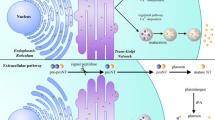
Abstract
Nitric oxide (NO) is a short lived diatomic free radical species synthesized by nitric oxide synthases (NOS). The physiological roles of NO depend on its local concentrations as well as availability and the nature of downstream target molecules. At low nanomolar concentrations, activation of soluble guanylyl cyclase (sGC) is the major event initiated by NO. The resulting elevation in the intracellular cyclic GMP (cGMP) levels serves as signals for regulating diverse cellular and physiological processes. The participation of NO and cGMP in diverse physiological processes is made possible through cell type specific spatio-temporal regulation of NO and cGMP synthesis and signal diversity downstream of cGMP achieved through specific target selection. Thus cyclic GMP directly regulates the activities of its downstream effectors such as Protein Kinase G (PKG), Cyclic Nucleotide Gated channels (CNG) and Cyclic nucleotide phosphodiesterases, which in turn regulate the activities of a number of proteins that are involved in regulating diverse cellular and physiological processes. Localization and activity of the NO-cGMP signaling pathway components are regulated by G-protein coupled receptors, receptor and non receptor tyrosine kinases, phosphatases and other signaling molecules. NO also serves as a powerful paracrine factor. At micromolar concentrations, NO reacts with superoxide anion to form reactive peroxinitrite, thereby leading to the oxidation of important cellular proteins. Extensive research efforts over the past two decades have shown that NO is an important modulator of axon outgrowth and guidance, synaptic plasticity, neural precursor proliferation as well as neuronal survival. Excessive NO production as that evoked by inflammatory signals has been identified as one of the major causative reasons for the pathogenesis of a number of neurodegenerative diseases such as ALS, Alzheimers and Parkinson diseases. Regenerative therapies involving transplantation of embryonic stem cells (ES cells) and ES cell derived lineage committed neural precursor cells have recently shown promising results in animal models of Parkinson disease (PD). Recent studies from our laboratory have shown that a functional NO-cGMP signaling system is operative early during the differentiation of embryonic stem cells. The cell type specific, spatio-temporally regulated NO-cGMP signaling pathways are well suited for inductive signals to use them for important cell fate decision making and lineage commitment processes. We believe that manipulating the NO-cGMP signaling system will be an important tool for large scale generation of lineage committed precursor cells to be used for regenerative therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitric oxide (NO) is a short lived diatomic free radical species that is synthesized by nitric oxide synthases (NOS). NOS catalyze the oxidation of l-arginine resulting in the formation of NO and l-citruline. Active NOS enzymes are dimeric and their activity also requires other factors such as NADPH, tetrahydrobiopterin (BH4), FAD, FMN, heme and calmodulin (CaM). Three major isoforms of nitric oxide synthase are known. These are nNOS or NOS1, iNOS or NOS II and eNOS or NOSIII. nNOS and eNOS are constitutively expressed enzymes and are regulated in a Ca2+ dependent manner. Expression of iNOS is induced in response to inflammatory stimuli and the enzyme is not regulated by Ca2+ as it has calmodulin associated. From the moment it is synthesized, NO takes part in a number of physiological processes such as smooth muscle relaxation, blood pressure and volume regulation, platelet aggregation, neurotransmission, immunomodulation, axon outgrowth and guidance mechanisms as well as cellular growth, survival, apoptosis, proliferation and differentiation. NO is produced as needed and is not stored as other messengers. However, NO complexes may exist as stored precursors to release NO.
Like all other short lived free radicals, NO is highly reactive. In vivo concentrations of NO range from low nanomolar to low micromolar. The downstream targets and cellular actions of NO depend on its local concentrations and the availability of target molecules. However, it can be safely stated that at low nanomolar concentrations, activation of soluble guanylyl cyclase (sGC), the major NO receptor, results in the elevation of intracellular cyclic GMP (cGMP) [3, 55]. This process is the major event triggered by low concentrations of NO. Other low concentration targets are transcription factors, Cytochrome C oxidase, and Catalase as well as thiol groups in various proteins which are nitrosated by NO on the Cystein residues. NO synthases may also generate superoxide anion (O .-2 ) or reactive nitrogen species other than NO under a variety of conditions particularly if the cofactor BH4 is limiting [1]. At sufficiently higher concentrations, NO rapidly reacts with superoxide anion to form the very reactive peroxinitrite causing nitration of proteins involved in diverse cellular physiological processes [8, 17]. This process becomes particularly significant when superoxide dismutase (SOD) is unable to scavenge O .-2 . Indeed the interaction of NO with superoxide anion is almost diffusion limited and more rapid than the dismutation of O .-2 by SOD. Downstream actions of NO can therefore be classified into cGMP-dependent and cGMP-independent. Here we review some of the advances made in NO and cGMP signaling including subjects related to central nervous system functions, diseases and the current trends in regenerative medicine utilizing stem cells. We have attempted to include key findings wherever possible. Our sincere apologies for any omissions made, for it would be a mammoth effort to discuss the vast amount of literature available on NO and cGMP signaling, which presently exceeds about 77,000 publications.
NO and cyclic GMP
Cyclic GMP-dependent functions of NO starts with its activation of sGC [3, 55, 56]. This functional NO receptor, sGC, is a heme containing heterodimeric enzyme, consisting of α and β subunits. In the absence of NO, soluble GC exhibits very low basal activity. Conformational changes following the binding of NO to heme result in marked activation of the enzyme. The resulting elevation in the intracellular cGMP concentrations triggers the activation of a number of signal transduction pathways that are responsible for regulating diverse physiological processes. The most extensively studied processes regulated by NO and cGMP are smooth muscle relaxation, neurotransmission, blood pressure regulation, inhibition of platelet aggregation and immunomodulation. At the cellular level NO and cGMP regulate important processes such as growth, survival, differentiation, proliferation, migration, axon guidance and other processes through a variety of downstream signaling cascades.
How does a simple elevation of cellular cyclic GMP levels resulting from the activation of sGC by NO regulate so many physiological processes? The answer perhaps lies in the cell type specific spatiotemporal regulation of sGC activity and the choice of downstream signaling cascades regulated by NO or cyclic GMP. This is achieved by regulation of gene expression and activities at the level of NO synthases, sGC, cGMP hydrolyzing phosphodiesterases and downstream effectors such as PKG. Activities of a number of proteins involved in the NO and sGC pathway are modified by components of some of the most widely used signal transduction pathways including G-protein coupled receptors, serine-threonine kinases, receptor and non receptor tyrosine kinases as well as phosphatases. For example, during nervous system development, secreted ligands for cell surface receptors help axons of developing neurons to find their correct target at the correct time. Thus class 3 semaphorins takes part in patterning sensory projections by selectively repelling axons away from sites where its concentration is high [79]. In xenopus spinal neurons, repulsion of the growth cone away from a site of semaphorin 3A gradient can be converted into attraction by elevating the intracellular cGMP levels [104], indicative of an important role of cyclic GMP signaling in nervous system development. A recent study also reveals close cooperation between semaphorin 3F mediated signals and the cyclic GMP signaling pathway. This study suggests that in dentate granule cells, collapse of axonal and dendritic growth cones in response to semaphorin 3F involves elevation of intracellular cyclic GMP levels [116]. Sonic hedgehog (Shh) along with other factors is known to play important roles in patterning the dorso-ventral axis of developing nervous system. Exposure of intermediate zone explants from developing chick embryos to cyclic GMP enhances the effect of Shh by increased differentiation into ventral neural cell types [97]. It appears that NO-cGMP signaling and its cross talk with a variety of signal transduction pathways are important in numerous vital cellular and developmental processes. In the following section, we will describe how signal specificity and diversity is achieved in NO and cGMP signaling and hence how NO and cGMP function as important mediators for regulating cellular and physiological processes.
