Abstract
LPS-induced inflammation and changes in protein phosphorylation and the JAK-STAT pathway accompanying glial activation after LPS treatment, were followed by analyzing secreted proinflammatory cytokine levels. The administration of LPS caused tyrosine phosphorylation of STAT3 in retinae and induced glial fibrillary acidic protein. (GFAP) from the nerve fiber layer to the ganglion cell layer. Our results suggest that the LPS-induced activation of the JAK2/STAT3 signaling pathway may play a key role in the induction of astrogliosis. However, no significant increase in vimentin, OX-42 or inducible nitric oxide synthase (iNOS) expressions were observed after LPS administration.
Sphingosine kinase catalyzes the conversion of sphingosine to sphingosine-1–phosphate (So-1-P), a sphingolipid metabolite that plays important roles in angiogenesis, inflammation, and cell growth. In the present study, it was found that sphingolipid metabolite levels were elevated in the serum and retinae of LPS-injected rats. To further investigate the chronic effect of increased So-1-P in the retina, So-1-P was infused intracerebroventricularly (i.c.v.) into rats using an osmotic minipump at 100 pmol/10 μl h-1 for 7 days, and was found to increase retinal GFAP expression. These observations suggest that LPS induces the activation of retinal astrocytes via JAK2/STAT3 and that LPS affects So-1-P generation. Our findings also suggest that elevated So-1-P in the retina and/or in serum could induce cytochemical alterations in LPS treated or inflamed retinae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipopolysaccharide (LPS) is a toxin derived from gram-negative bacteria and is largely used to mimic strong and acute inflammatory responses in rodents. LPS provokes a range of behavioral and endocrine alterations that are regulated in the CNS, which are collectively referred to as host response inflammation. Effects of LPS are believed to be mediated by proinflammatory cytokines, such as, interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), which are released in response to LPS endotoxemia [1]. The intracellular signaling mechanisms related to the effects of LPS have been well studied in several types of cells including macrophages, microglia, and astrocytes. Briefly, LPS is known to activate protein kinase C, protein-tyrosine kinases, mitogen-activated protein kinases, and NF-kB, which have been implicated in the release of immune-related cytotoxic factors, such as, NO and proinflammatory cytokines [2].
Cytokines regulate numerous aspects of hematopoiesis and immune response, and proinflammatory cytokines mediate their responses by activating the JAK/STAT signaling pathway. Signal transducers and activators of transcription compose a family of seven structurally and functionally related proteins, namely, STAT 1, STAT 2, STAT 3, STAT 4, STAT 5a, STAT 5b and STAT 6. Janus kinases (JAK) are a family of four non-receptor tyrosine kinases, i.e., JAK1, JAK 2, JAK 3, and Tyk2. These kinases selectively phosphorylate STATs, which leads to their activation, and once activated, STATs play a critical role in regulating innate and acquired host immune responses. Moreover, signaling through the JAK/STAT pathway is initiated when a cytokine binds to its receptor [3]. Analyses of the transcription factors essentially activated by inflammatory cytokines should provide more detailed mechanistic information. STAT3 recently appeared as a marker of brain cells which are directly activated by some cytokines. It is known that STAT3 activation is mediated by cytokine mobilizing gp130 receptor subunits, followed by recruitment of cytosolic JAKs. The latter phosphorylate STAT3, which then forms hetero- or homodimers, and translocates into the nucleus. These alter gene expression by binding to STAT-responsive element. Therefore, studies of STAT3 activation in the brain and retina after LPS application provide a means of identifying the cells targeted by circulating cytokines [1].
All types of central nervous system injuries result in reactive gliosis, a cellular response characterized predominantly by astrocytic hypertrophy and the attendant accumulation of glial filaments, the major protein component of which is GFAP [4, 5]. Many toxic stimuli activate astrocytes, as is demonstrated by dramatic morphological changes and by the elevation of GFAP expression [6, 7]. Moreover, activated astrocytes may differ from non-activated astrocytes with respect to their ability to protect neurons from excitotoxicity. Indeed, recent studies show that excitotoxicity is associated with the activations of astrocytes and microglia in vivo and in vitro. Furthermore, astrocyte activation becomes a detrimental factor in conditions of chronic neurodegeneration [6], because activated astrocytes secrete NO, interleukin (IL)-1α, IL-1β, and TNF-α, which exert strong neurotoxic effects that may counteract the neuroprotective effects of astrocyte-derived growth factors [7, 8].
