group, a p < 0.05; *p < 0.0001, as determined by one-way ANOVA followed by Tukey’s test
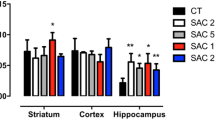
Locomotory and exploratory activity did not change in the rats that received vitamin A for 3 days (Fig. 3a). Surprisingly, supplementation with vitamin A at 2,500, 4,500, or 9,000 IU/kg for 7 days induced a decrease (1.3- to 1.5-fold) in locomotory activity (Fig. 3a). After 28 days of treatment, vitamin A at any dose tested induced a decrease (1.7- to 2.3-fold) in locomotion (Fig. 3a). Vitamin A supplementation at 9,000 IU/kg for 7 days decreased (1.7-fold) the number of rearings (Fig. 3b). Vitamin A at any dose for 28 days induced a decrease (1.9- to 2.4-fold) in the number of rearings performed by the rats in the open field (Fig. 3b).
Discussion
Our results show, for the first time, that vitamin A at both therapeutic and excessive doses alter the nigral redox environment after both acute and chronic supplementation. The brain as a whole is sensitive to oxidative stress due to its high content of peroxidizable fatty acids and relative decreased antioxidant defenses [12]. However, substantia nigra is particularly vulnerable to an oxidative insult due to: (1) its high content of iron and copper ions; (2) increased H2O2 production via MAO function during the degradation of dopamine (DA); and (3) DA is a source to DA-quinones, which react with thiol groups oxidizing it [32–35].
Here, we found that the level of lipid peroxidation (TBARS levels) increased after either acute (7 days) or chronic (28 days) vitamin A supplementation (Fig. 1a). In addition, an exacerbated level of protein carbonylation was also detected in this experimental model (Fig. 1b). Different from lipid peroxidation, protein carbonylation, and decreased protein thiol content (Fig. 1b, c, respectively) occurred earlier, indicating an increased vulnerability of nigral proteins to the oxidative insult induced in this experimental model. Increased TBARS and protein carbonylation levels, and decreased protein thiol content facilitate intra- and inter-molecular cross-links of proteins [36], which in turn induce conformational changes in proteins leading to increased hydrophobicity and aggregation of the protein is very likely to occur. Furthermore, these oxidative alterations on proteins favor the formation of protein aggregates, inducing generalized cellular dysfunction [37].
In situations of increased oxidative stress in substantia nigra, other than prooxidant molecules attack may also decreases its protein thiol content. It was demonstrated that DA-quinones covalently bind to thiol groups in proteins, oxidizing it [33, 34]. Consequently, this oxidation decreases the measurable content of protein thiol content (Fig. 1c). Previous reports suggested that lipid peroxidation participates in the non-specific DA oxidation, since DA is storage within acidic vesicles to avoid auto-oxidation, and loss of membrane integrity facilitates DA release to cytosol [33].
Non-protein thiol content, mainly represented by the reduced-form of glutathione (GSH), was decreased by vitamin A at some doses after 3 or 7 days of treatment (Fig. 1d). This indicates that: (1) there is a decrease in the reduced form of glutathione given the prooxidant circumstances imposed by vitamin A supplementation or (2) a possible action of a detoxifying system, such as glutathione-S-transferase (which uses GSH to conjugate to xenobiotics, eliminating them from the cell), upon vitamin A or its metabolites in substantia nigra and are conjugated with GSH to be exported from neuronal cells when in excess [38]. Non-protein thiol content was decreased by vitamin A at any dose after 28 days of supplementation, suggesting that long periods of exposition to vitamin A decrease an important non-enzymatic antioxidant defense.
In this work, we also show that vitamin A supplementation induced an imbalance in the ratio between SOD activity and CAT activity (SOD/CAT ratio) without any change in GPx activity (Fig. 1a–d). Increased SOD/CAT ratio suggests that there is an increased H2O2 production, since SOD metabolizes O −•2 to H2O2, but CAT converts H2O2 to water at lower rates. In addition, increased H2O2 availability is favored since GPx activity did not change after vitamin A supplementation. This H2O2 excess may reacts with iron and/or copper, which are found in high contents in substantia nigra, and produces OH• via Fenton reaction [12]. Actually, we found increased levels of oxidative damage markers in this experimental model (Fig. 1a–d), as mentioned above.