Regulation of NOS activity and localization
Because NO-cGMP signaling begins with the generation of NO, we will describe first, how NO synthesis by NOS is regulated both spatially and temporally. This is assuming that NO synthases represent the first and primary site of endogenous NO synthesis in mammals. Regulation of NO synthases has been one of the most extensively studied subjects [1]. The expression of eNOS was first observed in endothelial cells but it is also present in other cell types [77, 90]. Neuronal nitric oxide synthase (nNOS), as the name suggests is primarily found in the brain, although its expression is also observed in skeletal muscle and other tissues. Interestingly, studies conducted in our laboratory have shown that eNOS and nNOS are also expressed relatively well in ES cells [63]. Both eNOS and nNOS are Ca2+ dependent enzymes and eNOS respond to CaM binding with an increased rate of electron transfer from NADPH to flavins bound at the reductase domain. Therefore eNOS and nNOS are designed to respond to local Ca2+ oscillations. In contrast, under normal physiological conditions, expression of iNOS is undetectable in most tissues. It is induced in many cell types including neuronal and glial cells in response to inflammatory signals. Activity of iNOS is not very sensitive to changes in Ca2+ levels. Although recent findings have shown that expression of iNOS in ES cells result in increased cardiomyogenesis, the exact mechanisms involved in this process are not known [54]. In view of our findings that very low or undetectable amounts of sGC are expressed in embryonic stem cells, cGMP-independent mechanisms may be operative.
Studies have demonstrated that eNOS is phosphorylated and activated by Akt downstream of PI3K activation and this process is important for angiogenesis, platelet adhesion and aggregation [27, 39, 109]. Activation of G-protein coupled receptors, receptor tyrosine kinases and mechanical stimuli have been shown to result in the activation of Akt. It is very likely that activation of the NO signaling pathway may be a common step by which extracellular stimuli transmit cell survival signals. There are numerous examples for receptor stimulated activation of NO synthases. In endothelial cells, kinins stimulate the synthesis of NO through B1 and B2 receptors [7, 68]. Steroid hormones such as estrogen stimulate NO synthesis from eNOS in a nongenomic fashion through downstream activation of the heterotrimeric G-protein Gαi [20, 125]. Action of estrogen also results in transcriptional upregulation of eNOS gene [2, 19, 88]. In neurons, NO production is sensitive to a number of stimuli. Neurotrophic factors such as NGF may regulate NO synthesis by nNOS through mechanisms operating via TrkA receptors [47, 65]. Other factors including glutamate, thrombin, histamine and acetylcholine, all have been shown to stimulate the production of NO under a variety of physiological or pathophysiological conditions. However, whether or not such mechanisms are operative in ES cells and ES cell derived precursor cells is not known.
NOS activities have also been shown to be regulated through protein–protein interactions and lipid modifications. These protein–protein interactions and posttranslational modifications enable NO synthases to target to specific intracellular compartments, thereby leading to spatial resolution of signals transmitted by NO. Thus, specific targeting of eNOS to the Golgi compartment in endothelial cells is achieved through a dual acylation [70]. Studies conducted in HEK293 cells suggested that such a subcellular targeting is necessary for eNOS to be stimulated in response to Ca2+ ionophores such as ionomycin. Subcellular distribution of eNOS is regulated in response to bradykinin induced changes in intracellular Ca2+ concentrations [92]. In resting endothelial cells, eNOS is primarily localized to the plasma membrane. Short exposure to bradykinin results in the translocation of eNOS from the membrane to the cytosolic fraction, which is abrogated by pretreatment with Ca2+ chelators. In central nervous system neurons, nNOS is recruited to the site of the NMDA receptor activation through its protein–protein interactions with one of the several PDZ domains of PSD-95 [98].
Localized NO production has important implications. For instance, during development sequential actions of gradients of diverse inductive signals play critical roles in cell fate determination and patterning processes [4]. Thus, specific precursor cells form, survive, proliferate, mature and migrate at the correct time frame to insure normal development. The tissue distribution of NO synthases and their regulation by diverse signals are well suited for such developmental cues to utilize them for multiple purposes. Recent studies have shown that mice lacking all three known NO synthases exhibited increased mortality associated with nephrogenic diabetes insipidus [81]. The survival rate of triply NOS−/− mice was about 23% during a 10 month follow up period, when compared to the wild type animals, all of which survived during this time. Additional data from different developmental stages of these animals are therefore eagerly awaited with interest.
Regulation of sGC expression and activity
NO-cGMP signaling is also regulated at the level of sGC in genomic and non genomic fashions. Early studies on the tissue distribution of sGC have shown that its expression levels are different in different tissues [59]. Soluble guanylyl cyclase is a ubiquitously expressed enzyme consisting of α and β subunits. At least two isoforms of α and β subunits are known. Recent reports on the tissue distribution of sGC suggest that the expression level of different α and β subunits varies with the tissue examined. Distribution of α2 subunit has its major occurrence in brain. Transcripts of α2 and β1 subunit were found widely distributed in 8 day old rat brain [42]. Both α1 and β1 mRNA is abundant in cortex, caudate-putamen, nucleus-accumbens, olfactory tubercle and around the purkinje cell layer of the cerebellum. Importantly, message for β1 and traces of α2 are detected in substantia nigra pars compacta. Transcripts of α1 subunit were low or undetectable in this region. These observations made in rat brain seem to be consistent with a recent report on the developmental appearance of cyclic GMP production and NO responsiveness in embryonic mice cortex and striatum [23]. The physiological significance of the differences in the expression levels and distribution of these subunits is not clearly understood. Additionally, information is lacking regarding the expression and tissue distribution of various α and β subunits in human. It certainly appears that normal development and functioning of the nervous system demands spatial and temporal regulation of sGC expression and activity.
The expression of sGC subunits are regulated at transcriptional as well as posttranscriptional levels. Two independent studies reported recently suggest that expression of the α1 subunit is regulated similar to that of β1 as these genes are near one another on the same chromosome [106, 117]. The identification and characterization of human α1 and β1 promoters suggest that these subunits may be targets for regulation by a number of transcription factors that play critical roles in a variety of cellular processes [107]. Of particular interest is the binding site for Sp1, which has recently been shown to be responsible for transcriptional upregulation of SUR1 in cerebral ischemia [108]. During the development of mouse, the highest level of Sp1 is expressed in developing hematopoietic cells, fetal cells and spermatids [100]. It is likely that regulation of sGC expression by Sp1 may be important for proper hematopoiesis.
Expression of sGC is also regulated at the posttranscriptional level by rapid degradation of mRNA by endonucleases. Earlier studies conducted in smooth muscle cells demonstrated a cyclic AMP-dependent regulation of mRNA for sGC α and β subunits [91]. Stability of α1 and β1 mRNAs is enhanced under basal conditions by the binding of HuR to the 3′ untranslated region [58]. This has been substantiated by RNA interference mediated methods which show that suppression of HuR expression leads to decreased levels of α1 mRNA without influencing the gene transcription. Others suggested that expression of HuR is regulated by cyclic AMP as well as cyclic GMP. Therefore both cyclic AMP and cyclic GMP levels may influence the sGC expression by regulating the stability of α1 and β1 mRNA [60].
Activity of sGC has also been found to be modified by phosphorylation, dephosphorylation and protein–protein interactions. Although some investigators suggested that purified sGC is phosphorylated by PKA as well as PKC, this needs to be further confirmed. Interestingly, a recent study has shown that the basal as well as NO stimulated cGMP levels were substantially lower in cells overexpressing the protein tyrosine phosphatase SHP-1 when compared to the controls [21]. However, the mechanism by which SHP-1 regulates sGC activity remains to be studied. Tyrosine kinase phosphorylation of sGC by a Src like kinase in response to H2O2 or pervanadate was reported recently [80]. The physiological implication of such a process remains unknown.