The mechanisms underlying the injury-induced conversion of astrocytes into their reactive form remain to be elucidated. However, the generalized nature of astroglial response to central nervous system injuries suggests that common signaling events are responsible for the initiation of this process, despite the regional- and cell type-specific targets of individual insults or disease states. Although a variety of signal transduction pathways have been shown to be involved in the activation of astrocytes, in vitro, few signaling modules have been linked to the induction of reactive gliosis, in vivo. The limited evidence obtained to date using traumatic and ischemic models of brain damage, points to the involvements of proinflammatory cytokine/chemokine signaling pathways in astroglial responses to injury. These potentially include the JNK, ERK, and JAK/STAT pathways as downstream effectors of reactive gliosis [9]. Interestingly, serine protease inhibitor 3 mRNA and pSTAT3 protein have been reported to be colocalized in retinal astrocytes after LPS treatment in the rat [10].
Sphingosine-1-phosphate (So-1-P) has been characterized as a lipid-signaling molecule with dual functions, i.e., it acts extracellularly by binding to five different So-1-P receptors that couple to a variety of G-proteins to regulate diverse biological functions, and on the other hand, it appears to act as an intracellular “second messenger.” However, the molecular targets to which it binds within cells, remain to be discovered. Nevertheless, So-1-P has been established to have several cellular and tissue functions, which include the regulation of cell survival and motility, angiogenesis, and inflammatory response [11]. Sphingosine kinases (SPHKs) types 1 and 2, the two enzymes identified so far in mammals that produce So-1-P by ATP-dependent phosphorylation of sphingosine, have therefore attracted considerable research interest [12]. For example, it has been reported that the enzyme activity of SPHK1 is upregulated by stimulation with either TNF-α or IL-1β in human A549 epithelial cells, and the time course of this activation was reminiscent of SPHK activation in TNF-α stimulated HEK cells and in endothelial cells [12]. Moreover, SPHK and So-1-P were characterized in INS-1 insulinoma cells and in isolated rat islets of Langerhans. SPHK activity was increased in INS-1 cell homogenates treated with IL-1β and/or TNF-α, and these responses were additive and increased endogenous So-1-P biosynthesis in 32Pi-prelabeled INS-1 cells [13]. In addition, exposure of endothelial cells to TNF-α resulted in the rapid activation of SPHK and in the release of So-1-P from endothelial cells [14]. Based on these results, we suppose that pro-inflammatory cytokines induced by LPS generate So-1-P.
Retinal neurodegenerative disease causes an inflammatory response in the retina characterized by an increase in inflammatory cytokines and in the activation of astrocyte/microglia. Moreover, the degree of this astrocyte/microglia activation may influence the extent of retinal injury following an inflammatory stimulus. In the present study, the adult rat retina was used as a model system to investigate LPS-mediated JAK/STAT signaling in neurons and glia. We investigated retinal damage and found that sphingolipid metabolites can be used as a biomarker of physiological change in peripheral blood and retinae after LPS administration. In addition, using a metabolic study approach, we observed functional responses to So-1-P in the rat retina.
Experimental procedures
Animals and reagents
Sprague-Dawley rats (male, 260–280 g) were obtained from Daehan Biolink (Eumsung, Korea), and kept under standard conditions (24 ± 3°C) under a 12-h light/dark cycle (light on at 08:00 a.m.) with free access to food and water. All procedures involving rats were performed according to the guidelines issued by the Animal Care and Use Committee of the College of Medicine, Ewha Womans University. Rats were injected with LPS (1500 μg/kg, i.p.) and decapitated 6 h later.
Sphingosine (So, d-sphingosine), sphinganine (Sa, dl-erythro- dihydrosphingosine), sphingosine-1-phosphate (So-1-P), and sphinganine-1-phosphate (Sa-1-P) were purchased from Biomol Research Laboratory, Inc. (Plymouth, PA, USA). Alkaline phosphatase and o-phthaldialdehyde (OPA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). C17-sphingosine (C17-So) and C17 sphingosine-1-phosphate (C17 So-1-P) were obtained from Avanti Polar Lipids, Inc. (Alabaster, AL, USA).
ELISA for TNF-α and IL-1β proteins
Serum was collected and assayed for TNF-α and IL-1β protein levels by ELISA using Quantikine Colorimetric Sandwich ELISA kits (R&D System, Minneapolis, MN, USA).
