Abstract
The present study sought to evaluate the effect of a newly synthesized selenium compound, dicholesteroyl diselenide (DCDS) and diphenyl diselenide (DPDS) on the activities of delta-aminolevulinate dehydratase and Na+/K+-ATPase in the rat brain. The glutathione peroxidase mimetic activity of the two compounds as well as their ability to oxidize mono- and di- thiols were also evaluated. The antioxidant effects were tested by measuring the ability of the compounds to inhibit the formation of thiobarbituric acid reactive species and also their ability to inhibit the formation of protein carbonyls. The results show that DPDS exhibited a higher glutathione peroxidase mimetic activity as well as increased ability to oxidize di-thiols than DCDS. In addition, while DPDS inhibited the formation of thiobarbituric acid reactive species and protein carbonyls, DCDS exhibited a prooxidant effect in all the concentration range (20–167 μM) tested. Also the activities of cerebral delta-aminolevulinate dehydratase and Na+/K+ ATPase were significantly inhibited by DPDS but not by DCDS. In addition, the present results suggested that the inhibition of Na+/K+ ATPase by organodiselenides, possibly involves the modification of the thiol group at the ATP binding site of the enzyme. In conclusion, the results of the present investigation indicated that the non-selenium moiety of the organochalcogens can have a profound effect on their antioxidant activity and also in their reactivity towards SH groups from low-molecular weight molecules and from brain proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is increasing evidence that the mammalian brain may be exceptionally vulnerable to oxidative stress through oxygen radical attack which possibly contribute to cerebral ischemic injury by promoting membrane lipid peroxidation and oxidative damage to DNA and proteins [1–5]. In fact, several reports have implicated free radical overproduction in the etiology of a variety of acute and chronic neurodegenerative situations [5–7]. However, clinically effective drugs for the treatment of these diseases are rare. Consequently, continued efforts geared towards the development and biological testing of new antioxidant compounds for the treatment of these neurological disorders have increased considerably in recent times [8–12].
Reports have shown that these selenium-containing organic compounds are generally more potent antioxidants than classical antioxidants and this fact serves as an impetus for an increased interest in the rational design of synthetic organoselenium compounds [13, 14]. The development of these organoselenium compounds is based on their ability to mimic the antioxidant enzyme glutathione peroxidase (GSHPx) activity in vitro. The glutathione peroxidase -mimetic of organoselenium compounds, which, like the native enzyme, rely on the redox cycling of selenium, have been reported in the literature [13, 15]. In addition, a recent report described the thioredoxin reductase and thioredoxin peroxidase mimetic activity of organoselenides and related these effects as another possible mechanism for their observed antioxidant actions [16].
Diphenyl diselenide is a synthetic organodiselenide with potent antioxidant potentials [15, 17–19]. Earlier reports have indicated that DPDS affects a number of neuronal processes [14, 20, 21]. Nogueira et al. reported that DPDS increases the basal activity of adenylyl cyclase and inhibits [3H]glutamate, [3H]MK-801, and unstable [3H] guanylyl-imidodiphate binding to rat synaptic membrane preparations after both in vitro and ex vivo exposure [10]. Further study reveals that DPDS effectively reduced 45Ca influx into isolated nerve endings when a nondepolarizating condition was used or when 4-aminopyridine (4-AP) was used as a depolarizing agent [15]. Recently DPDS was also reported to inhibit the cerebral Na+, K+-ATPase in a concentration dependent manner and that the inhibition may occur through a change in the crucial thiol groups of this enzyme [22].
There are strong points of evidence suggesting that modification of the organic moiety of organoselenium compounds can have a profound effect on their biological activity [15, 23]. On the other hand, there are reports indicating that compounds containing the steroidal molecules could possess pharmacological potency and that small alterations in the structure of these steroids can greatly affect their receptor binding affinity and biological activity [24]. In fact, several studies have demonstrated that the modulation of the various heterocyclic rings of various steroids was effective in the production of a variety of compounds possessing potent biological activities [25, 26]. These observations aforementioned prompted the synthesis of a novel organoselenide compound in the organic synthesis unit of our laboratory. This novel compound possesses the cholesterol steroidal molecule as the organic moiety component.
However, earlier observations [27, 28] have demonstrated that chronic exposure to high doses of diselenides may cause central effects in animal models, suggesting that the brain may be a potential target for the toxic effect of organoselenium compounds. In addition, recent reports from our laboratory have indicated that DPDS has proconvulsant effect in mice model, an effect that can be further related to its neurotoxicity [23, 29]. However, the mechanism(s) involved in the neurotoxicity of DPDS is still unknown. Although DPDS possess antioxidant activity in vitro and in vivo including neuroprotective effects [30], it can also be neurotoxic. One can speculate that the neuroprotective effect of organochalcogens may be related to their antioxidant activity and that its neurotoxic activity may be related to its ability to oxidize thiol groups in enzymes. Therefore, the present study was aimed at comparing the in vitro antioxidant potential of this novel compound, DCDS with that of DPDS in the rat brain. In addition, the possible inhibitory effects of these two compounds on the thiol containing enzymes from brain namely cerebral delta aminolevulinic acid dehydratase and Na+/K+-ATPase were also evaluated. In fact, the main objective of this study was to investigate how changes in the non-selenium moiety of organochalcogens could modify their reactivity towards thiols and also whether their antioxidant properties could be modified by these changes. Furthermore, we also carried out a detailed study on the possible protective effect of ligands and substrate on the inhibitory effect of DPDS on brain Na+,K+-ATPase.
