Abstract
Pathogenesis of Alzheimer’s disease (AD), which is characterised by accumulation of extracellular deposits of β-amyloid peptide (Aβ) in the brain, has recently been linked to vascular disorders such as ischemia and stroke. Aβ is constantly produced in the brain from amyloid precursor protein (APP) through its cleavage by β- and γ-secretases and certain Aβ species are toxic for neurones. The brain has an endogenous mechanism of Aβ removal via proteolytic degradation and the zinc metalloproteinase neprilysin (NEP) is a critical regulator of Aβ concentration. Down-regulation of NEP could predispose to AD. By comparing the effects of hypoxia and oxidative stress on expression and activity of the Aβ-degrading enzyme NEP in human neuroblastoma NB7 cells and rat primary cortical neurones we have demonstrated that hypoxia reduced NEP expression at the protein and mRNA levels as well as its activity. On contrary in astrocytes hypoxia increased NEP mRNA expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypoxia is known to have a significant effect on cellular proteins with an immediate effect resulting in changes in enzyme activities and long-term effects resulting in changes in gene expression and levels of protein synthesis [1]. Much data has accumulated to suggest that vascular factors and, in particular, the levels of oxygen supply to the brain are linked with AD pathogenesis and progression [2]. The strongest evidence for this connection is that patients with cerebrovascular disease or vascular dementia will often bear AD pathology at autopsy even when there was no clinical pre-existing AD [3]. Brain ischemia and associated hypoxia are among the most common complications resulting from impaired brain circulation and metabolism, which can affect animals and humans at various stages of their life and ischemic stroke is the third leading cause of death in Western countries, after heart disease and cancer [4]. Brain hypoxia affects both behavioral and cognitive abilities of individuals and leads to various neuropathologies [5].
Metabolism of the Alzheimer’s amyloid β-peptide (Aβ) is affected by ischemia and hypoxia and an increased level of the amyloid precursor protein (APP) is a part of the acute adaptive response of the brain to hypoxia [6]. In cell models chronic hypoxia was shown to alter processing of APP and a decrease in expression of ADAM10 possessing α-secretase activity [7, 8]. Ischemic-reperfusion brain injury was shown to induce over-expression of APP in reactive astrocytes, with expression peaking at 7 days and 6 months after the insult [9]. We have previously demonstrated that prenatal hypoxia and acute global brain ischemia in rats affect both levels of expression of APP and production of its soluble fragments as well as the levels of expression of the amyloid-degrading enzymes NEP and ECE-1 [10, 11]. In the present study we have used human neuroblastoma cells and rat primary neurones and astrocytes to assess the effect of chronic hypoxia on the levels of expression of the amyloid-degrading zinc metallopeptidase neprilysin (NEP). Since hypoxia initiates a complex cascade of events in the cells including generation of reactive oxygen species (ROS) (for review see (12)) the effect of hypoxia on NEP levels in neuroblastoma cells was analysed in parallel with the effects of oxidative stress initiated in the cells by addition of hydrogen peroxide [13].
Materials and methods
Cell culture
The NB7 (SJ-N-CG) neuroblastoma cell line, which expresses NEP, was kindly provided by Dr. Vincent Kidd (St Jude Children’s Research Hospital). The NB7 cells were cultured in RPMI-1640 media supplemented with 10% (v/v) fetal bovine serum, 50 units/ml penicillin, 50 μg/ml streptomycin and 2 mM glutamine (all from Cambrex Bio Science Ltd., Wokingham, Berkshire, UK) at 37°C in 5% (v/v) CO2 and subcultured every 7 days. After reaching the confluent stage cells were treated as described in the Results section.
Primary neuronal cultures
Cerebral cortices from 1–6 day old Wistar rats were finely cut and dispersed in PBS buffer containing 0.25 mg/ml trypsin (37°C). After 15 min trypsin digestion was halted by addition of an equal volume of buffer supplemented with 16 μg/ml soybean trypsin inhibitor (STBI), 2 Kunitz units/ml DNase I and 0.24 mM MgSO4 and the mixture was centrifuged at 5,000 g for 3 min. The cell pellet was resuspended in 2 ml of PBS buffer solution containing 100 μg/ml SBTI, 12.5 Kunitz units/ml DNase I and 1.5 mM MgSO4 using a fire-polished Pasteur pipette. After allowing larger pieces of tissue to settle for 5 min, the cell suspension was removed, centrifuged at 100 g for 3 min, resuspended in culture medium (Eagle’s minimal essential medium supplemented with 10% (v/v) fetal bovine serum, 50 units/ml penicillin, 50 μg/ml streptomycin and 2 mM glutamine) and aliquoted in 6- or 24-well tissue culture plates. Cells were kept in an incubator at 37°C (95% air; 5% CO2). 48 h after plating, 90% of the medium in the wells was replaced with Eagle’s minimal essential medium supplemented with 10% (v/v) heat inactivated horse serum, 50 units/ml penicillin, 50 μg/ml streptomycin and 2 mM glutamine; 80 μM floxuridine (FUDR) was added to the medium to inhibit non-neuronal cell proliferation. These preparations were referred to as “neuronal cultures”. For preparation of cortical astrocytes 4–6 h after plating the cells, they were washed vigorously several times with PBS buffer solution (pH 7.2) to remove non-adhered cells. Culture medium (Eagle’s minimal essential medium supplemented with 10% FCS, 1% penicillin–streptomycin, 2 mM l-glutamine) was exchanged every 3–4 days and cells grown for up to 14 days.
