Abstract
Objective
To determine the effects of iron-deficiency anemia on the development of non-rapid-eye-movement (NREM) sleep stages, as indexed by sleep spindles.
Study design
Patterns of sleep spindles during NREM sleep stages 2 and 3–4 (slow-wave-sleep, SWS) were compared in 26 otherwise healthy 6-month-old Chilean infants with iron-deficiency anemia and 18 non-anemic control infants. From polygraphic recordings, EEG activity was analyzed for sleep spindles to assess their number (density), duration, frequency, and inter-spindle interval.
Results
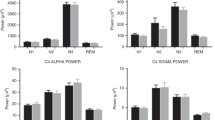
Iron-deficient anemic infants differed from the control group by having sleep spindles with reduced density, lower frequency, and longer inter-spindle intervals in NREM sleep stage 2 and SWS.
Conclusions
These results provide evidence of delayed sleep spindle patterns in iron-deficient anemic infants, suggesting that iron is an essential micronutrient for the normal progression of NREM sleep pattern development in the human.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pioneering work by Youdim and colleagues and others in the 1970s and 1980s suggested that iron-deficiency anemia produced sleep-wake cycle alterations in animal models [1, 2], perhaps due to effects of iron deficiency on dopamine systems [3, 4]. However, there has been little sleep-related research in human iron deficiency, despite the fact that iron-deficiency anemia continues to be the most common single nutrient deficiency in the world [5, 6]. In developing countries, over 50% of pregnant women are anemic [7, 8], as are 46–66% of children <4 years, with half attributed to iron deficiency [9]. Poor, minority and/or immigrant infants and toddlers in the US and other developed countries remain at increased risk for iron deficiency[10]. Infancy is a period of peak prevalence due to rapid growth and limited dietary sources of iron. Since the organization of sleep-wake states undergoes dramatic changes in early infancy, the sleep-wake cycle might be particularly vulnerable to the effects of iron deficiency in this age period.
Iron is required for proper function of many enzymes and proteins. Thus, there are several mechanisms by which iron deficiency could produce diffuse and subtle changes in the central nervous system. For instance, rodent studies have shown effects of iron deficiency on neurotransmitter systems, neurometabolism, dendritogenesis, and myelination (see reviews [11–13]). Rodent models have also shown decreased brain iron content, electrophysiological alterations, neurotransmitter changes, and behavioral alterations that persist despite iron treatment when iron-deficiency anemia occurs in infancy [11–13].
Finding direct evidence of central nervous system effects in human infants is challenging. We have tried to advance the field by using assessments that reflect patterns of functional development and integrity of the central nervous system. For instance, using auditory brainstem evoked potentials, we showed slower neural transmission throughout the auditory pathway [14] and using actigraphic recordings, altered spontaneous motor activity modulation as a function of sleep-wake states [15, 16] in otherwise healthy 6-month-old iron-deficient anemic infants. Here, we pursue the issue of sleep state maturation in more depth.
The first months of postnatal age show dramatic brain development in the human. One of the main sleep changes is the transition from quiet sleep to four differentiated stages of non-rapid-eye-movement (NREM) sleep by around 4 months [17–19]. The sleep spindle is one of the most characteristic EEG patterns during sleep and a hallmark of NREM sleep stage 2, with a known anatomic generator (the nucleus reticularis thalami) [20]. Sleep spindles are defined by discrete bursts of relatively sinusoidal 12–14 Hz waves, which become clearly distinguishable during quiet sleep between 4 and 9 weeks post-term age [19, 21–23]. Sleep spindles reach adult-like mature patterns at about 3 months, and their activity is maximal between 3 and 6 months [19, 22–25]. Spindles have been postulated to be a marker of normal brain functional development and integrity, and their absence or abnormality strongly suggests cerebral dysfunction or pathology. Indeed, deviations from normal maturational patterns have been observed among infant groups with conditions that put them at high risk for poorer health and development [26–28]. Thus, maturational patterns of sleep spindles provide a useful noninvasive tool for investigating central nervous system functioning and integrity during early development in the human.