Experimental procedure
Chemicals
DPDS and DCDS (Scheme 1) were synthesized according to literature methods [31]. These drugs were dissolved in 99% ethanol. Analysis of the 1HNMR and 13CNMR spectra showed that all the compounds obtained presented analytical and spectroscopic data in full agreement with their assigned structures. The purity of the compounds were assayed by high resonance mass spectroscopy (HRMS) and was higher that 99.9%. All chemicals used were of analytical grade and obtained from Sigma–Aldrich, FLUKA, BDH and other standard commercial suppliers. ATP (sodium salt) was purchased from Sigma–Aldrich Co.
Animals
Male adult Wistar rats (200–250 g) from our own breeding colony were used. Animals were kept in separate animal cages, on a 12-h light: 12-h dark cycle, at a room temperature of 22–24°C, and with free access to food and water. The animals were used according to the guidelines of the Committee on Care and Use of Experimental Animal Resources, the Federal University of Santa Maria RS, Brazil.
Preparation of tissue homogenate for thiobarbituric acid reactive species (TBARS) assay
Rats were decapitated under mild ether anesthesia and the cerebral tissue (whole brain) was rapidly removed, placed on ice and weighed. Tissues were immediately homogenized in cold 10 mM Tris–HCl, pH 7.4 (1/10, w/v) with 10 up-and-down strokes at approximately 1,200 rev/min in a Teflon-glass homogenizer. The homogenate was centrifuged for 10 min at 4,000g to yield a pellet that was discarded and a low-speed supernatant (S1). An aliquot of 100 μl of S1 was incubated for 1 h at 37°C in the presence of both organodiselenides (final concentrations range of 21–167 μM), with and without the prooxidants; iron (final concentration(10 μM) and sodium nitroprusside (SNP) (final concentration 3 μM). This was then used for lipid peroxidation determination. One rat brain was used per experiment. Production of TBARS were determined as described by method of Ohkawa et al. [32] excepting that the buffer of color reaction have a pH of 3.4. The color reaction was developed by adding 300 μl 8.1% SDS to S1, followed by sequential addition of 500 μl acetic acid/HCl (pH 3.4) and 500 μl 0.8% of thiobarbituric acid (TBA). This mixture was incubated at 95°C for 1 h. TBARS produced were measured at 532 nm and the absorbance was compared to that of a standard curve obtained using malondialdehyde (MDA).
Effect of organodiselenides on protein carbonyls formation
Brain was homogenized in a proportion of 1:20 in 10 mM Tris–HCl (pH 7.4). Homogenates were then incubated in the presence of organodiselenides (concentration range 20–80 μM) and iron (5 μM) and control groups were also prepared. The tubes were incubated for 18 h. After incubation, the protein carbonyls determination was carried out as described by Reznick and Packer [33] with some modifications. Briefly, three 0.8-ml brain homogenate samples were placed in glass tubes. Thereafter, 0.2 ml of 2,4-dinitrophenylhydrazine (DNPH) 10 mM in 2.0 M HCl was added to two of the tubes while the other tube contains only 0.2 ml of 2.0 M HCl solution (blank for DNPH-independent A365, to correct the background absorbances in brain samples). Tubes were incubated for 60 min at room temperature, in the dark. Samples were vortexed every 15 min. Then, 0.5 ml of denaturizing buffer (sodium phosphate buffer, pH 6.8, containing SDS 3%), 1.5 ml of ethanol and 1.5 ml of hexane were added, the mixture was vortexed for 40 s and centrifuged for 5 min at 2,000 rpm. The pellet obtained was separated and washed 3 times with 2 ml ethanol: ethyl acetate (1:1, v/v). The precipitate was dissolved in 1 ml denaturizing buffer solution and was incubated for 10 min at 37°C with mixing. Any insoluble material was removed by additional centrifugation. Carbonyl content was calculated from the peak absorbance of the spectra at 355–390 nm, using an absorption coefficient of 22,000 M-1 cm-1. Protein was measured by the method of Lowry et al. [34], using bovine serum albumin as standard. Results were expressed as nmol carbonyl/mg of protein.
Thiol peroxidase activity
The catalytic effects of the organodiselenides on the reduction of H2O2 by reduced glutathione were assessed using the rate of glutathione (GSH) oxidation. Free –SH groups were determined according to Ellman [35]. DPDS and DCDS at concentration range of 50–150 μM were incubated in the medium containing GSH (5.0 mM) with and without H2O2 (2 mM) at 2.5 mins intervals for 15 min. Aliquots of the reaction mixture (200 μl) were checked for the amount of GSH at the indicated time intervals.
Oxidation of mono and di-thiols
The rate of thiol oxidation was determined in the presence of 50 mM Tris–Cl, pH 7.4, and 250 μM of organodiselenides. The rate of thiol oxidation was evaluated by measuring the disappearance of –SH groups. Free –SH groups were determined according to Ellman [35]. Incubation at 37°C was initiated by the addition of the thiol compounds. Aliquots of the reaction mixture (100 μl) were checked for the amount of –SH groups at 412 nm after 90–120 min of addition of color reagent 5’5’-dithio-bis(2-nitrobenzoic) acid (DTNB).
ð-Aminolevulinate dehydratase (ð-ALA-D) activity
Cerebral ð-ALA-D activity was assayed according to the method of Sassa [36] by measuring the rate of product porphobilinogen (PBG) formation except that 100 mM potassium phosphate buffer and 2.5 mM ð-ALA were used. Rats cerebral were homogenized in Tris–HCl in the proportion 1:3 (w/v) and centrifuged at 4,000g for 15 min. An aliquot of 100 μl of supernatant (S1) was incubated for 3 h at 37°C. Reaction was linear in relation to protein and time of incubation. The reaction product was determined using modified Erlich’s reagent at 555 nm.