Hypoxia and oxidative stress
To study the effect of hypoxia on expression and localisation of proteins of interest, NB7 cells or rat primary neurones and astrocytes were incubated in an O2/CO2 incubator (MC0-175M, Sanyo) for 24 h under 2.5 % or 1% O2 depending on the conditions of the experiments and in accordance with previous reports [6]. To induce oxidative stress in the NB7 cells, H2O2 (Sigma-Aldrich Co., Poole, Dorset, UK) to a final concentration of 40 μM was added to the culture medium and the cells were kept in an incubator under normoxic conditions. The cells were collected 24 h or 48 h later, washed twice with 10 ml PBS, scraped into 10 ml of PBS (pH 7.2) and pelleted at 3,000 g for 5 min. Pellets were resuspended in 6 × vol of 50 mM Tris-1% Triton X-100 (pH 7.4) buffer containing an EDTA free cocktail of protease inhibitors in the concentration suggested by the manufacturer and lysed for 30 min at 4°C. Cell lysates were centrifuged at 3,000 g for 5 min and the supernatants were analysed for protein concentration and used either for activity assay or after mixing with 2× SDS-PAGE sample buffer for Western blotting. The amount of protein in the samples was measured by the bicinchinonic acid (BCA) method of Smith et al. [14], modified to use in 96-well microtitre plates.
Western blotting
Proteins from cell lysates were denatured for 5 minutes at 100°C with reducing agent in the sample buffer (10% v/v of 1 M Tris pH 6.8, 40% v/v of 10% SDS, bromophenol blue, 10–20% v/v glycerol, 20% H2O, 10% β-mercaptoethanol) and loaded (20–60 μg of protein per well) on to 6–8% (w/v) acrylamide-SDS gels in a Bio-rad electrophoresis system. Samples were run for 1–1.5 h at 120–150 V in a running buffer (10×: 30.2 g Tris, 188 g Glycine, 10 g SDS in 1 l of water) and the proteins were transferred onto an Immobilon-P (polyvinylidene difluoride, PVDF) membrane (VWR International Ltd., Dorset, UK) in a Nu-Page blot module for 1–1.5 h at 30 V. After blocking the membrane in phosphate-buffered saline containing Tween 20 (0.1% w/v) and 5 % (w/v) milk for 1 h at room temperature (or overnight at 4°), the membrane was incubated for 1 h (or overnight at 4°) with the appropriate primary antibodies in PBS-Tween or in 5% milk powder in PBS-Tween. Dilutions of primary antibodies were as follows: NEP (1:100, mouse monoclonal, NCL-CD10-270, Novocastra, Newcastle, UK or rabbit anti-rat polyclonal antibody, US Biological); GLUT-1 (1:500, rabbit polyclonal, from Prof. S. Baldwin, University of Leeds); anti-β-actin (1:500, rabbit polyclonal, Sigma-Aldrich Co., Poole, Dorset, UK); APP (1:4,000, mouse monoclonal, 22C11, Chemicon International Ltd., Harrow, UK), PS1 (1:1,000, rabbit polyclonal anti-human PS1 (N-terminal), Calbiochem, Merck Bioscience Ltd, Nottingham, UK). After washing the membrane 4 times for 15 min in PBS-Tween the blots were incubated with secondary peroxidase-linked antibodies (1:2,000, sheep anti-mouse and donkey anti-rabbit HRP-conjugated, Amersham Ltd., UK) for 1 h and then washed 4 × 15 min with PBS-Tween. Blots were visualised by an enhanced chemiluminescent (ECL) detection system (Perbio Science Ltd., Cramlington, Northumberland, UK). All incubations and washes were carried out on an orbital shaker. Recombinant NEP protein was a gift from Dr. David Nanus (Cornell University, New York, USA).