Recent findings suggest that localized activation of NOS and sGC is also achieved through protein–protein interactions. In rat brain, the α2 subunit of sGC has been shown to interact with PSD95, PSD93, SAP97 as well as SAP102. The interaction of PSD-95 with sGC involves the third PDZ domain of PSD-95 resulting in the recruitment of sGC to the synaptic membranes, where nNOS is located [98]. At the synaptic membrane the three proteins forms a signaling complex with the cytoplasmic domain of the NMDA receptor. Although the recruitment of sGC to the site of nitric oxide synthesis by PSD-95 is well accepted, the contribution of such a phenomenon to the overall activation of sGC and elevation in cellular cGMP levels observed after NMDA receptor activation is not completely understood. Several PDZ domain containing proteins have intrinsic serine-threonine kinase activities while many show protein tyrosine phosphatase activity, guanine nucleotide exchange factor activity or GTPase activity [87]. A number of this PDZ domain containing proteins function downstream of G-protein coupled receptors and takes part in regulating diverse cellular processes. Whether or not the activity and/or localization of sGC are also modified by other PDZ containing proteins is not known. A recent study has reported that sGC may also interact with a protein named AGAP1, a prototype of ArfGAP protein [82]. However, the importance of this interaction is not known. An exciting possibility is that diversity and specificity achieved in NO and cGMP signal transduction pathways activated by cell surface receptors depend on the dynamic regulation of NOS and/or guanylyl cyclase activity by a variety of participants. Our yeast two hybrid screens have also yielded a variety of proteins that interact with the α1 and β1 subunits of sGC ([45] and our unpublished results). Thus CCTη, a protein that is expressed in rat hippocampus is found to inhibit NO stimulated cGMP synthesis.
Temporal regulation of cellular cyclic GMP synthesis by sGC and the resolution of signals downstream of cGMP are also achieved through tightly regulated activation and desensitization cycles [6]. Upon encounter with NO, sGC is instantaneously activated in milliseconds with an EC50 for NO of about 45 nM. Dissociation of NO from sGC may mark the inactivation of the enzyme. Independent studies on NO dissociation from purified sGC resulted in differing estimates ranging from 2 s to min [15, 57]. However determination of the time scale of inactivation of the enzyme as measured by cGMP synthesis provides a more physiologically realistic value of about 10–30 s [51, 76]. In intact cells, sGC inactivation follows much faster kinetics. Experiments conducted in cerebellar cells have shown a complex inactivation profile, which is a slow exponential decay in cGMP synthesis following an immediate 40% loss of enzyme activity.
It can be postulated that in addition to the signal diversity and specificity observed at the level of NO synthesis, regulation of sGC activity by multiple factors gives rise to a situation where a high degree of spatial and temporal resolution in signals downstream of cGMP is achieved. However, are cells equipped sufficiently well enough to respond to such a high resolution achieved in cGMP synthesis? The answer to a large extent is perhaps yes. Signals downstream of cGMP are transmitted by an array of downstream effectors.
Downstream effectors of the NO-cGMP pathway
The identification of cGMP as a major regulator of important physiological processes [83] triggered an extensive search for identifying its downstream effectors. To date numerous direct downstream targets of cGMP are known. In fact many of the downstream effectors of cGMP are now known to regulate diverse cellular and physiological processes downstream of NO. Cyclic GMP regulates these processes through three major direct downstream effectors. These are protein kinase G (PKG), cyclic nucleotide phosphodiesterases (PDEs) and cyclic nucleotide gated ion channels (CNGs). Each of these downstream effectors then transmit the signals to an array of intracellular signaling molecules, thereby regulating neurotransmission, cell migration, proliferation, differentiation, survival, axon outgrowth and guidance, visual signal transduction and many other processes. All of these effectors of cGMP have been found to be expressed in brain as well as other tissues and their role in regulating nervous system functions is well studied.
Protein kinase G
PKG was one of the first proteins to be identified as a target of cGMP. Pioneering studies conducted in the seventies and early eighties established that PKG could be activated by cGMP both in purified preparations as well as in vivo [34, 35]. These data established that activation of PKG by cGMP is a major mechanism by which endothelium derived relaxing factor (NO) relaxed smooth muscle tissue. Subsequent work has resulted in the delineation of numerous signal transduction pathways regulated and controlled by the cGMP-PKG axis.
Regulation of gene expression by PKG downstream of cGMP is one of the most extensively studied subjects. PKG has been shown to regulate the activity of transcription factors including ATF-1, CREB and TFII-I and SRF downstream of its activation [16, 43, 102]. Some of these pathways have also been found to regulate the gene expression of a second set of transcription factors such as AP-1, Egr-1, GAX, NF-kB, and NF/AT [53, 89]. Among these, AP-1, CREB and Egr-1 have been suggested to play important roles in neuronal cells. These transcription factors regulate important processes such as synaptic plasticity.
Following the discovery of the activation of sGC by the NO donor sodium nitropruside, pioneering studies established that cGMP synthesized in nervous system serves as a powerful neurotransmitter [36, 41, 121]. Subsequent studies revealed that PKG is widely expressed in many parts of the central and peripheral nervous system and phosphorylates multiple target proteins including CREB as well as a protein called G-substrate [71, 84, 120]. At present, two isoforms of PKG are known to exist, PKGI (PKG-Iα and PKG-Iβ) and PKG-II [29, 40, 115]. Both PKG-I and PKG-II contains catalytic and regulatory domains. Among these, PKG-I is primarily cytosolic, where as PKG-II is myrystoylated and is generally found in membrane associated form. On the basis of studies conducted in C6 glioma and PC12 cells it has been suggested that in neuronal and glial cells PKG-II is responsible for regulation of gene expression in response to NO and cGMP [44]. One of the interesting roles of signal transduction by NO is its involvement in synaptic plasticity. Extensive studies conducted in hippocampal slices or cultured neurons have shown that NO and cGMP signaling is important for both early and late phase long term potentiation (LTP) [10, 13, 73, 110, 119].
The role of PKG in regulating the localization and activities of proteins involved in actin cytoskeleton reorganization has been extensively studied during the past 10 years. Of particular interest are the Ena/VASP proteins. The Ena/VASP family proteins have emerged as important regulators of actin assembly and cell motility in a number of organisms. In cells they are primarily localized to areas of dynamic actin reorganization such as filopodial tips, lamellipodia and sites of focal adhesions where they form multiprotein complexes with other proteins [62, 103]. Ena/VASP family of proteins can also bind directly to F- and G-actin as well as to Profilin. Genetic studies have revealed that the Ena/VASP proteins are important for a number of physiological processes including epithelial morphogenesis, T cell activation, phagocytosis as well as neuronal migration. Early experiments on the regulation of VASP by cGMP were conducted in platelets. These experiments have shown that VASP is phosphorylated at three sites by PKG and this phosphorylation may be responsible for its regulation in response to cGMP [118]. Ena/VASP proteins are also localized in living cells to the “puncta” between stress fibers where they presumably form specific protein–protein complexes. At a molecular level the presynaptic and postsynaptic mechanism of LTP involves the expression of AMPA receptors in clusters or otherwise called “puncta” which are GluR1 positive [74]. Based on the dendritic spine changes associated with hippocampal long term synaptic plasticity, it was suggested that such microstructural changes occur both presynaptically and postsynaptically and are important for LTP [28]. Recent findings show that indeed NO, cGMP and PKG system is involved in such presynaptic formation of synaptophysin and GluR1 positive puncta and this process involves the phosphorylation of VASP by PKG [119].
A recent study has shown that in B5 and B19 neurons from the buccal ganglion of H. trivolvis, PKG regulates the filopodial length in growth cones through a mechanism that involves cyclic ADP ribose [122]. However, the exact mechanism by which PKG activates cyclic ADP-ribosyl cyclase in not known. The existence of such a mechanism in mammalian neurons and its universality remain to be tested. In an interesting new study, PKG has been shown to phosphorylate p21 activated kinase (Pak) in endothelial cells [37]. The resulting S21 phosphorylation of Pak releases its adapter protein Nck, while stimulating the formation of a new Pak/VASP complex. Several Pak isoforms are expressed in brain. It will be interesting to see if such a phenomenon exists in neuronal cells. Further, the relevance of PKG phosphorylation of Pak to its known interactions with other proteins remains to be studied. This is particularly interesting considering the fact that Pak plays an important role in axonal pathfinding and is required for depolarization mediated neuronal survival. It is also not known if this phosphorylation of Pak is restricted only to one isoform or not. Considering the fact that PKG-II is the predominant form that is expressed in brain and that Pak is expressed in nervous system, it is likely that they all act in concert to further diversify signals downstream of cGMP.