The purpose of the present study was to compare sleep spindle patterns as a function of NREM sleep stages in otherwise healthy 6-month-old iron-deficient anemic and non-anemic control infants. We predicted that iron-deficiency anemia would be associated with altered spindle patterns, reflecting irregular progression or delay in the normal maturation of NREM sleep development.
Procedures
Sample
The study was conducted in conjunction with a clinical trial of the developmental effects of preventing iron-deficiency anemia in infancy[29]. The sample was drawn from healthy infants receiving routine pediatric care in community clinics in 4 working-class communities on the southeastern outskirts of Santiago, Chile. The infants who received sleep studies at 6 months were identified during screening for the preventive trial. At the 4-month routine pediatric visit, infants were evaluated to make sure that those invited to participate were healthy. Entrance criteria included birth weight ≥3.0 kg, singleton birth, routine vaginal delivery, no major congenital anomalies, no major birth or neonatal complications, no jaundice requiring phototherapy, no hospitalization for other than an uncomplicated problem, no chronic illness, no iron therapy, and no evidence of failure to thrive or other nutrient deficiency. Other exclusion criteria were specific to successful completion of the study: residence outside the identified neighborhoods; no stable, literate caregiver available to accompany the infant for project appointments; another infant less than 12 months of age in the household; infant in day care. Mixed breast- and bottle-feeding was the norm, and infants were growing normally by US standards.
Between 5 and 6 months a finger stick hemoglobin determination was performed (HemaCue, Leo Diagnostics, Helsingborg, Sweden). If the HemaCue value was ≤ 103 g/l (10.3 g/dl), a venipuncture was performed for determination of iron status: hemoglobin, hematocrit, mean cell volume (using Coulter Model ZBI, Hialeah FL); serum ferritin (International Nutritional Anemia Consultative Group, Washington, D.C.); and erythrocyte protoporphyrin (Hematofluorometer, Helena Laboratories, Beaumont TX). Anemia at 6 months was defined as a venous hemoglobin ≤100 g/l (10.0 g/dl). Iron deficiency was defined as two or more of the following: mean cell volume <70 fl, erythrocyte protoporphyrin >1.77 μmol/l (100 μg/dl) packed red blood cells, serum ferritin <12 μg/l. As expected, iron-deficiency anemia was uncommon at this age, detected in only about 3% of over 2000 infants with HemaCue determinations. For each iron-deficient anemic 6-month-old, an infant with a normal HemaCue level was randomly selected to receive a venipuncture specimen. Those who were clearly non-anemic (hemoglobin ≥120 g/l [12.0 g/dl]) constituted the control group. These infants did not enter the preventive trial, participating instead in the neuromaturation component of the project.
The infants were studied during a spontaneous afternoon nap, with about half receiving recordings of brainstem auditory evoked potentials [14] and half, polygraphic recordings of sleep-wake parameters, with actigraphy for some [15, 16]. A variety of technical difficulties made data unusable for 12 other infants who received polygraphic recordings. All infants were given appropriate iron therapy and close pediatric supervision. The study was explained to parents of qualifying infants, and signed informed consent was obtained. The research protocol was approved by the Institutional Review Boards of the University of Michigan Medical Center, Ann Arbor, and INTA, University of Chile, Santiago. The present report is based on the polygraphic recordings and compares sleep spindle characteristics in both NREM sleep stage and SWS in 26 iron-deficient anemic infants and 18 infants without iron-deficiency anemia (non-anemic control) at 6 months of age.
Sleep Data Collection
All sleep data were acquired and processed without knowledge of whether a given infant was iron-deficient anemic or non-anemic control. The wake-up time and the length of the waking episode that preceded the nap recording were estimated from information provided by the mother. Polygraphic recordings were conducted in the sleep laboratory of INTA, University of Chile. The procedures were standardized to limit potential influences of ambient environment, circadian rhythms, and/or food intake on sleep-waking patterns and related physiological activities. Studies took place in a special quiet and comfortable room, during the infant’s spontaneous afternoon nap. Infants and their mothers were transported from home to the laboratory so that they arrived at least one hour before their habitual noon meal based on parental report of the infant’s daily routine. During this one-hour period, electrodes were attached according to routine procedures. Mothers then fed their infant and engaged in their own routines before putting the infant down for a nap in his or her own clothing. Infants were placed in the supine position. The ambient temperature was maintained constant at 22–23°C. Mothers stayed in the laboratory throughout.