Real time PCR
Total RNA was isolated using the RNeasy kit (Qiagen, Crawley, W.Sussex, UK) according to the manufacturer’s instructions. The concentration of nucleic acids was determined spectrophotometrically at 260 nm and 280 nm, taking into account the dilution factor. When required, automated DNA sequencing was carried out by Lark Technologies (UK). For the PCR first strand synthesis was performed using the iScript cDNA synthesis kit (Bio-rad). The resulting cDNA was then assayed by real time PCR. Real time PCR was performed in 0.2 ml thinwall PCR plates using the iCycler thermal cycler (Bio-rad) and carried out with iQ SYBR Green supermix (Biorad) according to the manufacturer’s instructions. The standard reaction mix consisted of iQ SYBR Green supermix, forward and reverse primers at a final concentration of 500 nM each, 10 pg DNA template, DNase free water to give final volume of 20 μl. The mixture was heated to 95°C for 3 min followed by 35 cycles with denaturation at 95°C for 30 s, annealing at 57°C for 30 s and extension at 72°C for 30 s. A series of dilutions of NEP plasmid from 0.01 pg to 100 pg was used as standards. GAPDH was used as reference gene. Primer sequences for real time PCR:
GAPDH-F:5′-AGC TGA ACG GGA AGC TCA CT-3′; GAPDH-R:5′-AGG TCC ACC ACT GAC ACG TTG-3′; NEP-F:5′-CCT GGA GAT TCA TAA TGG ATC TTG T-3′; NEP-R: 5′-AAA GGG CCT TGC GGA AAG-3′; rat NEP-F: 5′-GGA AGC CAT TCA GCT GGT-3′; rat NEP-R: 5′-TGG AGC ATA AAC AAC CAC TTC T-3′; rat 18S-F: 5′-GAG AGG GAG CCT GAG AAA CG-3′; rat 18S-R: 5′-GGC CTC GAA AGA GTC CTG TA-3′.
Fluorometric assay of NEP activity
NEP activity in cell lysates was analysed using a synthetic NEP substrate succinoyl-Ala-Ala-Phe-7-amido-4-methylcoumarin (Suc-Ala-Ala-Phe-AMC, Bachem, UK) [15]. The fluorogenic assay was carried out at room temperature in the presence or absence of the NEP inhibitor, thiorphan, at 10 μM. The assay was monitored continuously by measuring the increase in fluorescence over the time course (excitation = 380 nm, emission = 460 nm) of substrate hydrolysis using a Wallac Victor fluorescence plate reader (Turku, Finland). The standard assay mixture consisted of 1 μg of total protein cell lysate (50–100 μg of media proteins), 50 μM substrate, 50 mM HEPES buffer and 200 mM NaCl (pH 7.2), 4 munits of Leu-aminopeptidase (LAP), in a total volume of 100 μl. The NEP activity was determined as the difference between the fluorescence of the product compared with that occurring in the presence of 10 μM thiorphan. Linearity of progress of the reaction was routinely checked. Addition of further aliquots of Leu-aminopeptidase did not enhance fluorescence, ensuring that NEP was rate-limiting in the coupled assay.
Statistical analysis
All results are given as mean ± S.E.M. from n experiments unless otherwise stated. Student t-tests were used to ascertain statistical significance with threshold of P < 0.05.
Results
Characterization of NEP expression in neuroblastoma NB7 and SH-SY5Y cells
SH-SY5Y cells, which are widely used in the studies of amyloid metabolism, express negligible levels of NEP both at mRNA and protein levels (Fig. 1A, B). On the contrary the neuroblastoma NB7 cells derived from the SJ-N-CG cell line express significant amounts of NEP both at mRNA and protein levels and thus have been selected for the analysis of the effects of hypoxia and oxidative stress on this amyloid-degrading enzyme (Fig. 1A, B).