Effect of organodiselenides on Na+, K+-ATPase activity
Immediately after the sacrifice, the brain was removed and the homogenate was prepared in 0.05 M Tris–HCl, pH 7.4, sucrose buffer (pH 7.4). The homogenate was centrifuged at 2,000g at 4°C for 7 min and supernatant was again centrifuged at 12,000g for 10 min. The pellets (P2) were reconstituted in the homogenizing buffer to give a final protein concentration of 5 to 6 mg/ml and used for assay of Na+, K+-ATPase. The reaction mixture for Mg2+-dependent-Na+, K+-ATPase activity assay contained 3 mM MgCl, 125 mM NaCl, 20 mM KCl and 50 mM Tris–HCl, pH 7.4 and 100–120 μg of protein, in a final volume of 500 μl. The reaction was initiated by addition of ATP to a final concentration of 3.0 mM. Controls were carried out under the same conditions with the addition of 0.1 mM ouabain. Na+, K+-ATPase activity was calculated by the difference between the two assays. Released inorganic phosphorous (Pi) was measured by the method of Fiske and Subbarow [37].
To check whether pre-incubation of homogenates without a cationic component of the assay medium will affect the interaction of organodiselenides with the Na+/K+-ATPase activity, organodiselenides and enzyme (P2) were incubated at 37°C for 10 min, with the selective exclusion of each Mg2+, Na+, K+ in the preincubating medium. All the experiments were conducted at least three times and similar results were obtained. Protein was measured by the method of Lowry et al. [34], using bovine serum albumin as standard.
For all enzyme assays, incubation times and protein concentration were chosen to ensure the linearity of the reactions. All samples were run in duplicate. Controls with the addition of the enzyme preparation after mixing with trichloroacetic acid (TCA) were used to correct for nonenzymatic hydrolysis of substrates. Enzyme activity was expressed as nmol of phosphate (Pi) released min−1 mg protein−1.
Statistical analysis
Results were analyzed by one-way, two way or three-way analysis of variance (ANOVA) and this is indicated in text of results. Duncan’s Multiple Range Test and paired t test were applied where appropriate. Differences between groups were considered to be significant when P < 0.05.
Results
Effect of organodiselenides on lipid peroxidation induced by iron and sodium nitruprusside
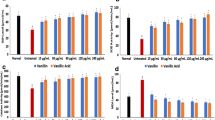
Three-way ANOVA (2 (DPDS/DCDS) × 5 concentrations of selenides × 2 (basal/iron) revealed a significant third-order interaction (P < 0.0001). Interaction was significant because, as can be seen in Fig. 1A, DPDS was able to exert significant inhibitory effect on basal and iron induced lipid peroxidation in the low-speed supernatant from brain homogenate. However, DCDS exert a prooxidant like effect on basal and iron induced lipid peroxidation in the brain.
(A) Effect of different concentrations of DPDS and DCDS on basal and iron (II) (10 μM)—induced TBARS production in the low-speed supernatant from brain homogenate. (B) Effect of different concentrations of DPDS and DCDS on basal and SNP (3 μM)—induced TBARS production in the low-speed supernatant from brain homogenate. Data show mean ± SEM values average from four independent experiments performed in quadruplicate in different days. Data are presented as mean ± SEM and post-hoc comparisons were done by Duncan´s multiple range test. Different letters indicate a significant difference in relation to the control (no compounds, but with prooxidant) or to basal (no pro-oxidant) at P < 0.05
Three-way ANOVA (2 (DPDS/DCDS) × 5 concentrations of selenides × 2 (basal/sodium nitroprusside)) revealed a significant third-order interaction (P < 0.0001). Interaction was significant because, as can be seen in Fig. 1B, DCDS elicited a prooxidant effect on the basal production of TBARS by brain low-speed supernatants (S1), but not in the presence of sodium nitroprusside. Sodium nitroprusside caused a marked increase in TBARS production and this effect was not modified by the DCDS. In contrast, DPDS exerted a profound antioxidant effect both on the basal and sodium nitroprusside-stimulated TBARS production.
Effect of organodiselenides on formation of protein carbonyls induced by iron
Figure 2 shows the effect of the two diselenides on the formation of protein carbonyls in the low-speed supernatant from brain homogenate. Three-way ANOVA revealed that iron (II) induced a marked increase on the formation of protein carbonyls which was markedly inhibited by DPDS in a concentration dependent manner. However, the DCDS did not have any protective effect on the formation of protein carbonyls at all the concentrations tested. This was indicated by the significant third order interaction (2 (DPDS/DCDS) × 6 concentrations of selenides × 2 (basal/iron (II); P < 0.01)
Effect of DPDS and DCDS on the formation of protein carbonyls in the low-speed supernatant from brain homogenate in the absence and presence of 5 μM Fe2+. Data are presented as mean ± SEM for 5 independent experiments done in duplicate in different days. Three-way ANOVA was followed by Duncan´s multiple range test. Different letters indicate a significant difference in relation to the control (no compounds, but with prooxidant) at P< 0.05
Glutathione peroxidase mimetic activity of organodiselenides
Three way ANOVA of glutathione peroxidase activity of selenides (2 compounds × 2 conditions: with and without H2O2 × 7 sample times) revealed a significant third-order interaction (P < 0.001). In line with this, the rate of GSH oxidation in the absence of peroxide was similar for control (no selenide) and for the selenides (Fig. 3A); however, in the presence of hydrogen peroxide, the oxidation of GSH was significantly higher in the presence of 100 μM DPDS during all the sampling period (Fig. 3B). In contrast, 100 μM DCDS increased the oxidation of GSH only at 2.5 and 5 min sampling times (Fig. 3C, with minus without H2O2).