Recordings were made continuously using a TECA lA97 18-channel polygraph as follows: the electroencephalographic (EEG) activity with electrodes placed according to the international 10–20 system, with bipolar montages Fp1-C3, C3-O1, Fp2-C4, C4-O2, and Cz-Pz; rapid eye movements, monitored by electro-oculogram; tonic chin and diaphragmatic electromyograms, using surface electrodes; motor activity of both upper and lower limbs recorded independently by piezo-electric crystal transducers; abdominal respiratory movements, using a mercury strain gauge; nostril airflow, by a thermistor; ECG using surface electrodes. For some infants, rectal and axillary temperatures and oximetry were also recorded. All data were simultaneously recorded on paper and computer, converted on-line from analog-to-digital signals, collected on hard disk, and then transferred to laser media for off-line analyses. Infant behavior was also observed directly and noted on the polygraphic paper.
Data Processing and Analysis
Coding waking and sleep states
Waking and sleep (NREM sleep with its four stages, REM sleep, and indeterminate sleep) states were visually coded by the temporal concordance of EEG, EOG and EMG criteria according to Rechtschaffen and Kales’ criteria [30], adapted for infants by Guilleminault and Souquet [31]. The minimum length of a state was 1 min. Interruption of the concordance between the given parameters for 1 min or more was considered as an interruption of the state; shorter changes were included in the preceding state. Recordings were terminated after a spontaneous waking episode lasting 15 min or more. Two independent scorers analyzed all recordings visually without knowledge of the infants’ iron status. Overall inter-scorer agreement was 94.6% for sleep-waking states. Discrepancies were discussed and codes thus agreed upon were used in the data analysis.
Sleep spindle measures from EEG signal processing
Sleep spindles were defined as follows: duration >0.5 s, amplitude >10 μV, frequency 11–15 s. The beginning and end of each individual spindle were identified in one of the Fp-C derivations (Fp1-C3 or Fp2-C4) by two observers, who followed the same rules to resolve scoring discrepancies. The following spindle parameters were then determined for NREM sleep stages 2 and SWS:
-
duration: time between the beginning and end of each spindle (in seconds),
-
frequency: number of waves (or cycles) within each spindle expressed as cycles/s (or Hz),
-
spindle index (density): number of spindles expressed as spindles/min,
-
inter-spindle interval: time spent between the end of each spindle and the beginning of the next one (in seconds).
Statistical methods
For each individual infant, the duration of waking and NREM sleep stages was calculated. The following spindle variables were computed for NREM sleep stage 2 and SWS: frequency, duration, index (spindles/min), and inter-spindle interval. A series of independent t tests and χ 2 tests were conducted to identify significant differences between iron-deficient anemic and control groups in background characteristics. Any background characteristic that was even weakly associated (p < 0.10) with more than one sleep variable was considered for covariate control. Due to colinearity among measures of growth and family characteristics, the set of covariates was simplified to age, gender, birth weight, weight-for-age z-score, and mother’s IQ. Because some of the sleep spindle variables were significantly skewed, general linear regressions with a log normal distribution model were performed utilizing generalized estimated equation (GEE) methodology [32]. The regressions tested for differences in sleep spindle variables between iron-deficient anemic and non-anemic infants controlling for the above covariates. All tests of statistical significance were two-sided, with alpha set at 0.05. Statistics analyses were conducted using SAS v9.1.
Results
Table 1 shows the infant and family background characteristics of the iron-deficient anemic and non-anemic control groups. Iron-deficient anemic infants were somewhat shorter and lighter at birth, as has been observed in some other studies [33–35]. They were also slightly older at the time of assessment. There was some indication that they came from less advantaged family backgrounds, as has been considered previously [36–37].