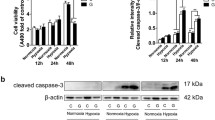
Levels of NEP and GLUT1 expression in neuroblastona NB7 and SH-SY5Y cell lines. (A) NEP mRNA levels in NB7 (1) and in SH-SY5Y (2) cells. The mean results ± S.E.M. (n = 3) are shown graphically. (B) NEP protein levels analysed by immunoblotting with a mouse monoclonal antibody against NEP. Immunoreactive bands were visualized with enhanced chemiluminescence as described in “Materials and Methods”. 1—NB7, 2—SH-SY5Y, 3—NEP positive control (human recombinant NEP). (C, D) Effect of chronic hypoxia (2.5% or 1% O2 for 4 or 24 h, respectively) or oxidative stress (40 μM H2O2 for 4 h or 24 h) on levels of expression of GLUT-1 in NB7 cells. (C) representative data of immunoblotting with a rabbit polyclonal antibody against GLUT-1. (D) graphical representation of the results of densitometric analysis. Data are presented as % of control levels, ± S.E.M. n = 3, *P < 0.05
GLUT1 as a marker of hypoxia in NB7 cells
In order to ensure strictly hypoxic conditions, a suitable marker was sought to demonstrate that under experimental conditions of chamber hypoxia cells underwent appropriate hypoxic treatment. It is known that that hypoxia inducible factor (HIF) [16] and tyrosine hydroxylase (TH) [17] are up-regulated after hypoxia in many cell lines. However, in NB7 cells these proteins were barely detectable using Western blotting analysis. Hence, another hypoxia marker up-regulated after chronic hypoxia [18], namely the glucose transporter GLUT-1, was used in these studies. GLUT-1 was highly expressed in NB7 cells and hypoxic conditions dramatically up-regulated its expression (Fig. 1C, D). The phenomenon of GLUT-1 up-regulation under hypoxic conditions was time- and oxygen content-dependent and was not observed under conditions of oxidative stress initiated by addition to the cells of 40 μM H2O2 (Fig. 1C, D).
Effect of hypoxia and oxidative stress on expression of APP and PS1 in NB7 cells
Although levels of expression of APP and PS1 have been well characterised in neuroblastoma SH-SY5Y cells there are no data on the levels of expression of these proteins in NB7 cells. APP is expressed in NB7 cells (Fig. 2) at comparable levels to SH-SY5Y cells (data not shown). PS1 protein levels were much higher in NB7 cells than in SH-SY5Y cells, even in those cells stably over-expressing the PS1 gene (Fig 2C,D). Although we have observed an increase of APP expression in NB7 cells under chronic hypoxic conditions (Fig. 1A, B), levels of PS1 were found not to be changed either under hypoxic conditions or oxidative stress (Fig. 2E, F).
Level of APP and PS1 protein expression in NB7 cells under normal conditions and after chronic hypoxia. NB7 cells were incubated for 24 h in atmosphere with reduced oxygen content to 2.5%. Control cells were incubated for 24 h under normal conditions (37°C, 5% CO2, 95% atmospheric air). (A) levels of APP expression under normal (1) and hypoxic conditions (2.5% O2, 24 h) revealed by immunoblotting with a mouse monoclonal antibody against APP (22C11). (B) graphical representation of results of densitometric analysis of the blots. 1—control cells, 2—hypoxia. Data are presented as % of control levels, ±S.E.M. n = 3, *P < 0.05. (C) PS1 protein expression in different cell lines revealed by immunoblitting with a rabbit polyclonal antibody against PS1. 1—NB7 cells, 2—SH-SY5Y cells, 3—SH-SY5Y cells stable over-expressing PS1. (D) graphical representation of the results of densitometric analysis. 1—NB7 cells, 2—SH-SY5Y cells, 3—SH-SY5Y cells stably over-expressing PS1. (E) levels of PS1 protein expression in NB7 cells (revealed by a rabbit polyclonal antibody against PS1) after hypoxia ad oxidative stress. NB7 cells were incubated for 4–24 h in atmosphere with reduced oxygen content to 2.5% or 1%, or in the presence of 40 μM H2O2. Control cells were incubated for 24 h at standard tissue culture conditions (37°C, 5%, CO2, 95% atmospheric air). (F) graphical representation of the results of the analysis of PS1 expression under control conditions and after hypoxia and oxidative stress (n = 3, % of control ±S.E.M)
Effect of hypoxia and oxidative stress on expression of NEP in NB7 cells
Analysing the effect of chronic hypoxia on NEP expression in neuroblastoma NB7 cells we have found that it was reduced compared to control cells both at the mRNA (by 40%) and protein level (on average by 25%) (Fig. 3A–C, bars 1, 2). In the cells subjected to oxidative stress the levels of NEP expression have been also decreased (by 20% at the mRNA level) and on average by 30% at the protein level compared to controls (Fig. 3A–C, bars 1, 3).