The peroxidase mimetic activity of DPDS and DCDS (at 100 μM) in the absence (A) and in the presence (B) of hydrogen peroxide. The peroxidase activity was evaluated at different times range from 0 min to 15 min at 2.5 min intervals. Data are the means of five to seven independent experiments carried out in different days. Data are presented as mean ± SEM and post-hoc comparisons were done by Duncan´s multiple range test. (C) The difference in the peroxidase mimetic activity of DPDS and DCDS (at 100 μM) in the presence of hydrogen peroxide. The difference in peroxidase activity was calculated at different times range from 0 min to 15 min at 2.5 min intervals. Data are the means of five to seven independent experiments done in different days. Data are expressed as mean ± SEM and post-hoc comparisons were done by Duncan´s multiple range test
We also performed a two-way ANOVA (2 compounds × 7 sample times) using as dependent variables the differences between the oxidation of GSH measured in the presence of 100 μM DPDS and DCDS to that in absence of selenides. These results are depicted in Fig. 3C and the results of statistical analysis revealed a significant selenides × sample times interaction (P < 0.001). In fact, there was a significant difference in the rate of GSH oxidation between the two selenides and this increase as a function of time of incubation (Fig. 3C). Thus, DPDS demonstrated thiol peroxidase-like activity higher than that of DCDS and similar results were obtained when 50 and 150 μM of organodiselenides were used (data not shown).
Effect of organodiselenides on oxidation of mono and dithiols
The rate of cystein oxidation was not modified by organodiselenides (data not shown). Two-way ANOVA (3 compounds: control, DPDS or DCDS × 4 sample times) revealed no significant effects (P > 0.10). Similarly, the rate of GSH oxidation was not modified by organodiselenides (data not shown).
The rate of DMPS oxidation was increased by DPDS and DCDS (Fig. 4). Two-way ANOVA (3 compounds: control, DPDS or DCDS × 4 sample times) revealed a significant organoselenides × sample time interaction (P < 0.01). In fact, the oxidation of DMPS was increased as a function of time and its oxidation was accelerated by organoselenides (Fig. 4).
Effects of DPDS and DCDS on the rate of DMPS (a dithiol) oxidation. The rate of oxidation was evaluated at the indicated times. Data are the means of five to seven independent experiments carried out in different day. Data are expressed as mean ± SEM and post-hoc comparisons were done by Duncan´s multiple range test. Different letters indicate a significant difference in relation to the control
The rate of DTT oxidation was increased by DPDS (Fig. 5). Two-way ANOVA (3 compounds: control, DPDS or DCDS × 5 sample times) revealed a significant organoselenides × time interaction (P < 0.01). In fact, the oxidation of DTT was increased as a function of time, but only in the presence of DPDS (Fig. 5).
Effects of DPDS and DCDS on the rate of DTT (a dithiol) oxidation. The rate of oxidation was evaluated at the indicated times. Data are the means of five to seven independent experiments carried out in different day. Data are expressed as mean ± SEM and post-hoc comparisons were done by Duncan´s multiple range test. Different letters indicate a significant difference in relation to the control
Effect of organodiselenides on the activity of ð-aminolevulinic acid dehydratase
Analysis of cerebral ð-aminolevulinic acid dehydratase activity revealed that DPDS caused a significant inhibition of enzyme activity. In contrast, DCDS did not significantly inhibit cerebral ð-aminolevulinic acid dehydratase. These results were confirmed by a significant organoselenides × concentration interaction (Fig. 6, P < 0.01).
Effect of DPDS and DCDS on the activity of δ-ALA-D in the low-speed supernatant from brain homogenate. Each point represents the mean ± SEM for four independent assays with different supernatant preparations carried out in different days. Two-way ANOVA was followed by Duncan´s multiple range test. Different letters indicate a significant difference in relation to the control (no compounds) at P< 0.05
Effect of organodiselenides on the activity of Na+/K+-ATPase
Three-way ANOVA of Na+/K+-ATPase activity (6 pre-incubation conditions × 2 organoselenides × 5 concentrations) revealed a significant third-order interaction that was a consequence to the fact that the inhibitory effect of diselenides (mainly of DPDS) was markedly affected by the pre-incubation condition (Fig. 7). In order to better explore the influence of pre-incubation condition on the inhibitory effect of selenides, we performed separate statistical analyses (2 organoselenides × 5 concentrations) for each pre-incubation condition. When the enzyme were pre-incubated for 10 min in the presence of Na+, K+ and Mg2+ (pre-incubation without ATP), two-way ANOVA revealed a significant organoselenide type × concentrations interaction (P < 0.001). Interaction was significant because DPDS caused a concentration dependent inhibition of ATP hydrolysis, whereas DCDS did not modify the enzyme activity (Fig. 7a). In the absence of pre-incubation (reaction started by the simultaneous addition of ATP and enzyme), two-way ANOVA yielded no significant main or interaction effects (Fig. 7b). Two-way ANOVA of the ATPase activity after pre-incubation of the enzyme for 10 min in a medium containing Na+, K+ and ATP (pre-incubation without Mg2+) revealed a significant interaction of organoselenide type × concentrations. In a similar way to that observed when pre-incubation was carried out in the absence of ATP, DPDS caused a concentration dependent inhibition of the ATP hydrolysis and DCDS did not inhibit the enzyme activity (Fig. 7c). Two-way ANOVA of enzyme activity when Na+ was omitted from the pre-incubation medium yielded a significant diselenide type × concentration interaction (P<0.05). This was significant because DPDS caused a significant inhibition of ATP hydrolysis when tested at 200 μM (Fig. 7d). Two-way ANOVA of ATP hydrolysis determined after pre-incubation in the absence of K+ yielded non-significant main or interaction effects (P > 0.10; Fig. 7e). Two-way ANOVA of Na+, K+-ATPase determined after 10 min of pre-incubation in the absence of Na+, K+ and Mg2+ yielded a significant diselenide type × concentration interaction (P < 0.01). Interaction was significant because DPDS caused a concentration dependent inhibition of ATP hydrolysis, whereas DCDS caused a significant inhibition of enzyme activity only when tested at 200 μM (P > 0.05, Fig. 7f).