Reverse transcription – polymerase chain reaction (RT-PCR)
Total RNA was isolated from retinae using TRIzol (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. For cDNA synthesis, 2 μg of total RNA was reverse-transcribed using the SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). cDNA was amplified by PCR using primers for IL-1β, TNF-α and β-actin as follows: IL-1β (forward, AAG CTC TCC ACC TCA ATG GA; reverse, TGC TTG AGA GGT GCT GAT GT), TNF-α (forward, AGC CCT GGT ATG AGC CCA TGT A; reverse, CCG GAC TCC GTG ATG TCT AAG T), β-actin (forward, CTC CTT AAT GTC ACG CAC GAT TTC; reverse, GTG GGG CGC CCC AGG CAC CA). PCR products (10 μl) were separated by 2% agarose gel electrophoresis and visualized by ethidium bromide staining.
Western blot analysis
Western blot analysis was preformed as described by Towbin et al. [15] with modification. Briefly, tissues were homogenized in buffer [10 mM Tris·Cl, pH 7.4, 0.5 mM EDTA, pH 8.0, 0.25 M sucrose, 1 mM PMSF, 1 mM Na4VO3 containing protease inhibitor cocktail (Complete Roche-Boehringer-Mannheim)]. After centrifugation in a microcentrifuge at 13000 rpm for 15 min at 4°C, supernatant was subjected to SDS-PAGE. Protein content was determined using the Bradford method [16] and proteins were western blotted. Proteins present in supernatant were loaded at 20–60 μg and size separated in 8–10% sodium dodecyl sulfate by polyacrylamide gel electrophoresis. Gels were processed using the appropriate antigens after blotting onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). Blots were blocked in TBS containing 5% non-fat dry milk and 0.1% Tween 20, and incubated with the appropriated primary antibodies {STAT1, pSTAT1, STAT3 and pSTAT3 (1:2000), Cell Signaling, Beverly, MA; JAK1 (1:1000), pJAK 1 (1:500), and JAK2 (1:4000), Upstate, CA, USA} followed by secondary antibody (1:2000) conjugated to horseradish peroxidase (Santa Cruze, CA, USA). The detection was performed using an ECL-detection system (Amersham Pharmacia Biotech, CA, USA).
Immunohistochemistry
Rats were anesthetized with ether and perfused through the heart with 0.05 M phosphate buffered saline (PBS), followed by 4% paraformaldehyde (in 0.1 M phosphate buffer). Eyes were enucleated and immersion fixed for 30 min in 4% (w/v) paraformaldehyde. Retinae were cryoprotected by infiltration with increasing concentrations of sucrose (10–30%), frozen in freezing medium, cut into 5 μm thick sections on a cryostat (LEICA CM 3000), and collected on 3-aminopropyltriethoxylilane-coated slides. For light microscopy every 10th slide was stained with hematoxylin and eosin. Tissue sections were then rinsed in PBS, immersed in 0.3% hydrogen peroxide (in PBS) for 20 min at room temperature, preincubated in 10% normal horse serum (Vector Lab) for 1 h, and incubated in mouse anti-GFAP antiserum (1:800, Chemicon), mouse anti-vimentin antiserum (1:100, Chemicon), mouse anti-iNOS antiserum (1:100, Santa Cruz), or OX-42 (1:100, Serotec) for 15 h at 4°C. The second incubation with HRP-conjugated anti-mouse IgG (1:50, Santa Cruz) was performed for 1 h at room temperature. Finally, immunocomplexes were visualized with 0.025% 3,3-diaminobenzidine in PBS containing 0.005% hydrogen peroxide. Appropriate controls for the immunohistochemical staining procedure included substituting primary antiserum with normal rabbit serum or omitting primary antiserum. Immuno-positive cells were measured and the certain area of particle (pixel) was calculated using analysis software (Soft Imaging System, Munster, Germany).
Measurement of sphingolipids in serum and retinae
Six hours after LPS injection, blood was collected from hearts and serum was separated. The concentrations of sphingoid bases (So, Sa) and of their phosphated forms (So-1-P, Sa-1-P) in plasma and retinae were determined by high performance liquid chromatography (HPLC) using a modification of a previously described procedure [17]. For lipid extraction, 700 μl of chloroform/methanol-1 M NaCl (1:1, v/v), 3N-NaOH, and C17 So-1-P and C17 So as internal standards were added to 50 μl plasma and incubated for 5 h. Aqueous and chloroform phases were separated by centrifugation at 15,000 g for 10 min. The alkaline aqueous phase containing sphingoid base 1-phosphate, but devoid of sphingoid bases and the majority of hydrophobic phospholipids, was transferred to a fresh tube. To analyze sphingoid bases, 400 μl of 0.15 M methanolic KOH was added to the chloroform layer. Sample tubes were incubated at 37°C for 1 h and then partitioned with 400 μl of 2N NH4OH. Chloroform phases were washed three times with alkaline water (150 μl of 2N NH4OH in 700 ml of distilled water, pH 10.0), and the lower organic phases were transferred to fresh tubes and dried under a stream of nitrogen.