Effect of hypoxia and oxidative stress on NEP mRNA and protein levels and NEP relative activity. NB7 cells were grown until 60–70% confluence and treated with 40 μM H2O2 or incubated in an incubator with reduced O2 content to 2.5% or 1% for 24 h. (A) levels of NEP mRNA expression analysed by real-time PCR with iQ SYBR Green supermix (Biorad). The mean results ±S.E.M. (n = 10 for H2O2 and n = 12 for hypoxia) are shown graphically. *P < 0.05 1—control, 2—40 μM H2O2, 3—hypoxia. (B) NEP protein expression analysed by immunoblotting with a mouse monoclonal antibody against NEP. Expression of GLUT-1, analysed with a rabbit polyclonal antibody against GLUT-1, was used as a marker of hypoxia. (C) graphical representation of results of densitometric analysis of the blots. Data are presented as % of control levels, ±S.E.M. n = 12, *P < 0.05. 1—control cells, 2—cells treated with 40 μM H2O2 (24 h), 3—cells subjected to hypoxia (2.5% O2, 24 h)
Effect of hypoxia on NEP mRNA levels in rat primary cortical neurones and astrocytes
Before the analysis of the effect of hypoxia on the levels of NEP expression in primary cortical neurones and astrocytes the levels of NEP specific activity in these cells have been compared to those in human neuroblastoma NB7 cells demonstrating that in neurones and astrocytes NEP activity was significantly lower than in NB7. Thus, mean NEP activity in NB7 cells determined by a fluorescent assay using Suc-Ala-Ala-Phe-AMC as a substrate, was 7.73 ± 0.57 nmol/mg protein/min compared with 0.09 ± 0.004 units in neurones and 0.82 ± 0.18 in astrocytes. Incubation of primary cortical neurones (10 days in culture) and astrocytes (7 days in culture) for 24 h under 1% of oxygen resulted in a decrease of NEP mRNA levels in neurones by 23% and an increase by 35% in astrocytes compared to control cells incubated for 24 h under normoxic conditions (Fig. 4A, B).
Effect of hypoxia on NEP mRNA expression in rat primary neurones and astrocytes. Cells were incubated under reduced O2 content to 1% for 24 h and NEP expression was analysed by real-time PCR as described in the Material and Methods using ribosomal S18 RNA as a house-keeping gene. (A) Neurones, (B) astrocytes. 1—control, 2—hypoxia. The mean results ±S.E.M. (n = 6 for neurones, n = 3 for astrocytes) are shown graphically. *P < 0.05
Discussion
Although much work on amyloid metabolism has been carried out using the human neuroblastoma SH-SY5Y cell line (originally sub-cloned from a neuroblastic (N)-type human neuroepithelioma cell line, SK-N-SH) which expresses APP and is a convenient and commonly used model for analysing APP processing by secretases under various experimental conditions by Youdim and colleagues [19, 20] and others [8, 21], the present study revealed that levels of the amyloid-degrading enzyme NEP protein expression in these cells are negligible. Further analysis of various human neuroblastoma cell lines demonstrated that NB7 cells derived from neuroblastic (N-type) SJ-N-CG cell line possessed a significantly high level of endogenous NEP and other amyloid-degrading enzymes e.g. ECE-1 [22] and IDE [23] and detectable levels of APP and PS1 which makes it a convenient neuronal model for studying regulation of expression of NEP in various in vitro models. Neuroblastoma NB7 cells are neuroepithelial-like cells derived from a solid tumour of the sympathetic nervous system [24]. These tumours arise from neuroblasts, which represent pluripotent sympathetic cells. Neuroblastomas differ in their origin and morphology and sometimes demonstrate spontaneous lineage conversion changing properties from neuronal to epithelial-like cells [25]. NB7 is the cell line with the greatest proliferation rate, also expressing the greatest quantity of survivin and has the highest level of the oncogene N-myc per cell [26]. Although NB7 cells do not represent morphologically typical differentiated neuronal cells and lack developed neurites, serum withdrawal or addition of a cholinergic agonist, carbachol, resulted in inhibition of cell proliferation and initiation of their differentiation towards the neurone-like phenotype (data not shown). Similar phenomena have previously been reported in the literature for SH-SY5Y [27].
Recently it has been suggested that in the brain there is an endogenous mechanism for removal of amyloid β-peptides, which are believed to be involved in pathogenesis of AD. There are several enzymes capable of degrading Aβ (see [28], for review) and two of them, namely NEP and IDE were shown to be reduced in the brain of AD patients [29] as well as in normally ageing brain [11, 30]. NEP was also found to be reduced in rat brain cortex after global ischemia [11]. Taking into account that ischemia and stroke predispose to the development of AD [31], analysis of the effect of hypoxia on NEP expression in neuronal cells is of particular interest since it might provide an insight into the mechanisms of regulation of this enzyme in neuronal cells under normal and pathological conditions. In this study we have demonstrated that in response to hypoxia NB7 cells increase expression of the glucose transporter GLUT1, in agreement with previous data on other cell lines [32, 33] and to decrease the levels of expression of NEP. Under conditions of oxidative stress caused by incubation of NB7 cells in the presence of 40 μM H2O2 there was no increase in GLUT1 levels which additionally provides specificity of this marker for hypoxia.