Influence of pre-incubation conditions on the inhibitory effects of DPDS and DCDS on Cerebral Na+/K+-ATPase. (a) reaction was started by addition of ATP after 10 min of pre-incubation in the presence of Na+, K+ and Mg2+; (b) reaction was started by the simultaneous addition of ATP, diorganoselenides and brain enzyme; (c) reaction was started by addition of Mg2+ after 10 min of pre-incubation in the presence of Na+, K+ and ATP; (d) reaction was started by addition of Na+ after 10 min of pre-incubation in the presence of Mg2+, K+ and ATP; (e) reaction was started by addition of K+ after 10 min of pre-incubation in the presence of Mg2+, Na+ and ATP; (f) reaction was started by addition of Na+, K+ and Mg2+ after 10 min of pre-incubation in the presence of ATP. Results are expressed as nmol Pi min−1 mg protein−1. Data represent the mean ± SD of three different experiments carried out in different days and were tested by two-way ANOVA followed by Duncan’s test. *Indicates a significant difference from control (no diselenide) at P < 0.05
Discussion
In our earlier studies we have demonstrated that the substitution of an aromatic moiety of diorganyl chalchogenides could modulate their effects [15, 23]. Here we also verified that the substitution of a cholesteryl moiety in place of phenyl moiety on diselenides changes their antioxidant and thiol peroxidase like-properties. The thiol peroxidase-like activity of diorganyl chalcogenides can explain, at least in part, the in vitro antioxidant properties of these compounds [38–42]. The results indicate that DPDS presented higher thiol peroxidase activity (Fig. 3) and demonstrated better antioxidant potential than DCDS. In addition, DPDS demonstrated higher potential for –SH group oxidation than DCDS (Figs. 4, 5). It is interesting to note that GSH was not oxidized by DPDS or DCDS in the absence of peroxide. This may be a consequence to the fact that GSH has a lower redox potential than dithiols, consequently, GSH has a weak potency to open the Se–Se bound of DPDS, but it can do this when the selenenic acid is formed after reaction with peroxide.
The mechanism by which iron causes deleterious effect is by reacting with superoxide anion (O •2 ) and hydrogen peroxide (H2O2) to produce the hydroxyl radical (OH•) via the Fenton reaction [43]. These radicals can also lead to the formation of other reactive oxygen species (ROS) [44]. The overproduction of ROS can directly attack the polyunsaturated fatty acids of the cell membranes and induce lipid peroxidation. However the synthetic DCDS did not protect against iron induced lipid peroxidation (Fig. 1) or protein carbonyls formation (Fig. 3) in the rat brain. In fact, our results (Fig. 1) demonstrate that it acts synergistically to augment lipid peroxidation products in brain. DPDS on the other hand was able to significantly protect the formation of TBARS in the rat brain homogenate subjected to iron (Fig. 1). In addition, attack by ROS upon proteins can damage several amino acid residues, including histidine, tryptophan, cysteine, proline, methionine, arginine and lysine. Oxidative damage to several of these amino acid residues and/or to the peptide backbone of proteins can generate carbonyl products [45]. However, the result presented in Fig. 2 shows that DPDS was able to exert a significant inhibitory effect on the formation of protein carbonyls; on the other hand, the DCDS had no protective effect on formation of carbonyls at all the concentration tested.
Photodegradation process of sodium nitroprusside (SNP) ultimately produces NO*, [(CN)5-Fe]3+ and [(CN)4-Fe]2+ species [46–48]. There is a growing number of studies concerning the role of NO, a molecule that is regarded as universal neuronal messenger in the central nervous system, in the pathophysiology of such disorders as Alzheimer’s and Parkinson’s diseases, stroke, trauma, seizure disorders, etc. [49–52]. NO is a free radical with short half-life (<30 s). Although NO acts independently, it also may cause neuronal damage in cooperation with other reactive oxygen species (ROS) [49, 53]. Thus, the protective effect of DPDS can be related to the ability of its selenol intermediate to react with the potentially toxic NO. Recently, we have observed that DPDS protects endothelial cells from the toxic effect of NO, which strongly indicate that DPDS can scavenge NO.