A behavioral analysis revealed that the animals that received vitamin A supplementation for 7 days walked and explored less a new place (the open field). Chronically, there is a drastic decreased in both locomotion and exploration of open field (Fig. 3a, b). These results indicate that vitamin A even at therapeutic doses (even considered high these doses are used therapeutically, as mentioned in the “Introduction” section) is effective in inducing a behavioral disturbance in adult rats. There are data reporting deleterious effects of excessive vitamin A use regarding behavior in humans [8, 39]. However, further studies would be useful to elucidate whether oxidative stress is a causative factor in the behavioral disturbances observed here.
We did not performed an analysis to investigate retinoids levels in plasma or substantia nigra because it is almost impossible to point to the retinoid responsible for the effects herein demonstrated, since there is a vast number of metabolites derived from vitamin A [40]. Moreover, case reports of vitamin A toxicity have shown serum retinol concentrations within normal limits [41–43], suggesting that serum retinol could not be a good parameter to analyze vitamin A toxicity.
In summary, we show that vitamin A, at therapeutic doses, is able to impairs the redox homeostasis of substantia nigra and to induce a disturbance in a nigral-related behavior. In some parameters, chronic vitamin A was deleterious to substantia nigra, showing no adaptation of the neuronal structure to the insult. Then, we suggest that vitamin A utilization at high doses, even therapeutically, must be rethought, mainly when administered to children or elderly, since some cognitive disturbances, which might be induced by vitamin A, are very difficult to be diagnosed in these stages of life.
References
Wolf G (1984) Multiple functions of vitamin A. Physiol Rev 64:873–937
Semba RD (1998) The role of vitamin A and related retinoids in immune function. Nutr Rev 56:S38–S48
McCaferry P, Zhang J, Crandall JE (2005) Retinoic acid signaling and function in the adult hippocampus. J Neurobiol 66:780–791
Krezel W, Ghyselinck N, Samad TA, Dupe V, Kastner P, Borrelli E, Chambon P (1998) Impaired locomotion and dopamine signaling in retinoids receptor mutant mice. Science 279:863–867
Samad TA, Krezel W, Chambon P, Borrelli E (1997) Regulation of dopaminergic pathways by retinoids: activation of the D2 receptor promoter by members of the retinoic acid receptor-retinoid X receptor family. Proc Natl Acad Sci USA 94:14349–14354
Wang HF, Liu FC (2005) Regulation of multiple dopamine signal transduction molecules by retinoids in the developing striatum. Neuroscience 134:97–105
Bendich A, Langseth L (1989) Safety of vitamin A. Am J Clin Nutr 49:358–371
Myhre AM, Carlsen MH, Bohn SK, Wold HL, Laake P, Blomhoff R (2003) Water-miscible, emulsified, and solid forms of retinol supplements are more toxic than oil-based preparations. Am J Clin Nutr 78:1152–1159
Geelen JA (1979) Hypervitaminosis A induced teratogenesis. CRC Crit Rev Toxicol 6:351–375
O’Reilly KC, Shumake J, Gonzalez-Lima F, Lane MA, Bailey SJ (2006) Chronic administration of 13-cis-retinoic acid increases depression-related behavior in mice. Neuropsychopharmacol 31:1919–1927
Hazen PG, Carney JF, Walker AE, Stewart JJ (1983) Depression- a side effect of 13-cis-retinoic acid therapy. J Am Acad Dermatol 9:278–279
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Abou-Sleiman P, Muqit MMK, Wood NW (2006) Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci 7:207–219
Zaidi SMKR, Banu N (2004) Antioxidant potential of vitamins A, E, and C in modulating oxidative stress in rat brain. Clin Chim Acta 340:229–233
Moreira JCF, Dal-Pizzol F, Von Endt D, Bernard EA (1997) Effect of retinol on chromatin structure in Sertoli cells: 1,10-phenanthroline inhibit the increased DNAse I sensitivity induced by retinol. Med Sci Res 25:635–638