It was previously reported that acute hypoxia resulted in an increased expression of APP both in animal brain and in cell cultures (including neuroblastoma SH-SY5Y cells), which is considered as an adaptive reaction of cells to hypoxia [7, 10, 34]. In experiments with NB7 cells it was also found that 2.5% O2 hypoxia resulted in increased levels of membrane bound APP, which can also be considered as a marker of hypoxic conditions in this cell line. Although in their later work Webster and colleagues have not found an increase in total APP expression in SH-SY5Y cells under the conditions of chronic hypoxia, they have reported that the level of soluble APPα released from these cells was decreased by 30% after cultivation of the cells for 24 h under 2.5% O2 [8]. Decreased levels of sAPPα have also been reported in the cortex of animals subjected to prenatal hypoxia [10].
Using NB7 cells it was demonstrated that hypoxia (either 2.5% or 1% for 24 h) decreases both protein expression of NEP and its mRNA level. For NEP it has also been found that hypoxia reduces the activity of NEP measured by using a fluorogenic substrate. Utilization of 2.5% O2 for cell cultures has widely been accepted as an adequate model of hypoxia and there are several reports that under these conditions there are changes in calcium homeostasis and amyloid metabolism in neuroblastoma SH-SY5Y cells [7, 8, 35]. However, neuroblastoma cells, as other solid tumour cells, adapt to acute hypoxic stress and resist lower levels of hypoxia. Some authors have therefore suggested that 1% O2 is a more adequate hypoxic condition for such cells [36]. Hence, in this work, both 2.5% and 1% O2 have been used to treat NB7 and primary neuronal cells.
Expression and activity of NEP under hypoxic conditions have been extensively studied in non-neuronal tissues. It was shown that NEP plays an important role in hypoxic response of the carotid body to hypoxia [37] and inhibition of NEP activity augmented this response [38]. In the lungs a decrease in NEP activity was observed 24 h and 48 h after hypoxia leading to increased pulmonary leak. This decrease in enzyme activity in the lungs was associated both with reduced NEP protein content and mRNA expression after hypoxia [39]. On the contrary, in the vitreous fluid of patients with proliferative diabetic retinopathy, which is often accompanied by retinal hypoxia, NEP activity was found to be elevated [40]. This increase in NEP activity has been accompanied by a decreased level of Aβ, further implicating NEP in Aβ catabolism. Previously, these authors [41] failed to demonstrate any effect of hypoxia on NEP expression at the mRNA level in neuroblastoma SH-SY5Y cells although they have observed a decrease in its activity detected by an HPLC assay. However, this method would not distinguish between NEP activity and that of its homologue NEP2 (SEP), which is expressed at high levels in SH-SY5Y cells (A. Hunter and A.J. Turner, University of Leeds, unpublished observations).
Decreased expression of NEP and its homologue endothelin-converting enzyme ECE-1 at the protein level has been reported in the model of global ischaemia in rats where an ECE-1 decrease was more pronounced than that of NEP in the cortex and striatum of rats submitted to prenatal hypoxia [11]. This study also suggested that preconditioning to mild hypoxia before the episode of acute hypoxia had a positive effect on the levels of NEP and ECE-1 in the cortex and striatum of animals, which can partially explain why hypoxic preconditioning might have a neuroprotective effect.
The data obtained in this study and the data in the literature suggest that, in general with age, there is a decline in the expression and activity of amyloid-degrading enzymes and also of non-amyloidogenic processing of APP, which will shift amyloid metabolism towards accumulation of higher amounts of amyloid peptide. Hypoxia is one of the factors that will shift this balance even more towards accumulation of Aβ-peptide. Thus up-regulation of α-secretase and amyloid-degrading enzymes represents strategies to improve amyloid metabolism and reduce the rate of its accumulation.
Recent works by Youdim and colleagues have demonstrated that the main constituent of green-tea (−)-epigallocatechin-3-gallate (EGCG) has a neuroprotective effect in AD reducing generation of toxic Aβ [42]. This EGCG effect might be not only via alteration of post-translational modification of APP as suggested by these authors but also due to stimulation of NEP activity which was demonstrated by Melzig and colleagues in SK-N-SH neuroblastoma cells [43, 44]. Our preliminary data confirm stimulating effect of EGCG on NEP expression in neuroblastoma NB7 cells both at the protein level and enzyme activity. Since up-regulation of NEP is currently considered as one of the viable therapeutic strategies in AD [45] these studies open an avenue in the search for natural compounds possessing desirable therapeutic efficacy in the prevention of Aβ-related neuropathology.