The aqueous fraction of the sphingoid bases 1-phosphate extract was mixed thoroughly with reaction buffer [200 mM Tris–HCl (pH 7.4), 75 mM MgCl2 in 2 M glycine buffer, pH 9.0] and 50 units of alkaline phosphatase and then incubated at 37°C for 1 h. This procedure completely converted sphingoid base 1-phosphate into the corresponding sphingoid bases by the action of alkaline phosphatase. The dephosphorylated sphingoid bases were then extracted with chloroform. Lipid solution in methanol was mixed well with 15 μl of OPA reagent [50 mg OPA, 1 ml ethanol, 100 μl 2-mercaptoethanol, and 50 ml 3% (w/v) boric acid solution adjusted to pH 10.5] and then incubated at room temperature for 15 min. HPLC analysis was performed using a Shimadzu (Tokyo, Japan) Model LC-10AT pump and SIL-10AXL autosampler system and an analytical Radial-Pak cartridge packed with Nova-Pak C18 (reversed-phase column; 4 μm X 100 mm X 8 mm). An isocratic eluent composition of methanol-distilled water (90:10 v/v) and a flow rate of 1 ml/min were accurately controlled during HPLC. A Shimadzu RF-10XL fluorescence detector was set at an excitation wavelength of 340 nm and an emission wavelength of 455 nm. The resulting data and chromatographic profiles were evaluated using Borwin system manager software (JMBS, France).
Infusion of So-1-P and LPS into ventricles
Rats were implanted with guide cannulae for drug infusion. Briefly, rats were anesthetized with ketamine (50 mg kg-1) and xylazine (1 mg/kg) i.p., and standard stereotaxic surgery was performed using a Kopf stereotaxic frame. A 21-gauge stainless steel cannula was implanted in the right lateral ventricle (L: 1.3 mm; A–P: -0.5 mm; D–V: -4.5 mm) using bregma as the stereotaxic reference point [18]. The cannula was held in place with rapid-setting dental acrylic and anchored to the skull with an aluminum protective cap and steel screws. Rats were allowed 1 week to recover before osmotic minipump implantation. Minipumps were implanted (s.c.) as previously described [19] with minor modification. Briefly, under ether anesthesia, a small incision was placed behind the ears and the subcutaneous space was expanded with a hemostatic forceps. Saline, So-1-P or LPS was filtered through a 0.2 μm sterile syringe filter and then used to fill an osmotic minipump (Alzet 2ML 1, Alza, Palo Alto, CA, USA). The minipump was then implanted and connected directly to the cannula via 6-cm long PE-60 polyethylene tubing. The rates and durations of infusion were; 100 pmol So-1-P/10 μl h−1 or 25 ng LPS/10 μl h−1 for 7 days. The incision made on the back was closed with cyanoacrylate glue, and dental acrylic was layered on top of the polyethylene tubing.
Statistical analysis
Data are expressed as means ± SEM and comparisons between groups were performed using analysis of variance followed by the t-test; p values of <0.05 were considered significant.
Results
Levels of TNF-α and IL−1β in serum and retinae after LPS administration
The level of TNF-α and IL-1β in the serum was measured. A ELISA kit (R&D System, Minneapolis, MN) was used to determine proinflammatory cytokines 6 h after LPS injection (1500 μg kg−1, i.p.). The levels of IL-1β in serum was remarkably elevated after LPS treatment, and TNF-α levels were significantly elevated (Table 1). To investigate the expressions of TNF-α and IL-1β, we examined the mRNA levels of both cytokines by RT-PCR in retinae 6 h after LPS injection. The results obtained showed that the expressions of TNF-α and IL-1β mRNA in retinae were highly induced after LPS injection (Fig. 1).