The iron moiety of SNP may have a free iron coordination site for H2O2, which could trigger the generation of highly reactive oxygen species, such as hydroxyl radicals (OH*) via the Fenton reaction [43]. Therefore following a short-lasting release of NO in the brain, iron moiety of SNP could cause a long-lasting generation of OH* radicals and oxidant stress/injury similar to that of ferrous citrate iron complexes which may initiate a lipid peroxidation chain reaction and oxidative brain injury [54]. The result presented in Fig. 1B indicated that while the DPDS exerted an antioxidant effect on in vitro sodium nitroprusside induction of lipid peroxidation in brain homogenate, whereas DCDS had no antioxidant effect. The absence of antioxidant activity of DCDS may be related to its low glutathione-peroxidase like activity. As consequence of this low activity, DCDS is a weaker scavenger of OH* radical. Furthermore, since the thiol-peroxidase activity and the ability of organoselenium compounds to scavenge free radicals will depend in their ability to form a selenol [15], we can suppose that the bulky moiety DCDS hampers the opening of the Se–Se bond and accounts for its low antioxidant activity.
δ-Aminolevulinate dehydratase (δ-ALA-D) is a sulfhydryl containing enzyme that is inhibited by a variety of sulfhydryl reagents [5, 15, 55–58]. This enzyme catalyzes the condensation of two ð-aminolevulinic acid (ALA) molecules with the formation of porphobilinogen, which is a heme precursor [59]. Consequently, δ-ALA-D inhibition may impair heme biosynthesis [60] and can result in the accumulation of ALA, which may affect the aerobic metabolism and may have some prooxidant activity [61]. DPDS inhibited the activity of the enzyme in a concentration dependent manner and at 100 μM, it exerted a complete inhibition on its activity (Fig. 6). However the novel DCDS exerted only a weak (< 10%) non-significant inhibitory effect on the activity of ALA-D. Earlier studies from our laboratory [15] have shown that the inhibition of δ-ALA-D by selenium compounds involves the oxidation of essential SH groups of the enzyme. In line with this, DPDS oxidized DTT (a dithiol), while DCDS was not effective as oxidant of DTT. Therefore with reference to our earlier observation, the rate of oxidation of DTT could explain the observed inhibition of the thiol containing protein ð-ALA-D.
The effect of the organodiselenides on another thiol containing protein, Na+K+-ATPase was also studied. Our present results (Fig. 7a, c, f) clearly indicate that DPDS inhibit Na+, K+-ATPase activity while DCDS inhibited the enzyme only at the highest concentration and only after the pre-incubation was carried out in the absence of Na+, K+ and Mg2+. Borges et al. [22] observed that dithiothreitol was able to revert the inhibition caused by DPDS and suggested that the possible mechanism of inhibition of the ATPase enzyme by organodiselenides may be related to their interaction with cysteinyl residues that are important for the enzyme activity. Other reports have demonstrated the importance of thiol groups for Na+, K+-ATPase catalysis. In fact, –SH groups of this enzyme is highly susceptible to oxidizing agents [62–64]. In addition, further studies (Fig. 7) with selective exclusion of each of the component of the cations in the incubating medium reveal that the inhibition of Na+/K+ ATPase by DPDS may possibly involve the modification of the ATP binding site of the enzyme. In conformity with our earlier observation, we may further conclude that the observed inhibition of Na+/K+ ATPase by organodiselenides, possibly involves the modification of the critical –SH group at the ATP binding site of the enzyme. In fact, the exclusion of magnesium from the incubating medium possibly impose a structural deformity on the magnesium dependent ATP, thereby preventing the competitive binding of the ATP to its site in the presence of organodiselenides. This hypothesis is strongly supported by the fact that inclusion of magnesium cation in the incubating medium abolished the observed inhibitory effect by DPDS (Fig. 7b, c, f). This is the first study in our laboratory suggesting the possible interaction of DPDS with the critical –SH at the ATP binding site of the cerebral Na+ K+ ATPase.
In conclusion, the results of the present investigation indicate that DPDS can be considered more potent oxidant of thiols than DCDS. However, DPDS also can use thiols to reduce peroxides, which can accounts for its pharmacological properties as potential scavenger of free radicals. Since DCDS presented a weak ability to oxidize thiols and to decompose hydrogen peroxide, we can expect that it will be less effective than DPDS as a potential scavenger of oxygen free radical. In contrast, since the toxicity of diselenides can be related to their ability in oxidize thiol groups we can suppose that DCDS should be less toxic than DPDS.