In xenopus spinal neurons cAMP/cGMP dependent modulation of Ca2+ channels sets the polarity of growth cone turning, presumably through PKG and PKA dependent mechanisms [86]. In cultured cerebellar granule cells, nerve growth cone turning is also controlled by Ca2+ and CaM dependent upregulation of Cdc42/Rac and downregulation of RhoA activities [52]. It is interesting to note that both eNOS and nNOS are calcium-calmodulin dependent enzymes and changes in the intracellular calcium concentrations may therefore influence the NO synthesis and hence sGC activity. The precise roles of cGMP in these processes are yet to be determined. Interestingly, the cerebral cortex of homozygous PKG-I mutant mice exhibited heterotopic collection of neurons in the upper cortical layers and abnormal invaginations in layer I [25]. It is likely that the presence of PKG-II in the mutant mice may at least in part compensate for the loss of PKG-I. Therefore, a precise understanding of the roles of PKG in nervous system development will require the generation of a mice lacking both PKG-I and PKG-II.
Cyclic nucleotide gated channels
Early studies on the biological actions of cGMP were focused on PKG and its downstream effectors. However in 1985, it was found that the light dependent calcium channels in rod photoreceptors were directly regulated by cGMP [33]. These channels were named by a general term CNG, since they are directly regulated by cyclic nucleotides. Almost all of the CNGs bind both cAMP and cGMP. Their ligand specificities and functions are still being debated and are subjects of extensive investigations.
In dark, cGMP is synthesized in rod outer segments at a relatively steady rate. However, phptoreception causes conformational changes in rhodopsin, leading to the dissociation of the βγ subunits from transducin. This leads to the activation of PDE6 and hydrolysis of cGMP, resulting in a change in the activity of cGMP gated Ca2+ channels. This and the subsequent events serve as signals responsible for vision. CNG channels are also expressed in the inner segment and synaptic terminals of cones. Expression of NO synthase and NO activated sGC in the inner segment of cones suggests that modulation of CNG channels by NO and cGMP may play important roles in synaptic transmission in the axon terminals of cones [61, 101]. Although the expression of CNG channels have also been reported in several parts of the brain including hippocampus the exact mechanisms of their regulation or their physiological function is not known.
Cyclic GMP regulated phosphodiesterases
At least 11 families of phosphodiesterases are known to be expressed in mammals [12]. PDE 5, 6 and 9 are cGMP specific where as PDEs 3, 4, 7, 8 and 10 show more selectivity towards hydrolyzing cAMP. PDE 1, 2 and 11 are known to hydrolyze both cAMP and cGMP. PDE2 is expressed in adrenal gland, liver, lung and platelets. Binding of cGMP to the allosteric sites activates PDE2 resulting in enhanced hydrolysis of cellular cGMP and cAMP. PDE3 is a cGMP inhibited cAMP selective enzyme that is expressed in heart, lung, liver, platelets, adipose tissue and inflammatory cells. Cyclic GMP binding to PDE3 results in an inhibition of cAMP hydrolysis. Binding of cGMP to the allosteric sites in PDE5 directly or indirectly activates the enzyme resulting in enhanced activity. A recent study reported that treatment of hippocampal slices with a specific PDE5 inhibitor resulted in increased cGMP immunoreactivity, which was accompanied by an improvement in memory performance [99]. PDE10 is a cGMP sensitive cAMP selective phosphodiesterase. Although PDE10 is expressed in brain, its role in nervous system function is not yet clear.
In short, although NO is a simple diatomic free radical species, cellular machinery seems to be well equipped for context specific interpretation of spatio-temporal signals provided by NO. If this is true, controlled differentiation of ES cells and ES cell derived precursor cells may be achieved through the pharmacological manipulation NO and cGMP signaling.
NO and cGMP in neurodegenerative diseases
From the nature and regulation of NO synthesis, sGC activation, cGMP synthesis, degradation and downstream activation of signal transduction pathways by cGMP, it is clear that the system is devised to respond to minuscule changes in the cellular environment. Such changes are reflected in its role in an array of physiological processes. While a tightly regulated NO signaling system seems to be required for normal physiological functions, alterations in NO and cGMP signaling pathway components are evident in life threatening diseases such as cancer, numerous cardiovascular, immunological and neurodegenerative diseases. The nervous system directly or indirectly regulates numerous physiological processes in mammals. Since NO is an important regulator of nervous system functions, substantial changes in NO and cGMP synthesis may lead to nervous system degeneration. Regulation of NO and cGMP formation in the central nervous system has been the subject of extensive studies for many years [5]. Numerous studies have shown that NO may directly or indirectly play an important role in the pathogenesis of a number of neurodegenerative diseases including Alzheimers, ALS and Parkinson diseases [31, 48, 85, 96].
The pathogenesis of Parkinson disease involves slow death or degeneration of dopaminergic neurons from substantia nigra, which synthesize and supply dopamine to the striatum of basal ganglia. Diminished availability of dopamine in the striatum results in the manifestation of symptoms of PD. Evidence accumulated over the past two decades has established that the majority of the cases of PD can be classified due to either (1) genetic or (2) environmental factors. Among some of the genetic factors that seems to contribute to PD are mutations in PINK1, DJ1, UCHL1, SNCA, PARK2 and LRRK2 [31]. The two forms of PD can be recognized by the time frame of its onset. In general PD due to genetic factors show early onset of the symptoms and that caused by environmental factors are characterized by its late onset nature. Two most recent studies conducted in Swedish twins seem to suggest that heritability is less of a factor in pathogenesis of late-onset PD [123, 124].
The role of NO in the development of Parkinson disease has been extensively studied. Pioneering studies in the early eighties showed that the environmental toxicant MPTP lead to a selective and progressive death of dopaminergic neurons from substantia nigra. Since then, this agent has been widely used to induce PD in animal model systems [14, 46, 66]. Postmortem studies in MPTP models revealed that NO plays an important role in development of PD. MPTP causes glial reaction and clustering of microglia around neurons in the substantia nigra. This is accompanied by an up-regulation of iNOS gene expression in glial cells [69]. Interestingly, significant resistance to MPTP induced cytotoxicity was exhibited by mice lacking either iNOS or nNOS [24, 93]. These studies suggest that MPTP induced cytotoxicity in DA neurons of substantia nigra are caused by NO or other species generated by nNOS rather than iNOS. In glial cells, MPTP is converted into MPP+, which then enters DA neurons through dopamine transporters. The majority of the studies conducted toward identifying the fundamental mechanism of MPP+ induced cytotoxicity in substantia nigra focused on cGMP independent mechanisms of NO’s action. These cGMP-independent actions of NO include oxidation of thiols as well as nitrosation and nitration of proteins. A recent study shows that MPP+ inhibits complex I of the mitochondrial electron transport chain, leads to the formation of superoxide anion and peroxinitrite thereby causing DNA damage, PARP-I activation and neuronal cell death [75]. Other studies have found that a number of important proteins including α-synuclein and Parkin are either tyrosine nitrated or S-nitrosylated in PD and these modified proteins may play a role in the neuronal cell death.