Table 2 shows the comparisons between iron-deficient anemic and non-anemic infants in the duration of relevant sleep-wake states and the sleep spindle parameters, controlling for covariates. Statistically significant differences were the same with and without covariate control. On average, as noted before [15], the iron-deficient anemic group had been awake for a shorter time before napping than non-anemic controls, but the groups did not differ in the duration of NREM sleep stage 2 or SWS sleep during the nap. When spindle patterns were compared as a function of NREM sleep stages, both groups showed the same pattern: spindle index, spindle duration, spindle frequency, and the inter-spindle interval were similar in NREM sleep stage 2 and SWS. However, iron-deficient anemic infants showed a reduced spindle index during NREM sleep stage 2 and SWS relative to non-anemic control infants. The effect sizes were large (0.6–1.1 SD). Since spindle duration was similar across groups, the differences in the spindle index appears to be due to the significantly longer inter-spindle intervals in the iron-deficient anemic group during both NREM sleep stage 2 and SWS (effect sizes 0.6–0.8 SD). In addition, sleep spindle frequency was lower in iron-deficient anemic infants than in non-anemic control infants in both NREM sleep stage 2 and SWS. The effect sizes were very large in both stages (1.7 SD).
Discussion
This study shows altered sleep spindle patterns in 6-month-old infants with iron-deficiency anemia. During a spontaneous afternoon nap, their NREM stage 2 and SWS were characterized by reduced spindle index, longer inter-spindle interval, and lower spindle frequency, compared to non-anemic controls. The duration of sleep stages and sleep spindles did not differ between the groups.
To the best of our knowledge, no data on sleep spindle patterns in iron deficiency have been reported previously. Although the results must be replicated, our findings are reminiscent of the developmental changes in sleep spindles that have been observed in several other biological risk conditions. NREM sleep appears to be a vulnerable state to a variety of insults occurring during the first months of postnatal life [38–42]. Infant groups considered at higher than average epidemiological risk for poorer developmental or neurological outcome also present different patterns of sleep spindle organization. For example, infants with mental retardation, prematurity, congenital hyperthyroidism, hyperbilirubinemia, phenylketonuria, or autism are characterized by different patterns of sleep spindles [26–28, 42–46].
The differences in sleep spindle patterns observed in the iron-deficient anemic group correspond to less mature characteristics. Since spindles have been shown to arise from synchronized activities in functionally important neuronal networks linking the thalamus and the cortex [20, 47, 48], our results suggest that iron is required to assure the normal progression of the oscillating thalamocortical network that regulates the spindle patterning within NREM sleep stages.
Promising structural explanations for the observed sleep spindle alterations relate to impaired myelination and dendritic growth. Early iron deficiency alters myelin content and compaction [49–53] by directly affecting oligodendrocytes, which are responsible for myelin formation (see reviews [12, 13]). Myelination is at least partly postnatal in the human [54], coinciding with the period of quiet sleep-NREM sleep restructuring [17–19]. Disrupted growth and organization of dendrites has also been reported in early iron deficiency in rodent models [55]. Although sleep spindles represent one of the few EEG patterns with a known anatomic generator [20], rapidly developing changes in early infancy appear to reflect myelination and dendritic growth [56] within the neuronal networks that link the thalamus and the cortex. Since adequate iron is required for normal myelination and dendritogenesis, alterations in these processes due to iron-deficiency anemia could impact function in many brain systems, including those that underlie the occurrence of sleep spindles at the macroscopic EEG level. Future research in animal models may be particularly useful in evaluating the developmental profile of spindles in iron-deficiency anemia and understanding the NREM sleep stage differences observed in iron-deficient anemic infants.
Other mechanisms should be considered as well, particularly those related to a potential imbalance of brain neurotransmitter systems with early iron deficiency [12, 13]. Beginning with the work of Youdim and colleagues in rodent models [4], iron deficiency has been implicated in later functioning of the dopamine (DA) system, especially the D2 receptor [12, 13]. Recent studies of developmental iron deficiency suggest that transporters for DA, serotonin, and norepinephrine, DA levels, and D1 receptors are affected as well [57–59]. These neurotransmitter systems are involved in sleep spindle patterns. For example, there is a progressive disappearance of EEG sleep spindles in the rat under dietary-induced reduction of endogenous brain serotonin levels [60]. In experimental models of hyperphenylalaninemia, serotonin (5-HT2) receptors decrease together with D2 receptors [61]. Therefore, if brain neurotransmitter imbalance results from iron-deficiency anemia, there might be effects on the biochemical substrate of spindle generation.