The changes in the expressions of JAK/STAT in retinae after LPS injection
Of the potential signaling modules involved in LPS-induced astrogliosis, the JAK2/STAT3 pathway appeared to be a likely candidate because it was found to be associated with the enhanced expression of GFAP in a model of gliogenesis [9]. Therefore, we analyzed changes in the expression and phosphorylation of STAT3 following LPS treatment. To identify kinases involved in the signaling pathways associated with LPS-induced astrogliosis, we analyzed changes in the phosphorylations of several kinases, including JAK1 and JAK2. The expressions of JAK2 and pSTAT3 were found to be significantly elevated in retinae after LPS injection (1500 μg kg−1, i.p.) (Fig. 2).
Retinal expressions of JAK1, pJAK1, JAK2, STAT1, STAT3 and pSTAT3 6 h after LPS administration (1500 μg kg-1, i.p.). Protein blots were quantified versus β-actin, which was used as a loading control., Data are means ± SEs of three individual experiments. * p < 0.05 compared with non-treated controls
Expression of GFAP in retinae after i.c.v. LPS infusion
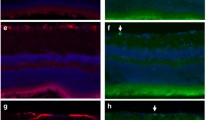
LPS infusion is known to trigger the release of pro-inflammatory cytokines in vivo. After filling an osmotic minipump with LPS it was implanted and connected directly to the cannula in ventricle. The rate and duration of infusion was 25 ng LPS/10 μl h−1 for 7 days. To examine retinal changes using GFAP and vimentin antibodies, retinae were removed and sectioned at 5 μm on a cryostat. The expression of GFAP, but not of vimentin, was found to be significantly elevated in the nerve fiber layer–ganglion cell layer of retinae after LPS-infusion (Fig. 3). However, the expressions of OX-42 and iNOS were not altered by LPS-infusion (Fig. 4).
Retinal GFAP and vimentin staining in sections from a rat administered LPS (25 ng 10 μl h-1) for 7 days. (A) Strong GFAP and vimentin immunoreactivities were observed from the nerve fiber layer to the ganglion cell layer (original magnification, X200) Bar = 1 mm. (B) Quantitative analysis of immunostaining from the nerve fiber layer to the ganglion cell layer. * p < 0.05 compared with non-treated controls (each group n = 6) GCL: ganglion cell layer, IPL: inner plexiform layer
Changes in sphingolipid in serum and retinae after LPS treatment
Serum was separated from blood and retinae were collected 6 h after injecting saline or LPS (1500 μg kg−1, i.p.). Levels of sphingolipids (So, Sa, So-1-P, Sa-1-P) in serum and of phosphated sphingosine (So-1-P) in retinae were measured by HPLC. Levels of So-1-P and Sa-1-P were found to be significantly elevated in LPS treated animals, as were levels of So and Sa in serum (Fig. 5A). In retinae, levels of So and So-1-P were significantly increased by LPS injection (Fig. 5B). Interestingly, the ratio of So-1-P/So was low in retinae but high in the serum. However, we could not detect Sa-1-P in retinae (detection limit 1 pmol mg-1 protein).
Elevations of sphingolipids in serum and phosphated sphingolipid in retinae of LPS-treated rats. Serum was separated from blood and retinae were collected 6 h after LPS treatment (1500 μg kg-1, i.p.), So-1-P: sphingosine-1-phosphate, Sa-1-P: sphinganine-1-phosphate, So: sphingosine, Sa: sphinganine, * p < 0.05 compared with controls (each group n = 6)
Elevation of GFAP expression by So-1-P infusion
The above results show that So-1-P levels in serum were increased by LPS. To determine whether So-1-P affects retinal function, So-1-P was infused into a ventricle at 100 pmol/10 μl h-1 for 7days, and then histological retinal changes were examined using GFAP and vimentin antibodies. The expression of GFAP, a reactive gliosis marker, was found to be significantly elevated in the nerve fiber layer–ganglion cell layer after So-1-P infusion (Fig. 6). However, vimentin immunoreactivity was unchanged versus the So-1-P non-treated control.
GFAP and vimentin staining of retinae in sections from a rat infused with So-1-P (100 pmol 10 μl h-1) for 7 days. (A) Strong GFAP immunoreactivities were observed from the nerve fiber layer to the ganglion cell layer (original magnification, X200) Bar = 1 mm. (B) Quantitative analysis of immunostaining from nerve fiber layer to the ganglion cell layer. * p < 0.05 compared with non-treated controls (each group n = 6), GCL: ganglion cell layer, IPL: inner plexiform layer
Discussion
Inflammation is a natural response to disturbed homeostasis caused by infection, injury or trauma. Astrocytes and microglia are the main effector cells of innate immune response in the central nervous system (CNS) and can be activated in neurodegenerative diseases to produce an array of inflammatory mediators. The generalized nature of reactive gliosis suggests that the origin of glial activation in signaling mechanisms common to many types of CNS injuries [9]. However, the identities of such signaling mechanisms in the retina has eluded discovery. In the present study, LPS was used to induce retinal inflammation, and the study produced strong evidence that reactive gliosis occurs via a JAK2/STAT3 pathway was obtained in the subsequent astroglial response.