Recent findings shed more light into the pathogenic mechanism of PD in MPTP models. First, pretreatment with COX inhibitors inhibited the augmented expression of iNOS and nNOS in rat hippocampal slices treated with LPS [26]. Second, like iNOS and nNOS deficient mice, neurons in substantia nigra of COX-2 deficient mice are less prone to MPTP induced cytotoxicity than wild type mice [32]. Thirdly, JNK2 and JNK3 deficient mice are less prone and JNK2/3 double knockout mice are resistant to MPTP induced cell death of nigral dopaminergic neurons [49]. Moreover, mice lacking both JNK2 and JNK3 display virtually no defects in motor neuron functions measured on a rotarod. In this study, elevated expression of COX-2 was only found in MPTP treated WT mice compared to that in JNK deficient mice. Based on these findings, the authors suggest that induction of Cox-2 is required for neurodegeneration in MPTP induced PD. However, whether or not administration of COX-2 inhibitors to in JNK2-/- and/ or JNK3-/- mice will improve MPTP induced impairment in motor neuron functions in these animals is not known. Additionally, an independent report shows that although COX-2 expression is elevated in the striatum in response to MPTP, Cox-2 inhibitor administration resulted in no significant neuroprotective effect [94]. Thus, the role of elevated COX-2 levels found in MPTP models of PD need to be further analyzed.
Although iNOS and nNOS are critically implicated in a number of studies on MPTP models of Parkinson disease, reports are rare on the regulation of sGC gene expression and activity. Recent studies suggested that expression of sGC at the level of mRNA may be present in substantia nigra pars compacta of rat brain [42]. Autoradiographic studies with [3H]cGMP has been conducted to locate the presence of downstream targets of cGMP in rat brain [11]. Although independent verification is lacking, this study shows that substantia nigra represented the region of highest [3H]cGMP binding. In an isolated study, sGC gene expression and activity was measured in the striatum of MPTP-induced mouse model of PD [18]. Interestingly, protein levels of the sGC heterodimer showed a steady increase with time. This was correlated with a corresponding increase in cGMP content, the profile of which was not altered by the presence or absence of the PDE inhibitor IBMX. However, whether or not the changes in cGMP content plays a role in PD is not known. The expression, activity and regulation of sGC subunits in DA neurons of substantia nigra also remain to be studied. Considering the fact that substantial differences exist between the promoters for α and β subunits from mouse and human, regulation of their expression in brain under physiological and pathophysiological conditions warrant new studies.
Embryonic stem cell based therapies for neurodegenerative diseases
The unraveling of molecular mechanisms leading to neurodegeneration as in the case of PD, ALD and Alzheimers disease, have lead to developing therapeutic strategies. Administration of antioxidants has been attempted as a treatment for both Alzheimers disease as well as PD. However, these trials have not resulted in any major long term improvement in patients. In the case of PD, conventional therapies including delivery of neurotrophic factors such as GDNF or other alternative therapies showed only limited short term success [30, 105]. A second strategy involving transplantation of tissue from ventral mesencephalon also proved to be not greatly beneficial. In this case, severe adverse side effects including dyskinesias were also observed [64].
The identification of multipotent stem cells in adult tissue that are not lineage committed attracted the attention of both scientists as well as clinicians. One of the key features of these cells is their ability to differentiate into many, if not all cell types of the body. Thus, mesenchymal stem cells isolated from bone marrow or umbilical cord show the ability to differentiate into cardiac, neural and other cell types under specific conditions in vitro. Further, when injected into specific tissues, these cells showed the ability to engraft and to a large extent, differentiate into the corresponding cell type of tissue. Recent studies have shown that human umbilical cord mesenchymal stem cells in Wharton’s jelly are able to successfully differentiate into lineage committed neural precursor cells [38]. Further, these cells were able to engraft and partially correct the lesion when transplanted into amphetamine induced PD models. However, long term benefits of using adult stem cells or multipotent stem cell derived neural precursor cells for the treatment of PD remains to be seen. Although adult stem cells provide a unique opportunity for age matched allogenic transplantation, their rare occurrence and limited proliferation potential are serious impediments to overcome in the future.
Pluripotent embryonic stem cells have the unique potential for unlimited proliferation without undergoing a significant degree of differentiation. Most importantly, in vitro they have the ability to give rise to almost all types of cells in the body. This means that specific precursor cells can be generated and purified from ES cells in large scale, which then can be used for transplantation. Establishment of methods for isolation, purification and culture of ES cells accelerated research toward identification of factors regulating stem cell differentiation. These studies shed much light into the molecular mechanisms that regulate neural cell fate specification. In an important study, it was shown ES cells are capable of directly differentiating into neural precursor cells through a default mechanism [114]. Subsequently, transplantation of ES cells in Parkinson Disease models showed that the majority of the survived cells in the transplant are able to differentiate into functional dopaminergic neurons [9]. How dopaminergic neurons, that provided the “niche” for the transplanted ES cells influence its differentiation and lineage commitment is not clearly understood. Although transplantation of ES cells seems promising, such transplanted cells frequently gave rise to teratomas. Transplantation of a pure population of lineage restricted precursor cells circumvents the problem of teratoma formation. Subsequently several studies have attempted the derivation of lineage committed neural precursor cells from mouse and human ES cells [50, 67, 95]. Thus early neural precursors generated in vitro, when treated with Shh and FGF8 gave rise to a high percentage of cells with dopaminergic characteristics. These methods utilized earlier findings that during development, spatial and temporal identity of neural cells in the central nervous system is specified by inductive signals from the surrounding tissue.
Do NO signals exist early in the developing human nervous system? If it does, is it functional and what are its functional roles? Although some of these questions have not been answered yet, several studies have attempted to identify factors that regulate the proliferation of neural precursor cells and subsequent lineage specification. Recent findings suggest that NO regulates both proliferation and differentiation of neural precursor cells. In mammalian brain, NO may regulate neural progenitor proliferation and differentiation by acting in a positive feedback loop with BDNF [22]. Most recently, it was reported that BMP4 regulate cell numbers in the developing neural tube in a NO dependent manner [113]. Consistent with this finding, an independent study reported that inhibition of nitric oxide synthesis using NOS inhibitors results in increased proliferation of precursors isolated from postnatal mouse SVZ [78]. Although these studies show the involvement of NO in regulating neural precursor proliferation and differentiation, whether or not NO stimulated cGMP synthesis is important in this process is not clearly understood. Considering the complex nature of NO and cGMP signaling, additional studies are required to establish the precise roles they play in the formation, survival, proliferation and differentiation of neural precursor cells. Additionally, most of the reports on the role of NO in influencing the development of the nervous system come from studies conducted in rodents. Further, whether or not NO and cGMP play a role in differentiation of embryonic stem cells into neural precursor cells is not understood. It is also not known as to what roles NO and cGMP play in the proliferation and subsequent lineage commitment of early neural precursor cells derived from embryonic stem cells.
Recent studies have shown a promising future for ES cell derived neural precursor cells for the treatment of PD. Embryonic stem cell derived progenitor cells are also being widely considered for the treatment of other neurodegenerative diseases such as ALS. Early studies have shown that transplantation of lineage committed neural precursor cells prepared from ES cells were functional in an animal model of PD [72]. Most recently, when neural precursor cells derived from monkey ES were used for transplantation in a primate model of PD, reversal of symptoms and restoration of normal motor neuron functions were observed [112]. A separate study reported that NPCs derived from human embryonic stem cells, when transplanted into rat brain responded to endogenous cues by migrating into specific regions and differentiating into neurons, astrocytes and oligodendrocyte [111]. However, it is not known whether or not the transplanted precursor cells survive and function normally for long periods of time.
Summary
Regenerative therapies involving the usage of ES cells and ES cell derived lineage committed precursor cells are revolutionizing the concept of regenerative medicine. It is being increasingly accepted that generation and transplantation of lineage committed precursor cells are very important steps in the process. The complex nature of tightly controlled spatio-temporal regulation of NO and cGMP signaling is ideally suitable for stem cells and stem cell derived precursor cells to use as tools to interpret and respond to various developmental cues. Indeed, studies from our laboratory clearly indicate that NO-cGMP signals are well positioned from the beginning of ES cell differentiation to take part in cell fate specification [63]. We have initiated an effort toward identifying the role of NO and cGMP in the differentiation of embryonic stem cells into neural precursor cells and their subsequent lineage specification. We predict that understanding and manipulating NO and cGMP signaling in stem cells will provide a very important tool for large scale generation of lineage committed stage specific precursor cells that can be used in regenerative therapies for PD, ALS as well as many other diseases.