Sleep spindles appear to do more than reflect network properties (i.e., promoting the formation of thalamocortical networks by providing endogenous signals with repetitive and synchronized activity [62]). Some investigators have suggested that sleep spindles provide necessary conditions for the plastic modifications underlying memory formation [48, 63]. Although the functions of sleep remain largely unknown, one of the most exciting hypotheses is that sleep contributes importantly to processes of memory and brain plasticity (for review see ref. [64]). As the multifaceted relationships between sleep and memory were recognized, initial attention concentrated on rapid-eye-movement (REM) sleep [64]. More recently, NREM sleep stages 2 and SWS have become a major focus of attention, with particular emphasis on sleep spindles. There is evidence that the spindles are markers for ability to learn certain kinds of tasks [65–67] even during a daytime nap [68]. The role of sleep in learning and memory has been shown by studies at the behavioral, systems, cellular, and molecular levels, including the modulation during sleep of cerebral protein synthesis and expression of genes involved in neuronal plasticity [64]. However, research to date continues to be fragmentary and has been conducted almost exclusively in adults (human or animal). Large amounts of sleep in infancy suggest that sleep may play a role in brain maturation [69], and sleep state organization and especially quiet sleep-NREM sleep in early infancy correlate with measures of cognitive functioning and attention in later childhood and early adolescence [70]. Yet the relationships between sleep spindles and learning have not been characterized in infants and young children. If the connections between sleep spindles and learning also apply in infancy, it is possible that the altered patterns of sleep spindles in iron-deficient anemic infants restrict their cognitive and memory-related abilities and contribute to the poorer developmental outcome that is consistently observed [13, 71].
Finally, another aspect that deserves attention is the potential relationship between sleep spindles and motor control issues during sleep. Sleep spindles have been associated with a suppression of muscle tone. In the study of Chase and Harper [72], cats were trained to produce 12–14 Hz EEG activity during waking and, with acquisition of the spindle task; they showed concurrent loss of nuchal EMG together with entrainment of both respiratory and cardiac rhythms. This motoric relationship may be particularly relevant to our observations, since restless legs syndrome (RLS) and periodic limb movements during sleep (PLMS) have been associated with iron deficiency and conditions characterized by compromised iron status [73]. Moreover, dietary iron supplementation has been shown to alleviate RLS symptoms and to reduce PLMS in adult patients [74] and to be associated with clinical improvement in some children with PLMS [75, 76].
There are several limitations to our study. With a bipolar montage, we could not evaluate the precise location of spindle waves. Frontal and parietal spindles, for instance, appear to follow somewhat different developmental paths, suggesting the existence of different generators or a topographical difference during maturation of the thalamocortical network [77]. Because our recordings were performed during naturally-occurring early afternoon naps in healthy infants, we could not evaluate the influence of known modifiers of spindle characteristics, such as sleep restriction or deprivation, circadian phase, or pharmacologic effects [78]. Also, we could not evaluate the modulation of spindle characteristics as a function of the ongoing temporal structure within a long sleep episode [24, 78].
Despite these drawbacks, our results provide further evidence that iron deficiency adversely affects the functional development of the central nervous system in the human infant. The observation of delayed maturation of sleep spindles – a key characteristic of NREM sleep stages – suggests that iron is an essential micronutrient for the normal progression of sleep development in infancy.