Abbreviations
- AD:
-
Alzheimer’s disease
- NEP:
-
Neprilysin
- ECE:
-
Endothelin converting enzyme
- IDE:
-
Insulin degrading enzyme
- APP:
-
Amyloid precursor protein
- GLUT1:
-
Glucose transporter 1
- PS1:
-
Presenilin 1
References
Atkinson L, Boyle JP, Pearson HA et al (2006) Chronic hypoxia inhibits Na+/Ca2+ exchanger expression in cortical astrocytes. Neuroreport 17:649–652
Di Legge S, Hachinski V (2003) Prospects for prevention and treatment of vascular cognitive impairment. Curr Opin Invest Drugs 4:1082–1087
Kalaria RN (2000) The role of cerebral ischemia in Alzheimer’s disease. Neurobiol Aging 21:321–330
Camarata PJ, Heros RC, Latchaw RE (1994) “Brain attack”: the rationale for treating stroke as a medical emergency. Neurosurgery 34:144–157
Dolinak D, Smith C, Graham DI (2000) Global hypoxia per se is an unusual cause of axonal injury. Acta Neuropathol (Berl) 100:553–560
Baiden-Amissah K, Joashi U, Blumberg R et al (1998) Expression of amyloid precursor protein (β-APP) in the neonatal brain following hypoxic ischaemic injury. Neuropathol Appl Neurobiol 24:346–352
Webster NJ, Green KN, Peers C et al (2002) Altered processing of amyloid precursor protein in the human neuroblastoma SH-SY5Y by chronic hypoxia. J Neurochem 83:1262–1271
Webster NJ, Green KN, Settle VJ et al (2004) Altered processing of the amyloid precursor protein and decreased expression of ADAM 10 by chronic hypoxia in SH-SY5Y: no role for the stress-activated JNK and p38 signalling pathways. Brain Res Mol Brain Res 130:161–169
Pluta R (2002) Astroglial expression of the β-amyloid in ischemia-reperfusion brain injury. Ann N Y Acad Sci 977:102–108
Nalivaeva NN, Fisk L, Canet Aviles RM et al (2003) Effect of prenatal hypoxia on expression of amyloid precursor protein and metallopeptidases in the rat brain. Lett Peptide Sci 10:455–462
Nalivaeva NN, Fisk L, Kochkina EG et al (2004) Effect of hypoxia/ischemia and hypoxic preconditioning/reperfusion on expression of some amyloid-degrading enzymes. Ann N Y Acad Sci 1035:21–33
Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79:1431–568
Gille JJ, Joenje H (1992) Cell culture models for oxidative stress: superoxide and hydrogen peroxide versus normobaric hyperoxia. Mutat Res 275:405–414
Smith PK, Krohn RI, Hermanson GT et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Mumford RA, Strauss AW, Powers JC et al (1980) A zinc metalloendopeptidase associated with dog pancreatic membranes. J Biol Chem 255:2227–2230
Jogi A, Ora I, Nilsson H et al (2003) Hypoxia-induced dedifferentiation in neuroblastoma cells. Cancer Lett 197:145–150
Jogi A, Ora I, Nilsson H et al (2002) Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci USA 99:7021–7026
Zhang JZ, Behrooz A, Ismail-Beigi F (1999) Regulation of glucose transport by hypoxia. Am J Kidney Dis 34:189–202
Avramovich-Tirosh Y, Amit T, Bar-Am O et al (2007) Therapeutic targets and potential of the novel brain-permeable multifunctional iron chelator-monoamine oxidase inhibitor drug, M-30, for the treatment of Alzheimer’s disease. J Neurochem 100:490–502 [Epub ahead of print Nov 27, 2006]
Weinreb O, Amit T, Bar-Am O et al (2006) Involvement of multiple survival signal transduction pathways in the neuroprotective, neurorescue and APP processing activity of rasagilinr and its propargyl moiety. J Neuroal Transm Suppl 70:457–465
Cedazo-Minguez A, Bonecchi L, Winblad B et al (1999) Nicergoline stimulates protein kinase C mediated α-secretase processing of the amyloid precursor protein in cultured human neuroblastoma SH-SY5Y cells. Neurochem Int 35:307–315
Fisk L, Nalivaeva NN, Turner AJ (2006) Regulation of endothelin-converting enzyme-1 expression in human neuroblastoma cells. Exp Biol Med (Maywood) 231:1048–1053
Nalivaeva NN, Fisk L, Kochkina EG et al (2006) Different mechanisms of secretion of amyloid-degrading enzymes neprilysin and insulinase by human neuroblastoma cells in culture. J Neurochem 98(Suppl):19
Seeger RC, Siegel SE, Sidell N (1982) Neuroblastoma: clinical perspectives, monoclonal antibodies, and retinoic acid. Ann Intern Med 97:873–884