LPS has been used extensively in inflammatory studies [20, 21], and it has been shown that LPS activates astroglia and exerts neurocytotoxic effects both in vitro and in vivo [22, 23]. Reactive gliosis is the most prominent response to diverse forms of CNS injury, and increased GFAP expression is recognized as a marker of reactive gliosis. The cellular events typifying this response include the proliferation of astrocytes with hypertrophy of cell bodies and the development of astrocytic processes, and an apparent increase in the levels of intermediate glial filaments, GFAP [24]. Astrocytes show several distinctive characteristics during neurological damage, such as an intense proliferative response, enlargement, and greater cytoplasmic branching complexity, and in addition, molecules not normally expressed are synthesized [25]. To investigate the activation of astrocytes among glial cells, the western blotting using GFAP and vimentin antibodies was performed using whole retinae. It was found that LPS treatment did not change GFAP or vimentin expression in retinae (data not shown), but it was believed that the method used was limited to detecting changes in specific retinal layers. Thus, we sectioned retinae on a cryostat and used a histological approach using appropriate antibodies. It was found that GFAP was more higher expressed in the retinae of LPS-infused rats, especially in the nerve fiber layer –ganglion cell layer.
It is known that sphingosine kinase-1 (SPHK1) catalyzes the conversion of sphingosine to sphingosine-1-phosphate (So-1-P), a sphingolipid metabolite that plays important roles in angiogenesis, inflammation, and cell growth [12]. In the present study, we found that the sphingolipid metabolite, So-1-P was elevated in the serum and retinae of LPS-treated rats. To further investigate the effect of increased So-1-P in retinae, So-1-P was infused intracerebroventricularly (i.c.v.) using an osmotic minipump at 100 pmol/10 μl h-1 for 7 days, and resultantly, GFAP expression was found to have increased in retinae. These findings suggest that LPS induces the activation of astrocytes via JAK2/STAT3 in retinae. It was also found to affect the generation of So-1-P in serum.
Recent work suggests the involvement of JAK-STAT signaling in multiple physiological processes in the nervous system [26]. The activation of STAT3 has been reported following excitotoxic injury, ischemia, cortical and nerve lesion administration, and after a peripheral challenge with bacterial lipopolysaccharide [9]. These findings suggest that STAT3 participates in response to neuronal stress [26]. The present study demonstrates that expression of JAK2 is temporally consistent with the subsequent phosphorylation of STAT3 [9]. Moreover, our results indicate that LPS predominantly activates STAT3 rather than STAT1 in the retina, and support the notion that STAT3 is the primary effector protein of LPS-mediated JAK-STAT signaling.
Whether translocated pSTAT3 serves as a transcription factor for GFAP was not established in this investigation; nevertheless, pSTAT3 has been reported to enhance the transcription of GFAP in vitro [9]. However, our results reinforce the idea that astrocytes are activated during inflammatory processes. During peripheral inflammation, STAT3 activation in astrocytes could trigger the transcriptions of genes involved in brain response to inflammation, especially GFAP, the main molecular marker of mature astrocytes. STAT3 regulates GFAP expression via a STAT-responsive element in GFAP promoter [1]. Furthermore, GFAP expression is known to be upregulated in response to brain injury and during the inflammation mediated by centrally or peripherally administered LPS. Further studies are needed to determine the nature of the correlation between STAT3 activation and GFAP expression and to evaluate the relationship between the activities of astrocytes and neurons during inflammation. In addition, it is possible that cytokines and chemokines that are not the upstream effectors of the gp130-JAK2/STAT3 pathway, but that they are linked to early responses to injury following chemical denervation, e.g., TNF-α may play an important role in the induction of astrogliosis. As such, the JAK2/STAT3 pathway serves as a potential target for pathological modification of astroglial response to injury [9].