References
Youdim MBH, Yehuda S, Ben-Uriah Y (1981) Iron deficiency-induced circadian rhythm reversal of dopaminergic-mediated behaviours and thermoregulation in rats. Eur J Pharmacol 74:295–301
Glover J, Jacobs A (1972) Activity pattern of iron-deficient rats. BMJ 2:627–628
Youdim MBH, Yehuda S (1985) Iron deficiency induces reversal of dopamine dependent circadian cycles: differential response to d-Amphetamine and TRH. Peptides 6:851–855
Youdim MBH (1988) Brain iron: neurochemical and behavioural aspects. Taylor & Francis, London
Beard J, Stoltzfus R (2001) Iron-deficiency anemia: reexamining the nature and magnitude of the public health problem. J Nutr 131:563S–703S
Mannar V, Bellamy C (2004) Vitamin and mineral deficiency – a global progress report. UNICEF, New York
WHO (1998) Global Database on Child Growth and Malnutrition. WHO, Geneva
Administrative Committee on Coordination Sub-Committee on Nutrition (ACC/SCN) (2000) Fourth Report on the World Nutrition Situation. ACC/SCN in collaboration with IFPRI, Geneva
Stoltzfus RJ, Mullany L, Black RE (2004) Iron deficiency anaemia. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL (eds) Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. World Health Organization, Geneva, pp 163–209
Brotanek JM, Halterman J, Auinger P et al (2005) Iron deficiency, prolonged bottle-feeding, and racial/ethnic disparities in young children. Arch Pediatr Adolesc Med 159:1038–1042
Smith LB, Thelen E, Titzer R et al (1999) Knowing in the context of acting: the task dynamics of the A-not-B error. Psychol Bul 106:235–260
Beard JL, Connor JR (2003) Iron status and neural functioning. Ann Rev Nutr 23:41–58
Lozoff B, Georgieff MK (2006) Iron deficiency and brain development. Semen Pediatr Neurol 13:158–165
Roncagliolo M, Garrido M, Walter T et al (1998) Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: delayed maturation of auditory brain stem responses. Am J Clin Nutr 68:683–690
Angulo-Kinzler RM, Peirano P et al (2002) Spontaneous motor activity in human infants with iron-deficiency anemia. Early Hum Dev 66:67–79
Angulo-Kinzler RM, Peirano P, Lin E et al (2002) Twenty-four-hour motor activity in human infants with and without iron deficiency anemia. Early Hum Dev 70:85–101
Fagioli I, Salzarulo P (1982) Sleep states development in the first year of life assessed through 24-h recordings. Early Hum Dev 6:215–228
Coons S, Guilleminault C (1982) The development of sleep-wake patterns and non-rapid eye movement stages during the first six months of life in normal infants. Pediatrics 69:793–798
Louis J, Cannard C, Bastuji H et al (1997) Sleep ontogenesis revisited: a longitudinal 24-h home polygraphic study on 15 normal infants during the first two years of life. Sleep 20:323–333
Steriade M, Deschenes M, Domich L et al (1985) Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol 54:1473–1497
Metcalf DR (1970) EEG sleep spindle ontogenesis. Neuropadiatrie 1:428–433
Curzi-Dascalova L (1977) Waking and sleeping E.E.G. in normal babies before 6 months of age. Revue d’Electroencephalographie et de Neurophysiologie Clinique 7:316–326
Ellingson RJ (1982) Development of sleep spindle bursts during the first year of life. Sleep 5:39–46
Louis J, Zhang JX, Revol M et al (1992) Ontogenesis of nocturnal organization of sleep spindles: a longitudinal study during the first 6 months of life. Electroencephalogr Clin Neurophysiol 83:289–296
Hughes JR (1996) Development of sleep spindles in the first year of life. Clin Electroencephalogr 27:107–115
Monod N, Eliet-Flesher J, Dreyfus-Brisac C (1977) Le sommeil du nouveau-ne et du premature. III. Les troubles de l’organization du sommeil chez le nouveau-ne pathologique: analyse des etudes polygraphiques. Biol Neonatorum 11:216–247
Dreyfus-Brisac C, Curzi-Dascalova L (1975) The EEG during the first year of life. In: Remond A (ed) The evolution of the EEG. Handbook of electroencephalography. Elsevier, Amsterdam, pp 24–30
Shibagaki M, Kiyono S, Watanabe K (1982) Spindle evolution in normal and mentally retarded children: a review. Sleep 5:47–57