References
Siesjo B, Agardh CD, Bengtsson F (1989) Free radicals and brain damage. Cerebrovasc Brain Metab Rev 1:165–211
Taystman RJ, Kirsch JR, Koehler RC (1991) Oxygen radical mechanisms of brain injury following ischaemia and reperfusion. J Appl Physiol 71:1185–1995
Dawson VL, Dawson TM (1996) Free radicals and neuronal cell death. Death Differ 3:71–76
Puntel RL, Nogueira CW, Rocha JBT (2005) Krebs cycle intermediates modulate thiobarbituric acid reactive species (TBARS) production in rat brain in vitro. Neurochem Res 30:225–235
Alejandro S, Borges N, Cerejo A, Sarmento A, Azevedo I (2005) Catalase activity and thiobarbituric Acid reactive substances (TBARS) production in a rat model of diffuse axonal injury. Effect of Gadolinium and Amiloride. Neurochem Res 30:635–631
Schwarcz R, Whetsell Jr WO, Mangano RM (1983) Quinolinic acid: an endogenous metabolite that produces axons paring lesions in rat brain. Science 219:316–318
Bruyn RPM, Stoof JC (1990) The quinolinic acid hypothesis in Huntington’s chorea. J Neurol Sci 95:29–38
Anderson CM, Hallberg A, Brattsand R et al (1993) Glutathione peroxidase-like activity of diaryl tellurides. Bioorg Med Chem Lett 3:2553–2558
Anderson CM, Brattsand R, Hallberg AR et al (1994) Diaryl tellurides as inhibitors of lipid peroxidation in biological and chemical systems. Free Rad Res 20:401– 410
Nogueira CW, Rotta LN, Perry ML et al (2001) Diphenyl diselenide and diphenyl ditelluride affect the rat glutamatergic system in vitro and in vivo. Brain Res 906:157–163
Moretto MB, Franco J, Posser T, Nogueira CW, Zeni G, Rocha JBT (2004) Ebselen protects Ca2+ influx blockage but does not protect glutamate uptake inhibition caused by Hg2+. Neurochem Res 29:1801–1806
Kalayci M, Coskun, Cagavi F, Kanter K et al (2005) Neuroprotective effects of ebselen on experimental spinal cord injury in rats. Neurochem Res 30:403–410
Arteel GE, Sies H (2001) The biochemistry of selenium and the glutathione system. Environ Toxicol Pharmacol 10:153–158
Rossato JI, Ketzer LA, Centurião FB et al (2002) Antioxidant properties of new chalcogenides against lipid peroxidation in rat brain. Neurochem Res 27:297–303
Nogueira CW, Zeni G, Rocha JBT (2004) Organoselenium and organotellurium compounds: toxicology and pharmacology. Chem Rev 104:6255–6285
Zhao R, Holmgren A (2002) A novel antioxidant mechanism of ebselen involving ebselen diselenide, a substrate of mammalian thioredoxin and thioredoxin reductase. J Biol Chem 278:39456–39462
Puntel RL, Roos DH, Paixao MW et al (2007) Oxalate modulates thiobarbituric acid reactive species (TBARS) production in supernatants of homogenates from rat brain, liver and kidney: effect of diphenyl diselenide and diphenyl ditelluride. Chem Biol Interact 165:87–98
Meotti FC, Stangherlin EC, Nogueira CW, Rocha JBT (2004) Protective role of aryl and alkyl diselenides on lipid peroxidation. Environ Res 94:276–282
Borges LP, Nogueira CW, Panatieri RB et al (2006) Acute liver damage induced by 2-nitropropane in rats: effect of diphenyl diselenide on antioxidant defenses. Chem Biol Inter 160:99–107
Centuriao FB, Corte CLD, Paixao MW et al (2005) Effect of ebselen and organochalcogenides on excitotoxicity induced by glutamate in isolated chick retina. Brain Res 1039:146–152
Burger ME, Fachinetto R, Wagner C et al (2006) Effects of diphenyl-diselenide on orofacial dyskinesia model in rats. Brain Res Bull 70:165–170
Borges VC, Rocha JBT, Nogueira CW (2005) Effect of diphenyl diselenide, diphenyl ditellurite and ebselen on cerebral Na+/K+ ATPase activity in rats. Toxicology 215:191–197
Nogueira CW, Meotti FC, Curte EM et al (2003) Investigations in the potential neurotoxicity induced by diselenides in mice and rats. Toxicology 183:29–37
McLachlan JA (1980) The chemistry of estrogens and antiestrogens, relationships between structure, receptor binding and biological activity. In: McLachlan JA (ed) Estrogens in the environment. Elsevier, New York, pp 46–49
Jindal DP, Piplani P, Fajrak H et al (2001) Synthesis and neuromuscular blocking activity of 16 beta-piperidinosteroidal derivatives. Eur J Med Chem 36:195–202
Hoyte RM, Zhong J, Lerum R et al (2002) Synthesis of halogen-substituted pyridyl and pyrimidyl derivatives of [3,2-c] pyrazolo corticosteroids: strategies for the development of glucocorticoid receptor mediated imaging agents. J Med Chem 45:5397–5405
Maciel N, Bolzan RC, Braga AL, Rocha JBT (2000) Diphenyl diselenide and diphenyl ditelluride differentially affects aminolevulinate dehydratase from liver, kidney and brain of mice. J Biochem Mol Toxicol 14:310–319
Jacques-Silva MC, Nogueira CW, Broch LC et al (2001) Diphenyl diselenide and ascorbic acid changes deposition of selenium and ascorbic acid in brain of mice. Pharmacol Toxicol 88:119–125
Brito VB, Folmer V, Puntel GO et al (2006) Diphenyl diselenide and 2,3-dimercaptopropanol increase the PTZ-induced chemical seizure and mortality in mice. Brain Res Bull 68:414–418
Ghisleni G, Porciuncula LO, Cimarostia H et al (2003) Diphenyl diselenide protects rat hippocampal slices submitted to oxygen-glucose deprivation and diminishes inducible nitric oxide synthase immunocontent. Brain Res 986:196–199
Paulmier C (1986) Synthesis and properties of selenides. In: Baldwin JE (ed) Selenium reagents and intermediates. Organic synthesis. Pergamon Press, Oxford, pp 84–116