Ross RA, Spengler BA, Biedler JL (1983) Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J Natl Cancer Inst 71:741–747
Shapiro DN, Valentine MB, Rowe ST et al (1993) Detection of N-myc gene amplification by fluorescence in situ hybridization. Diagnostic utility for neuroblastoma. Am J Pathol 142:1339–1346
Rosner H, Vacun G, Rebhan M. (1995) Muscarinic receptor-mediated induction of actin-driven lamellar protrusions in neuroblastoma cell somata and growth cones. Involvement of protein kinase C. Eur J Cell Biol 66:324–334
Carson JA, Turner AJ (2002) β-amyloid catabolism: role for neprilysin (NEP) and other metallopeptidases? J Neurochem 81:1–8
Caccamo A, Oddo S, Sugarman MC et al (2005) Age- and region-dependent alterations in Aβ-degrading enzymes: implications for Aβ-induced disorders. Neurobiol Aging 26:645–654
Nalivaeva NN, Fisk L, Kochkina EG et al (2006) Developmental dynamics of amyloid-degrading enzymes in the rat brain under normal and hypoxic conditions. Abstracts of FENS meeting. Vienna, Austria 3:335–336
Breteler MM (2000) Vascular risk factors for Alzheimer’s disease: an epidemiologic perspective. Neurobiol Aging 21:153–160
Boado RJ, Pardridge WM (2002) Glucose deprivation and hypoxia increase the expression of the GLUT1 glucose transporter via a specific mRNA cis-acting regulatory element. J Neurochem 80:552–554
Vannucci SJ, Seaman LB, Vannucci RC (1996) Effects of hypoxia-ischemia on GLUT1 and GLUT3 glucose transporters in immature rat brain. J Cereb Blood Flow Metab 16:77–81
Hoyer A, Bardenheuer HJ, Martin E et al (2005) Amyloid precursor protein (APP) and its derivatives change after cellular energy depletion. An in vitro-study. J Neural Transm 112:239–253
Smith IF, Boyle JP, Vaughan PF et al (2001) Effects of chronic hypoxia on Ca2+ stores and capacitative Ca2+ entry in human neuroblastoma (SH-SY5Y) cells. J Neurochem 79:877–884
Holmquist L, Jogi A, Pahlman S (2005) Phenotypic persistence after reoxygenation of hypoxic neuroblastoma cells. Int J Cancer 116:218–225
Kumar GK, Runold M, Ghai RD et al (1990) Occurrence of neutral endopeptidase activity in the cat carotid body and its significance in chemoreception. Brain Res 517:341–343
Kumar GK, Kou YR, Overholt JL et al (2000) Involvement of substance P in neutral endopeptidase modulation of carotid body sensory responses to hypoxia. J Appl Physiol 88:195–202
Carpenter TC, Stenmark KR (2001) Hypoxia decreases lung neprilysin expression and increases pulmonary vascular leak. Am J Physiol Lung Cell Mol Physiol 281:L941–L948
Hara H, Oh-hashi K, Yoneda S et al (2006) Elevated neprilysin activity in vitreous of patients with proliferative diabetic retinopathy. Mol Vis 12:977–982
Oh-hashi K, Nagai T, Tanaka T et al (2005) Determination of hypoxic effect on neprilysin activity in human neuroblastoma SH-SY5Y cells using a novel HPLC method. Biochem Biophys Res Commun 334:380–385
Reznichenko L, Amit T, Zheng H et al (2006) Reduction of iron-regulated amyloid precursor protein and beta-amyloid peptide by (−)-epigallocatechin-3-gallate in cell cultures: implications for iron chelation in Alzheimer’s disease. J Neurochem 97:527–536
Melzig MF, Janka M (2003) Enhancement of neutral endopeptidase activity in SK-N-SH cells by green tea extract. Phytomedicine 10:494–498
Ayoub S, Melzig MF (2006) Induction of neutral endopeptidase (NEP) activity of SK-N-SH cells by natural compounds from green tea. J Pharm Pharmacol 58:495–501
Golde TE (2006) Disease modifying therapy for AD?. J Neurochem 99:689–707
Acknowledgements
This work was supported by Medical Research Council, UK and The Biochemical Society (Mrs L Fisk).
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue dedicated to Dr. Moussa Youdim.
Rights and permissions
About this article
Cite this article
Fisk, L., Nalivaeva, N.N., Boyle, J.P. et al. Effects of Hypoxia and Oxidative Stress on Expression of Neprilysin in Human Neuroblastoma Cells and Rat Cortical Neurones and Astrocytes. Neurochem Res 32, 1741–1748 (2007). https://doi.org/10.1007/s11064-007-9349-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9349-2