Lozoff B, De Andraca I, Castillo M et al (2003) Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics 112:846–854
Rechtschaffen A, Kales A (1968) A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. UCLA Brain Research Institute/Brain Information Services, Los Angeles
Guilleminault C, Souquet M (1979) Sleep states and related pathology. In: Korobkin R, Guilleminault C (eds) Advances in perinatal neurology. Spectrum, New York, pp 415–426
McCullagh P, Nelder JA (1989) Generalized linear models. Chapman and Hall, New York
Lozoff B, Brittenham GM, Viteri FE, Wolf AW, Urrutia JJ (1982) The effects of short-term oral iron therapy on developmental deficits in iron deficient anemic infants. J Pediatr 100:351–357
Lozoff B, Brittenham GM, Wolf AW et al (1987) Iron deficiency anemia and iron therapy: effects on infant developmental test performance. Pediatrics 79:981–995
Lozoff B, Walter T, Kaciroti N (2006) Predicting iron deficiency in infancy: application of a physiologic framework. Am J Clin Nutr 84:1412–1421
Lozoff B (1998) Considering environmental factors in research on nutrient deficiencies and infant development. In: Perman JA, Rey J (eds) Clinical trials in infant nutrition. Lippincott-Raven Publishers, Philadelphia, pp 203–218
Pollitt E (2000) Developmental sequel from early nutritional deficiencies: conclusive and probability judgments. J Nutr 130:350S–353S
Salzarulo P, Fagioli I, Salomon F et al (1982) Developmental trend of quiet sleep is altered by early human malnutrition and recovered by nutritional rehabilitation. Early Hum Dev 7:257–264
Peirano P, Fagioli I, Singh BB et al (1989) Effect of early human malnutrition on waking and sleep organization. Early Hum Dev 20:67–76
Fagioli I, Peirano P, Bes F et al (1989) Sleep in early human malnutrition. In: Horne J (ed) Sleep 88. Gustav Fischer Verlag, Stuttgart, pp 59–62
Peirano P, Fagioli I, Singh BB et al (1990) Quiet sleep and slow wave sleep in malnourished infants. Brain Dysfunct 3:80–83
Fagioli I, Salzarulo P, Salomon F et al (1983) Sinus pauses in early human malnutrition during waking and sleeping. Neuropediatrics 14:43–46
Lenard HG, Schulte FJ (1974) Sleep spindles in hormonal and metabolic diseases of infancy and childhood. In: Petre-Quadens O, Schlag JD (eds) Basic sleep mechanisms. Academic Press, New York, pp 380–403
Gurses D, Kilic I, Sahiner T (2005) The effects of hyperbilirubinemia on sleep-spindle characteristics in infants. Sleep 28:644–648
De Giorgis GF, Nonnis E, Crocioni F et al (1996) Evolution of daytime quiet sleep components in early treated phenylketonuric infants. Brain Dev 18:201–206
De Giorgis GF, Nonnis E, Crocioni F et al (1972) Development of sleep patterns in autistic children. In: Clement C, Purpura DP, Mayer FE (eds) Sleep and the maturing mervous system. Academic Press, New York, pp 321–363
Contreras D, Destexhe A, Sejnowski TJ et al (1996) Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science 274:771–774
Steriade M (1999) Coherent oscillations and short-term plasticity in corticothalamic networks. Trends Neurosci 22:337–345
Beard JL, Wiesinger JA, Connor JR (2003) Pre- and postweaning iron deficiency alters myelination in Sprague-Dawley rats. Dev Neurosci 25:308–315
Larkin EC, Rao GA (1990) Importance of fetal and neonatal iron: Adequacy for normal development of central nervous system. In: Dobbing J (ed) Brain, behaviour, and iron in the infant diet. Springer-Verlag, London, pp 43–62
Kwik-Uribe CL, Gietzen D, German JB et al (2000) Chronic marginal iron intakes during early development in mice result in persistent changes in dopamine metabolism and myelin composition. J Nutr 130:2821–2830
Ortiz E, Pasquini JM, Thompson K et al (2004) Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J Neurosci Res 77:681–689
Hill JM (1988) The distribution of iron in the brain. In: Youdim MBH (ed) Brain iron: neurochemical and behavioural aspects. Taylor and Francis, London
Carmody DP, Dunn SM, Boddie-Willis AS et al (2004) A quantitative measure of myelination development in infants, using MR images. Neuroradiology 46:781–786