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Reznick AZ, Packer L (1994) Oxidative damage to proteins—spectrophotometric method for carbonyl assay. Meth Enzymol 233:357–363
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin-phenol reagent. J Biol Chem 193:265–275
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Sassa S (1982) δ-aminolevulinic acid dehydratase assay. Enzyme 28:l33–145
Fiske CH, Subbarow YJ (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–381
Muller A, Cadenas E, Graf P, Sies H (1984) A novel biologically active seleno-organic compound I. Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ51 (Ebselen). Biochem Pharmacol 33:3235–3239
Wendel A, Fausel M, Safayhi H, Tiegs G (1984) A novel biologically active seleno-organic compound II. Activity of PZ 51 in relation to glutathione peroxidase. Biochem Pharmacol 33:3241–3245
Parnham MJ, Graf E (1991) Pharmacology of synthetic organic selenium compounds. Prog Drug Res 36:10–47
Sies H (1993) Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radic Biol Med 14:313–323
Schewe T (1994) Molecular actions of Ebselen-an anti-inflammatory antioxidant. Gen Pharmacol 26:1153–1169
Graf E, Mahoney JR, Bryant RG, Eaton JW (1984) Iron catalyzed hydroxyl radical formation: Stringent requirement for free iron coordination site. J Biol Chem 259:3620–3624
Klebanoff SJ, Gally JI, Goldstein IM, Snyderman R (eds) (1992) Oxygen metabolities from phagocytes. Raven Pres, New York, NY, pp 541–588
Amici A, Levine RL, Tsia L, Stadtman ER (1989) Comparative analysis of alternating purine-pyrimidine tracts and potential Z-DNA sequences in DNA plant viruses. J Biol Chem 264:3341–3346
Arnold WP, Longneeker DE, Epstein RM (1984) Photodegradation of sodium nitroprusside: biologic activity and cyanide release. Anesthesiology 61:254– 260
Bates JN, Baker MT, Guerra R, Harrison DG (1990) Nitric oxide generation from nitroprusside by vascular tissue. Biochem Pharmacol 42:157– 165
Singh RJ, Hogg N, Neese F et al (1995) Trapping of nitric oxide formed during photolysis of sodium nitroprusside in aqueous and lipid phases: an electron spin resonance study. Photochem Photobiol 61:325–330
Pryor WA, Squadrito GL (1995) The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol 268:L699–L722
Bolanos J, Almeida A (1999) Roles of nitric oxide in brain hypoxia–ischemia. Biochim Biophys Acta 1411:415–436
Castill J, Rama R, Davalos A (2000) Nitric oxide-related brain damage in acute ischemic stroke. Stroke 31:852–857
Prast H, Philippou A (2001) Nitric oxide as modulator of neuronal function. Prog Neurobiol 64:51–68
Huie RE, Padmaja S (1993) The reaction of NO with superoxide. Free Radic Res Commun 18:195–199
Mohanakumar KP, De Bartolomeis A, Wu RM et al (1994) Ferrous-citrate complex and nigral degeneration: Evidence for free-radical formation and lipid peroxidation. Ann NY Acad Sci 738:392–399
Rodrigues AL, Bellinaso ML, Dick T (1989) Effect of some metal ions on blood and liver delta-aminolevulinate dehydratase of Pimelodus malacatus (pisces, Pimelodidae). Comp Biochem Physiol B Comp Biochem 94:65–69
Rocha JBT, Tuerlinckx SM, Schetinger MRC, Folmer V (2004) Effect of Group 13 metals on porphobilinogen synthase in vitro. Toxicol Appl Pharmacol 200:169–176
Rocha JBT, Lissner LA, Puntel RL et al (2005) Oxidation of delta-ALA-D and DTT mediated by ascorbic acid: modulation by buffers depends on free iron. Biol Pharm Bull 28:1485–1489
Santos FW, Rocha JBT, Nogueira CW (2006) 2,3-dimercaptopropanol, 2,3-dimercaptopropane-1-sulfonic acid and meso-2,3-dimercaptosuccinic acid increase lead-induced inhibition of delta-aminolevulinate dehydratase in vitro and ex vivo. Toxicol Vitro 20:317–323
Jaffe EK (1995) Porphobilinogen synthase, the first source of heme’s assymmetry. J Bioenerg Biomembr 27:169–179
Sassa S, Fujita H, Kappas A (1989) Genetic and chemical influences on heme biosynthesis. In: Kotyk A, Skoda J, Paces V, Kostka V (eds) Highlights of modern biochemistry, vol.1. VSP, Utrecht, pp 329–338
Bechara EJH, Medeiros MHG, Monteiro HP et al (1993) A free radical hypothesis of lead poisoning and inborn porphyrias associated with 5-aminolevulinic acid overload. Quim Nova 16:385–392
De Assis DR, Ribeiro CA, Rosa RB et al (2003) Evidence that antioxidants prevent the inhibition of Na_,K_-ATPase activity induced by octanoic acid in rat cerebral cortex in vitro. Neurochem Res 28:1255–1263
Bavaresco C, Calcagnotto T, Tagliari B et al (2003) Brain Na_,K_-ATPase inhibition induced by arginine administration is prevented by vitamins E and C. Neurochem Res 28:825–829
Carfagna MA, Ponsler GD, Muhoberac BB (1996) Inhibition of ATPase activity in rat synaptic plasma membranes by simultaneous exposure to metals. Chem Biol Interact 100:53–65
Acknowledgement
IJK is specifically grateful for the financial support of TWAS and CNPq. IJK is a beneficiary of the TWAS-CNPq postgraduate (doctoral) fellowship. JBT gratefully acknowledge the financial supports by CNPq, FAPERGS, CAPES, CAPES/SAUX and VITAE, FINEP/IBNET
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kade, I.J., Paixão, M.W., Rodrigues, O.E.D. et al. Comparative Studies on Dicholesteroyl Diselenide and Diphenyl Diselenide as Antioxidant Agents and their Effect on the Activities of Na+/K+ ATPase and δ-Aminolevulinic acid Dehydratase in the Rat Brain. Neurochem Res 33, 167–178 (2008). https://doi.org/10.1007/s11064-007-9432-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9432-8