References
Alderton WK, Cooper CE, Knowles EG (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357:593–615
Armour KE, Ralston SH (1998) Estrogen upregulates endothelial constitutive nitric oxide synthase expression in human osteoblast-like cells. Endocrinology 139:799–802
Arnold WP, Mittal CK, Katsuki S, Murad F (1977) Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci 74:3203–3207
Ashe HL, Briscoe J (2006) Interpretation of morphogen gradients. Development 133:385–394
Baltrons MA, Pedraza C, Sardon T, Navarra M, Garcia A (2003) Regulation of NO-dependent cyclic GMP formation by inflammatory agents in neural cells. Toxicol Lett 139:191–198
Bellamy TC, Garthwaite J (2002) The receptor-like properties of nitric oxide-activated soluble guanylyl cyclase in intact cells. Mol Cell Biochem 230:165–176
Bhoola KD, Figueroa CD, Worthy K (1992) Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev 44:1–80
Bian K, Gao Z, Weisbrodt N, Muad F (2003) The nature of heme/iron-induced protein tyrosine nitration. Proc Natl Acad Sci USA 100:5712–5717
Bjorklund LM, Sanchez-Pernaute T, Chung S, Anderson T, Chen I, Chen Y, McNaught K, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, Isacson O (2002) Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci USA 99:2344–2349
Bohme GA, Bon C, Stutzmann JM, Doble A, Blanchard JC (1991) Possible involvement of nitric oxide in long-term potentiation. Eur J Pharmacol 199:379–381
Bonkale WL, Cowburn RF, Winblad B, Fastbom J (1997) Autoradiographic characterization of [3H] cGMP binding sites in rat brain. Brain Res 763:1–13
Boswell-Smith V, Spina D, Page CP (2006) Phosphodiesterase inhibitors. Brit J Pharmacol 147:S252–S257
Boulten CL, Southam E, Garthwaite J (1995) Nitric oxide-dependent long-term potentiation is blocked by a specific inhibitor of soluble guanylyl cyclase. Neuroscience 69:699–703
Bove J, Prou D, Perier C, Przedborski S (2005) Toxin-induced models of Parkinson’s disease. NeuroRx 2:484–494
Brandish PE, Buchler W, Marletta MA (1998) Regeneration of the ferrous heme of soluble guanylate cyclase from the nitric oxide complex: acceleration by thiols and oxyhemoglobin. Biochemistry 37:16898–16907
Casteel DE, Zhuang S, Gudi T, Tang J, Vuica M, Desiderio S, Pilz RB (2002) cGMP-dependent protein kinase 1 beta physically and functionally interacts with the transcriptional regulator TFII-I. J biol Chem 277:32003–32014
Castegna A, Thongboonkerd V, Klein JB, Lynn B, Markesbery WR, Butterfield DA (2003) Proteomic identification of nitrated proteins in Alzheimer’s disease brain. J Neurochem 85:1394–1401
Chalimoniuk M, Langfort J, Nadezda L, Marsala J (2004) Upregulation of guanylyl cyclase expression and activity in striatum of MPTP-induced parkinsonism in mice. Biochem Biophys Res Commun 324:118–126
Chambliss KL, Shaul PW (2002) Estrogen modulation of endothelial nitric oxide synthase. Endocrine Rev 23:665–686
Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH Mendelsohn ME, Shaul PW (1999) Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest 103:401–406
Chen ZJ, Che D, Vetter M, Liu S, Chang CH (2001) 17beta-estradiol inhibits soluble guanylate cyclase activity through a protein tyrosine phosphatase in PC12 cells. J Steroid Biochem Mol Biol 78:451
Cheng A, Wang S, Rao MS, Mattson MP (2003) Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol 258:319–333
Currie DA, de Vente J, Moody WJ (2006) Developmental appearance of cyclic guanosine monophosphate (cGMP) production and nitric oxide responsiveness in embryonic mouse cortex and striatum. Dev Dyn Online ahead of print, March 3
Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz JB (2000) Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J Neurochem 74:2213–2216
Demyenenko GP, Halberstadt AI, Pryzwansky KB, Werner C, Hoffmann F, Maness PF (2005) Abnormal neocortical development in mice lacking cGMP dependent protein kinase I. Develop Br Res 160:1–8
Di Girolamo G, Farina M, Riberio ML, Ogando D, Aisemberg J, de los Santos AR, Marti ML, Franchi AM (2003) Effects of cyclooxygenase inhibitor pretreatment on nitric oxide production, nNOS and iNOS expression in rat cerebellum. Brit J Pharmacol 139:1164–1170
Dimmeler S, Fleming I, Fisslthaler B, Herman C, Busse R, Zeiher AM, (1999) Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601–605
Engert F, Bonhoeffer T (1999) Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399:66–70
El-Husseini AE, Bladen C, Vincent SR (1995) Molecular characterization of a type II cyclic GMP-dependent protein kinase expressed in the rat brain. J Neurochem 64:2814–2817
Eslamboli A (2005) Assessment of GDNF in primate models of Parkinson’s disease: comparison with human studies. Rev Neurosci 16:303–310
Farrer MJ (2006) Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet 7:306–318
Feng Z, Dongdong L, Fung PCW, Pei Z, Ramsden DB, Ho S-L (2003) COX-2-deficient mice are less prone to MPTP-neurotoxicity than wild-type mice. Mol Neurosci 14:1927–1929
Fesenko EE, Kolesnikov SS, Lyubarski AL (1985) Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature 313:310–313
Fiscus RR, Murad F (1988) cGMP-dependent protein kinase activation in intact tissues. Methods Enzymol 159:150–159
Fiscus RR, Rapoport RM, Murad F (1983–84) Endothelium-dependent and nitrovasodilator-induced activation of cyclic GMP-dependent protein kinase in rat aorta. J Cyclic Nucleotide Protein Phosphor Res 9:415–425
Förstermann U, Gorsky LD, Pollock JS, Schmidt HH, Heller M, Murad F (1990) Regional distribution of EDRF/NO-synthesizing enzyme(s) in rat brain. Biochem Biophys Res Commun 168:727–732
Fryer BH, Wang C, Vedantam S, Zhou G-L, Jin S, Fletcher L, Simon MC, Field J (2006) PKG phosphorylates Pak1, inhibiting Pak/Nck binding and stimulating Pak/VASP association. J Biol Chem 281:11487–11495
Fu Y-S, Cheng YC, Lin MA, Cheng H, Chu P-M, Chou SC, Shih YH, Ko M-H, Sung M-S (2006) Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells 24:115–124
Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC (1999) Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399:597–601
Gamm DM, Francis SH, Angelotti TP, Corbin JD, Uhler MD (1995) The type II isoform of cGMP-dependent protein kinase is dimeric and possesses regulatory and catalytic properties distinct from the type I isoforms. J Biol Chem 270:27380–27388
Garthwaite J (1991) Glutamate, nitric oxide and cell–cell signaling in the nervous system. Trends Neurosci 14:60–67
Gibb BJ, Garthwaite J (2001) Subunits of the nitric oxide receptor, soluble guanylyl cyclase, expressed in rat brain. Eur J Neuroscience 13:539–544
Gudi T, Casteel DE, Vinson C, Boss GR, Pilz RB (2000) NO activation of fos promoter elements requires nuclear translocation of G-kinase I and CREB phosphorylation but is independent of MAP kinase activation. Oncogene 19:6324–6333