Dal-Pizzol F, Klamt F, Frota MLC Jr, Moraes LF, Moreira JCF, Benfato MS (2000) Retinol supplementation induces DNA damage and modulates iron turnover in rat Sertoli cells. Free Rad Res 33:677–687
Klamt F, Dal-Pizzol F, Ribeiro NC, Bernard EA, Benfato MS, Moreira JCF (2000) Retinol-induced elevation in ornithine decarboxylase activity in cultured Sertoli cells is attenuated by free radical scavenger and by iron chelator. Mol Cell Biochem 208:71–76
Dal-Pizzol F, Klamt F, Benfato MS, Bernard EA, Moreira JCF (2001) Retinol supplementation induces oxidative stress and modulate antioxidant enzyme activity in rat Sertoli cells. Free Rad Res 34:395–404
Frota MLC Jr, Klamt F, Dal-Pizzol F, Schiengold M, Moreira JCF (2004) Retinol-induced mdr1 and mdr3 modulation in cultured rat Sertoli cells is attenuated by free radical scavengers. Redox Rep 9:161–165
Klamt F, De Oliveira MR, Moreira JCF (2005) Retinol induces permeability transition and cytochrome c release from rat liver mitochondria. Biochim Biophys Acta 1726:14–20
Gelain DP, Cammarota M, Zanotto-Filho A, De Oliveira RB, Dal-Pizzol F, Izquierdo I, Bevilaqua L, Moreira JCF (2006) Retinol induces the ERK1/2-dependent phosphorylation of CREB through a pathway involving the generation of reactive oxygen species in cultured Sertoli cells. Cell Signal 18:1685–1694
Cohen G, Farooqui R, Kesler N (1997) Parkinson disease: a new link between monoamine oxidase and mitochondrial electron flow. Proc Natl Acad Sci USA 94:4890–4894
Norum KR (1993) Acute myeloid leukaemia and retinoids. Eur J Clin Nutr 47:77–87
Lam HS, Chow CM, Poon WT, Lai CK, Yeung WL, Hui J, Chan AY, Ng PC (2006) Risk of vitamin A toxicity from candy-like chewable vitamin supplements for children. Pediatrics 118:820–824
Lowry OH, Rosebrough AL, Randal RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Hirsch EC (1992) Why are nigral catecholaminergic neurons more vulnerable than others in Parkinson’s disease. Ann Neurol 32:S88–S93
Lotharius J, Brundin P (2002) Pathogenesis of Parkinson’s disease: dopamine, vesicles and α-synuclein. Nat Rev Neurosci 3:932–942
Graham DG (1978) Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol 14:633–643
Dawson TM, Dawson VL (2003) Molecular pathways of neurodegeneration in Parkinson’s disease. Science 302:819–822
Goetz ME, Gerlach M (2004) Formation of radicals. In: Herdegen T, Delgado-Garcia J (eds) Brain damage and repair. Kluwer, London, pp 135–164
Mattson MP, Magnus T (2006) Ageing and neuronal vulnerability. Nat Rev Neurosci 7:278–294
Fang YZ, Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition 18:872–879
Hathcock JN, Hattan DG, Jenkins MY, McDonald JT, Sundaresan PR, Wilkening VL (1990) Evaluation of vitamin A toxicity. Am J Clin Nutr 52:183–202
Napoli JL (1999) Interactions of retinoids binding proteins and enzymes in retinoids metabolism. Biochim Biophys Acta 1440:139–162
Frame B, Jackson CE, Reynolds WA, Umphrey JE (1974) Hypercalcemia and skeletal effects in chronic hypervitaminosis A. Ann Intern Med 80:44–48
Ellis JK, Russel RM, Makrauer FL, Schaefer EJ (1986) Increased risk for vitamin A toxicity in severe hypertriglyceridemia. Ann Intern Med 105:877–879
Croquet V, Pilette C, Lespine A (2000) Hepatic hypervitaminosis A: importance of retinyl ester level determination. Eur J Gastroenterol Hepatol 12:361–364
Acknowledgments
This work was supported by grants of CNPq, FAPERGS, and PROPESQ-UFRGS. M. R. de Oliveira and R. B. Silvestrin are recipients of a CNPq fellowship. T. Mello e Souza was supported by PROFIX (CNPq, Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Oliveira, M.R., Silvestrin, R.B., Mello e Souza, T. et al. Therapeutic Vitamin A Doses Increase the Levels of Markers of Oxidative Insult in Substantia Nigra and Decrease Locomotory and Exploratory Activity in Rats after Acute and Chronic Supplementation. Neurochem Res 33, 378–383 (2008). https://doi.org/10.1007/s11064-007-9438-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9438-2