It has recently been demonstrated using biochemical, immunohistochemical, and western blot approaches that the overproduction of NO in neurons causes noxious effects. iNOS can be induced by proinflammatory agents, such as, endotoxin (bacterial lipopolysaccharide), IL-1β, TNF-α, and interferon-γ in various cell systems, including retinal microglia [27]. According to a suggestion that inflammation causes retinal microglial activation [27], we used a histological method to follow OX-42 (a marker of microglial activation) and iNOS expression in retinae. However, we found that OX-42 and iNOS expressions were unaltered. To investigate cytokine-induced changes in retinae caused by LPS-induced pro-inflammatory cytokine release, we examined retinae after 7 days of i.c.v. LPS administration immunohistochemically using GFAP and vimentin. LPS was found to increase GFAP expression in the nerve fiber layer – ganglion cell layer. However, the expressions of OX-42 and vimentin were not altered by LPS. We believe that OX-42 and iNOS were transiently expressed in LPS-treated animals.
The SPHK/So-1-P pathway plays an important role during pathological conditions, such as, cancer, angiogenesis, and inflammation. SPHK1-mediates the generation of So-1-P and has been shown to be a signaling molecule, which mediates various activities of TNF-α [14]. Moreover, it has been demonstrated that SPHK1 activation in A549 cells is required for the transcriptions of proinflammatory proteins and for the full activation of the NF-κB pathway induced by TNF-α and IL-1β [12]. Cytokines such as IL-1β and TNF-α are also major stress inducers in β-cells, and have been implicated in the development of diabetes. Moreover, SPHK activity was found to be elevated in the membrane and nuclear subcellular fractions of INS-1 cells, and SPHK activity was found to be significantly activated in cytosolic fractions after treating the cells with IL-1β or TNF-α [13].
The role for SPHK in the regulation of inflammatory gene expression in the retina remains unclear. Our results show that levels of So-1-P and Sa-1-P were significantly elevated in serum, and that So-1-P levels were elevated in the retinae of LPS treated animals. It has been suggested that release of TNF-α and IL-1β activates a variable sphingosine kinase in platelets, which is then phosphorylated [11]. In the present study, LPS was also found to elevate the levels of sphingosine and sphinganine in serum. These results suggest that reactions initiated by endogenous LPS are dependent on pro-inflammatory cytokines.
In conclusion, the present study demonstrates that sphingolipid metabolites are significantly upregulated in rat serum and retinae after LPS-treatment, and that the direct i.c.v infusion of So-1-P elevates GFAP expression in the retinal nerve fiber layer–ganglion cell layer. In addition, the levels of JAK2 and pSTAT3 were elevated in retinae after LPS treatment. These observations indicate that LPS predominantly activates STAT3 rather than STAT1 in the retina, and support the notion that STAT3 is the primary effector protein, at least of LPS-mediated JAK-STAT signaling. In addition, it is possible that elevated So-1-P in retinae and/or serum could induce cytochemical alterations after LPS treatment or inflammation.
References
Gautron L, Lafon P, Chaigniau M, Tramu G, Laye S (2002) Spatiotemporal analysis of signal transducer and activator of transcription 3 activation in rat brain astrocytes and pituitary following peripheral immune challenge. Neuroscience 112:717–729
Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS (2004) NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem 279:1415–1421
Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW (2002) Signaling through JAK/STAT pathway, recent advances and future challenges. Gene 285:1–24
Eng LF, Ghirnikar RS (1994) GFAP and astrogliosis. Brain Pathol. 4:229–237
Oh TH, Markelonis GJ, Von Visger JR, Baik B, Shipley MT (1995) Acidic pH rapidly increase immunoreactivity of glial fibrillary acidic protein in cultured astrocytes. Glia 13:319–322
Ridet JL, Alonso G, Chauvet N, Chapron J, Koenig J, Privat A (1996) Immunocytochemical characterization of a new marker of fibrous and reactive astrocytes. Cell Tissue Res. 283:39–49