Gudi T, Hing GK-P, Vaandrager AB, Lohmann SM, Pilz RB (1999) Nitric oxide and cGMP regulate gene expression in neuronal and glial cells by activating type II cGMP-dependent protein kinase. FASEB J 13:2143–2152
Hanafy K, Martin E, Murad F (2004) CCTη, a novel soluble guanylyl cyclase-interacting protein. J Biol Chem 279:46946–46953
Heikkila RE, Manzino L, Cabbat FS, Duvoisin RC (1984) Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine by monoamine oxidase inhibitors. Nature 311:467–469
Holtzman DM, Kilbridge J, Bredt DS, Black M, Li Y, Clary DO, Reichardt LF, Mobley WC (1994) NOS induction by NGF in basal forebrain cholinergic neurones: evidence for regulation of brain NOS by a neurotrophin. Neurobiol Diseases 1:51–60
Hunot S, Hirsch EC (2003) Neuroinflammatory processes in Parkinson’s disease. Ann Neurol 53:49–60
Hunot S, Vila M, Teismann P, Davis R, Hirsch E, Przedborski S, Rakic P, Flavel RA (2004) JNK-mediated induction of cyclooxygenase-2 is required for neurodegeneration in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA 101:665–670
Ideda H, Osakada F, Watanabe K, Mizuseki K, Haraguchi T, Miyoshi H, Kamiya D, Honda Y, Sasai N, Yoshimura N, Takahashi M, Sasai Y (2005) Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci USA 102:11331–11336
Ishi K, Sheng H, Warner TD, Forstermann U, Murad F (1991) A simple and sensitive bioassay method for detection of EDRF with RFL-6 rat lung fibroblasts. Am J Physiol 26:H598–H603
Jin M, Guan CB, Jiang YA, Chen G, Zhao CT, Cui K, Song YQ, Wu CP, Poo MM, Yuan XB (2005) Ca2+-dependent regulation of rho GTPases triggers turning of nerve growth cones. J Neurosci 25:2338–2347
Jouvert P, Revel MO, Lazaris A, Aunis D, Langley K, Zwiller J (2004) Activation of the cGMP pathway in dopaminergic structures reduces cocaine-induced EGR-1 expression and locomotor activity. J Neurosci 24:10716
Kanno S, Kim PK, Sallam K, Li J, Biliiar TR, Shears LL (2004) Nitric oxide facilitates cardiomyogenesis in mouse embryonic stem cells. Proc Natl Acad Sci USA 101:12277–12281
Katsuki S, Arnold W, Mittal C, Murad F (1977) Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res 3:23–35
Katsuki S, Murad F (1977) Regulation of adenosine cyclic 3′,5′-monophosphate and guanosine cyclic 3′,5′-monophosphate levels and contractility in bovine tracheal smooth muscle. Mol Pharmacol 13:330–341
Kharitonov VG, Russwurm M, Magde D, Sharma VS, Koesling D (1997) Biochem Biophys Res Commun 239:284–286
Kloss S, Furneaux H, Mulsch A (2003) Post-transcriptional regulation of soluble guanylyl cyclase expression in rat aorta. J Biol Chem 278:2377
Kimura H, Murad F (1975) Two forms of guanylate cyclase in mammalian tissues and possible mechanisms for their regulation. Metabolism 24:439–445
Kloss S, Srivastava R, Mulsch A (2004) Down-regulation of soluble guanylyl cyclase expression by cyclic AMP is mediated by mRNA-stabilizing protein HuR. Mol Pharmacol 65:1440–1451
Koch K-W, Lambrecht H-G, Habercht M, Redburn D, Schmidt HH (1994) Functional coupling of a Ca2+/calmodulin-dependent nitric oxide synthase and a soluble guanylyl cyclase in vertebrate photoreceptor cells. EMBO J 13:3312–3320
Krause M, Dent EW, Bear JE, Lourero JJ, Gertler FB (2003) Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Ann Rev Cell Dev Biol 19:541–564
Krumenacker JS, Kots A, Murad F (2006) Differential expression of genes involved in cGMP-dependent nitric oxide signaling in murine embryonic stem (ES) cells and ES cell-derived cardiomyocytes. Nitric Oxide 14:1–11
Kuan W-L, Barker RA (2005) New therapeutic approaches to Parkinson’s disease including neural transplants. Nerorehabil Neural Repair 19:155–181
Lam HHD, Bhardwaj A, O’Connel MT, Hanley DF, Traystman RJ, Sofroniew MV (1998) Nerve growth factor rapidly suppresses basal, NMDA-evoked, and AMPA-evoked nitric oxide synthase activity in rat hippocampus in vivo. Proc Natl Acad Sci USA 95:10926–10931
Langston JW, Ballard PA (1983) Parkinson’s disease in a chemist working with 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. N Engl J Med 309:310
Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD (2000) Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol 18:675–679
Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL (2005) International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev 57:27–77
Liberatore GT, Jackson-Lewis V, Vuskosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S (1999) Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med 5:1403–1409
Liu J, Hughes TE, Sessa WC (1997) The first 35 amino acids and fatty acylation sites determine the molecular targeting of endothelial nitric oxide synthase into the Golgi region of cells: a green fluorescent protein study. J Cell Biol 137:1525–1535
Lohmann SM, Walter U, Miller PE, Greengard P, DcCamilli P (1981) Immunohistochemical localization of cyclic GMP-dependent protein kinase in mammalian brain. PNAS 78: 653–657
Lumelsky N, Lee S-H, Nguyen LJ, Sanchez-Pernaute R, Bankiewicz K, McKay R (2002) Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature 418:50–56
Lu YF, Kandel ER, Hawkins RD (1999) Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J Neurosci 19:10250–10261
Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Ann Rev Neurosci 25:103–126
Mandir AS, Przedborski S, Jackson-Lewis V, Wang Z-Q, Simbulanrosenthal CM, Samuelson ME, Hoffman BE, Guastella DB, Dawson VL, Dawson TM (1999) Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. PNAS 96:5774–5779
Margulis A, Sitaramaiah A (2000) Rate of deactivation of nitric oxide-stimulated soluble guanylate cyclase: influence of nitric oxide scavengers and calcium. Biochemistry 39:1034–1039
Massion PB, Pelat M, Belge C, Balligand JL (2005) Regulation of mammalian heart function by nitric oxide. Comparative Biochem Physiol Part A 142:144–150
Matarredona ER, Murillo-Carretero M, Moreno-Lopez B, Estrada C (2004) Nitric oxide synthesis inhibition increases proliferation of neural precursors isolated from the postnatal mouse subventricular zone. Brain Res 995:274–284
Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, Kolodkin AL (1995) Semaphorin III can function as a selective chemorepellent to pattern sensory projections in spinal chord. Neuron 14:949–959
Meurer S, Pioch S, Gross S, Muller-Esterl W (2005) Reactive oxygen species induce tyrosine phosphorylation of and Src kinase recruitment to NO-sensitive guanylyl cyclase. J Biol Chem 280: 33149–33156
Morishita T, Tsutsui M, Shimokawa H, Sabanai K, Tasaki H, Suda O, Nakata S, Tanimoto A, Wang K-Y, Ueta Y, Sasaguri Y, Nakashima Y, Yanagihara N (2005) Nephrogenic diabetes insipidus in mice lackingall nitric oxide synthase isoforms. Proc Natl Acad Sci USA 102:10616–10621
Muerer S, Pioch S, Wagner K, Muller-Esterl W, Gross S (2004) AGAP1, a novel binding partner of nitric oxide sensitive guanylyl cyclase. J Biol Chem 279:49346–49354.