Jorgenson LA, Wobken JD, Georgieff MK (2003) Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci 25:412–420
Schade JP, Meeter K (1963) Neuronal and dendritic patterns in the uncinate area of human hippocampus. Prog Brain Res 3:89–110
Lozoff B, Beard J, Connor J et al (2006) Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 64:S34–S43
Beard JL, Felt B, Schallert T et al (2006) Moderate iron deficiency in infancy: biology and behavior in young rats. Beba Brain Res 170:224–232
Felt BT, Beard JL, Schallert T et al (2006) Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res 171:261–270
Lanoir J, Ternaux JP, Pons C et al (1981) Long-term effects of a tryptophan-free diet on serotonin metabolism and sleep-waking balance in rats. Exp Brain Res 41:346–357
Hommes FA (1993) The effect of hyperphenylalaninaemia on the muscarinic acetylcholine receptor in the HPH-5 mouse brain. J Inherit Metab Dis 16:962–974
Jenni OG, Borbely AA, Achermann P (2004) Development of the nocturnal sleep electroencephalogram in human infants. Am J Physiol Regul Integr Comp Physiol 286:R528–R538
Rosanova M, Ulrich D (2005) Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci 25:9398–9405
Walker MP, Stickgold R (2006) Sleep, memory, and plasticity. Ann Rev Psychol 57:139–166
Schabus M, Gruber G, Parapatics S et al (2004) Sleep spindles and their significance for declarative memory consolidation. Sleep 27:1479–1485
Fogel SM, Smith CT (2006) Learning-dependent changes in sleep spindles and Stage 2 sleep. Sleep 15:250–255
Schabus M, Hodlmoser K, Gruber G et al (2006) Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. Eur J Neurosci 23:1738–1746
Schmidt C, Peigneux P, Muto V et al (2006) Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci 26:8976–8982
Peirano P, Algarin C, Uauy R (2003) Sleep-wake states and their regulatory mechanisms throughout early human development. J Pediatr 143:S70–S79
Parmelee AH, Sigman M, Garbanati J et al (1994) Neonatal electroencephalographic organization and attention in early adolescence. In: Dawson G, Fischer KW (eds) Human behavior and the developing brain. Guilford, New York, pp 537–554
Grantham-McGregor S, Ani C (2001) A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr 131:649S–668S
Chase MH, Harper RM (1971) Somatomotor and visceromotor correlates of operantly conditioned 12–14 c/sec sensorimotor cortical activity. Electroencephalogr Clin Neurophysiol 31:85–92
Trenkwalder C, Paulus W, Walters AS (2005) The restless legs syndrome. Lancet Neurol 4:465–474
Earley CJ, Heckler D, Allen RP (2004) The treatment of restless legs syndrome with intravenous iron dextran. Sleep Med 5:231–235
Simakajornboon N, Gozal D, Vlasic V et al (2003) Periodic limb movements in sleep and iron status in children. Sleep 26:735–738
Simakajornboon N (2006) Periodic limb movement disorder in children. Paediatr Respir Rev 7:S55–S57
Shinomiya S, Nagata K, Takahashi K et al (1999) Development of sleep spindles in young children and adolescents. Clin Electroencephalogr 30:39–43
De Gennaro L, Ferrara M (2003) Sleep spindles: an overview. Sleep Med Rev 7:423–440
Acknowledgements
The authors thank the infants and parents whose participation made this study possible. We also thank Miriam Dinamarca for valuable assistance, technicians for performing the polysomnographic recordings during the course of this study, drivers for providing careful transportation services to infants and parents, and Yuezhou Jing for statistical support. The work was supported by grants from the U.S. National Institutes of Health (R01 HD14122 and R01 HD33487, Betsy Lozoff, P.I.) and FONDECYT in Chile (1040945, Patricio Peirano, P.I.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue dedicated to Dr. Moussa Youdim.
Rights and permissions
About this article
Cite this article
Peirano, P., Algarín, C., Garrido, M. et al. Iron-Deficiency Anemia is Associated with Altered Characteristics of Sleep Spindles in NREM Sleep in Infancy. Neurochem Res 32, 1665–1672 (2007). https://doi.org/10.1007/s11064-007-9396-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9396-8