Ahlemeyer B, Kolker S, Zhu Y, Hoffmann GF, Krieglstein J, (2002) Increase in glutamate-induced neurotoxicity by activated astrocytes involves stimulation of protein kinase C J. Neurochem 82:504–515
Chao CC, Hu S, Sheng WS, Bu D, Bukrinsky MI, Peterson PK (1996) Cytokine-stimulated astrocytes damage human neurons via a nitric oxide mechanism. Glia 16:276–284
Sriram K, Benkovic SA, Hebert MA, Miller DB, O`Callaghan JP (2004) Induction of gp130-related cytokines and activation of Jak2/STAT3 pathway in astrocytes precedes up-regulation of glial fibrillary acidic protein in the 1-methyl-4-phentl-1,2,3,6-tetrahydropyridine model of neurodegeneration. J Biol Chem 279:19936–19947
Takamiya A, Takeda M, Yoshida A, Kiyama H (2001) Expression of serine protease inhibitor 3 in ocular tissues in endotoxin-induced uveitis in rat. Invest Opthalmol Vis Sci 42:2427–2433
Davaille J, Li L, Mallat A, Lotersztajin S (2002) Sphingosine-1-phosphate triggers both apoptotic and survival for human hepatic myofibroblasts. J Biol Chem 277:37323–37330
Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T (2005) Basal and induced sphingosine kinase 1 activity in A 549 carcinoma cells: function in cell survival and IL-1β and TNF-α-induced production of inflammatory mediated. Cell Signal 17:1203–1217
Mastrandrea LD, Sessanna SM, Laychock SG (2005) Sphingosine kinase activity and sphingosine-1-phosphate production in rat pancreatic islets and INS-1 cells: response to cytokines. Diabetes 54:1429–1436
Chen XL, Grey JY, Thomas S, Qiu FH, Medford RM, Wasserman MA, Kunsch C (2004) Sphingosine kinase-1 mediates TNF-α-induced MCP-1 gene expression in endothelial cells: upregulation by oscillatory flow. Am J Physiol Heart Circ Physiol 287:H1452–H1258
Towbin H, Staehelim T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Bradford MM (1976) A rapid sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:249–254
Min JK, Yoo HS, Lee EY, Lee WJ, Lee YM (2002) Simultaneous quantitative analysis of sphingoid base 1-phosphates in biological samples by o-phthalaldehyde precolumn derivatization after dephosphorylation with alkaline phosphatase. Anal Biochem 303:167–175
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd ed. Academic, press, Orlando, Florida
Kimura T, Miyaoka T, Saunders PA, Baker ML, Hume AS, Yamamoto I, Ho IK (1993) Induction of tolerance to and physical dependence on pentobarbital continuous intracerebroventricular administration. J Pharmacol Exp Ther 266:1300–1305
Carter DA, Dick AD (2003) Lipopolysaccharide/interferon-γ and not transforming growth factor β inhibit retinal microglial migration from retinal explant. Br J Ophthalmol 87:481–487
Maekawa S, Aibiki M, Si QS, Nakamura Y, Shirakawa Y, Kataoka K (2002) Differential effects of lowering culture temperature on mediator release from lipopolysaccharide-stimulated neonatal rat microglia. Crit Care Med 30:2700–2704
Hughes EH, Schlichtenbrede FC, Murphy CC, Broderick C, van Rooijen N, Ali RR, Dick AD (2004) Minocyclin delays photoreceptor death in the rds mouse through a microglia-independent mechanism. Exp Eye Res 78:1077–1084
Nakamura Y, Si QS, Kataoka K (1999) Lipopolysaccharide-induced microglial activation in culture: temporal profiles of morphological change and release of cytokines and nitric oxide. Neurosci Res 35:95–100
Lee YB, Shen D, Rhim H, Lee E, Markelonis GJ, Oh TH (2000) Rapid increase in immunoreactivity to GFAP in astrocytes in vitro induced by acidic pH is mediated by calcium influx and calpain I . Brain Res 864:220–229
Martinez-Contreras A, Huerta M, Lopez-Perez S, Grarcia-Estrada J, Luquin S, Zarate CB (2002) Astrocytic and microglia cells reactivity induced by neonatal administration of glutamate in cerebral cortex of the adult rats. J Neurosci Res 67:200–210
Peterson WM, Wang Q, Tzekova R, Wiegand SJ (2000) Ciliary neurotrophic factor and stress stimuli activate the JAK-STAT pathway in retinal neurons and glia. J Neurosci 20:4081–4090
Wang AL, Yu ACH, Lau LT, Lee C, Wu LM, Zhu XA, Tso MOM (2005) Minocycline inhibits LPS-induced retinal microglia activation. Neurochem Int 47:152–158
Acknowledgment
This work was supported by a KOSEF brain Neurobiology Grant (2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jang, S., Lee, JH., Choi, KR. et al. Cytochemical Alterations in the Rat Retina by LPS Administration. Neurochem Res 32, 1–10 (2007). https://doi.org/10.1007/s11064-006-9215-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9215-7