Murad F (1986) Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest 78:1–5
Nairn AC, Greengard P (1983) Cyclic GMP-dependent protein phosphorylation in mammalian brain. Fed Proc 42:3107–3113
Nathan C, Calingasan N, Nezezon J, Ding A, Lucia MS, La Perle K, Fuortes M, Lin M, Ehrt S, Kwon NS, Chen J, Vodovotz Y, Kipiani K, Beal MF (2005) Protection from Alzheimer’s-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp Med 202:1163–1169
Nishiyama M, Hoshino A, Tsai L, Henley JR, Goshima Y, Lavigne T, Poo MM, Hong K (2003) Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature 423:990–995
Nourry C, Grant SGN, Borg J-P (2003) PDZ domain proteins: plug and play. Sci STKE 179:re7
Nuelding S, Kahlert S, Loebbert K, Doevendans PA, Meyer R, Vetter H, Grohe C (1999) 17 Beta-estradiol stimulates expression of endothelial and inducible NO synthase in rat myocardium in-vitro and in-vivo. Cardiovascular Res 43:666–674
Pilz RB, Casteel DE (2003) Regulation of gene expression by cyclic GMP. Circ Res 93:1034–1046
Pollock JS, Forstermann U, Mitchell JA, Warner TD, Schmidt HHH, Nakane M, Murad F (1991) Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci USA 88:10480–10484
Papapetropoulos A, Marczin N, Mora G, Milici A, Murad F, Catravas JD (1995) Regulation of vascular smooth muscle soluble guanylate cyclase activity, mRNA, and protein levels by cAMP-elevating agents. Hypertension 26:696–704
Prabhakar P, Thatte HS, Goetz RM, Cho MR, Golan DE, Michel T (1998) Receptor-regulated translocation of endothelial nitric-oxide synthase. J Biol Chem 273:27383–27388
Przedborski A, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM (1996) Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. PNAS 93:4565–4571
Przybylkowski S, Kurkowska-Jastrzebska I, Joniec I, Ciesielska A, Czlonkowska A, Czlonkowski A (2004) Cyclooxygenases mRNA and protein expression in striata in the experimental mouse model of Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration to mouse. Brain Res 1019:144–151
Rathjen J, Haines BP, Hudson KM, Nesci A, Dunn S, Rathjen PD (2002) Directed differentiation of pluripotent cells to neural lineages: homogeneous formation and differentiation of a neurectoderm population. Development 129:2649–2661
Raoul C, Buhler E, Sadeghi C, Jacquier A, Aebischer P, Pettmann B, Henderson CE, Haase G (2006) Chronic activation in presymptomatic amyotrophic lateral sclerosis (ALS) mice of a feedback loop involving Fas, Daxx, and FasL. Proc Natl Acad Sci USA 103:6007–6012
Robertson CP, Gibbs SM, Roelink H (2001) cGMP enhances the sonic hedgehog responses in neural plate cells. Dev Biol 238:157–167
Russwurm M, Wittau N, Koesling D (2001) Guanylyl cyclase/PSD-95 interaction: targeting of the nitric oxide-sensitive alpha2beta1 guanylyl cyclase to synaptic membranes. J Biol Chem 276:44647
Rutten K, De Vente J, Şik A, Van Ittersum MM, Prickaerts J, Blokland A. (2005) The selective PDE5 inhibitor, sildenafil, improves object memory in Swiss mice and increases cGMP Levels in hippocampal slices. Behav Brain Res 164:11–16
Saffer JD, Jackson SP, Annarella MB (1991) Developmental expression of Sp1 in mouse. Mol Cell Biol 11:2189–2199.
Savchenko A, Barnes S, Kramer RH (1997) Cyclic-nucleotide-gated channels mediate synaptic feedback by nitric oxide. Nature 390:694–698
Sauzeau V, Rolli-Derkinderen M, Marionneau C, Loirand G, Pacaud PJ (2003) RhoA expression is controlled by nitric oxide through cGMP-dependent protein kinase activation. J Biol Chem 278:9472–9480
Scott JA, Shewan AM, den Elzen NR, Lourero JJ, Gertler FB, Yap AS. (2006) Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol Biol Cell 17:1085–1095
Song H-J, Ming G-L, He Z, Lehmann M, McKeracher L, Tessier-Lavigne M, Poo MM (1998) Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science 281:1515–1518
Suchowersky O, Gronseth G, Perlmutter J, Reich S, Zesiewicz T, Weiner WJ. (2006) Practice parameter: neuroprotective strategies and alternative therapies for Parkinson disease (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 66:976–982
Sharina IG, Krumenacker JS, Martin E, Murad F (2000) Genomic organization of alpha1 and beta1 subunits of the mammalian soluble guanylyl cyclase genes. Proc Natl Acad Sci USA 97:10878
Sharina IG, Martin E, Thomas A, Uray KL, Murad F (2003) CCAAT-binding factor regulates expression of the beta1 subunit of soluble guanylyl cyclase gene in the BE2 human neuroblastoma cell line. Proc Natl Acad Sci USA 100:11523–11528
Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, Tsymbalyuk N, West GA, Gerzanich V (2006) Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med 12:433–440
Skidgel RA, Stanisavljevic S, Erdos EG (2006) A phosphoinositide 3-kinase-AKT-nitric oxide-cGMP signaling pathway in stimulating platelet secretion and aggregation. Biol Chem 387:159–165
Son H, Lu YF, Zhuo M, Arancio O, Kandel E, Hawkins RD (1998) The specific role of cGMP in hippocampal LTP. Learn Mem 5:231–245
Tabar V, Panagiotakos G, Greenberg ED, Chan BK, Sadelain M, Gutin PH, Studer L. (2005) Migration and differentiation of neural precursors derived from human embryonic stem cells in the rat brain. Nat Biotechnol 23:601–606
Takagi Y, Takahashi J, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N (2005) Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest 115:102–109
Traister A, Abashidze S, Gold V, Yairi R, Plachta MN, McJinnell I, Patel K, Weil M (2004) BMP controls nitric oxide-mediated regulation of cell numbers in the developing neural tube. Cell Death Different 11:832–841
Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D (2001) Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron 30:65–78
Uhler MD (1993) Cloning and expression of a novel cyclic GMP-dependent protein kinase from mouse brain. J Biol Chem 268:13586–13591
Yamada RX, Matsuki N, Ikegaya Y (2006) Soluble guanylyl cyclase inhibitor prevents Sema3F-induced collapse of axonal and dendritic growth cones of dentate granule cells. Biol Pharm Bull 29:796–798
Yamamoto T, Suzuki N (2002) Promoter activity of the 5′-flanking regions of medaka fish soluble guanylate cyclase α1 and β1 subunit genes. Biochem J 361:337
Waldman R, Nieberding M, Walter U (1987) Vasodilator-stimulated protein phosphorylation in platelets is mediated by cAMP- and cGMP-dependent protein kinases. Eur J Biochem 167:441–448
Wang HG, Lu FM, Jin I, Udo H, Kandel ER, deVente J, Walter U, Lohmann SM, Hawkins RD, Antonova I (2005) Presynaptic and postsynaptic roles of NO, cGK, and RhoA in long-lasting potentiation and aggregation of synaptic proteins. Neuron 45:389–403
Wang X, Robinson PJ (1995) Cyclic GMP-dependent protein kinase substrates in rat brain. J Neurochem 65:595–604
Weight FF, Petzold G, Greengard P (1974) Guanosine 3′,5′-monophosphate in sympathetic ganglia: increase assoicated with synaptic transmission. Science 186:942–944
Welshhans K, Rehder V (2005) Local activation of the nitric oxide/cyclic guanosine monophosphate pathway in growth cones regulates filopodial length via protein kinase G, cyclic ADP ribose and intracellular Ca2+ release. Eur J Neurosci 22:3006–3016
Wirdefeldt K, Gatz M, Pawitan Y, Pederson NL (2005) Risk and protective factors for Parkinson’s disease: a study in Swedish twins. Ann Neurol 57:27–33
Wirdefeldt K, Gatz M, Schalling M, Pedersen NL (2004) No evidence for heritability of Parkinson disease in Swedish twins. Neurology 63:305–311
Wyckoff MH, Chambliss KL, Mineo C, Yuhanna IS, Mendelsohn ME, Mumby SM, Shaul PW (2001) Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through Galpha(i). J Biol Chem 276:27071–27076
Acknowledgments
Our studies reported here were supported by multiple grants and awards from the following: The National Institute of Health, The John S. Dunn Foundation, The Welch Foundation, NASA, The U.S. Department of Defense and the University of Texas.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue dedicated to John P. Blass.
Rights and permissions
About this article
Cite this article
Madhusoodanan, K.S., Murad, F. NO-cGMP Signaling and Regenerative Medicine Involving Stem Cells. Neurochem Res 32, 681–694 (2007). https://doi.org/10.1007/s11064-006-9167-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9167-y