Abstract
Ionized calcium-binding adapter molecule 1 (iba-1) is specifically expressed in microglia and plays an important role in the regulation of the function of microglia. We observed chronological changes of iba-1-immunoreactive cells and iba-1 level in the gerbil hippocampal CA1 region after transient ischemia. Transient forebrain ischemia in gerbils was induced by the occlusion of bilateral common carotid arteries for 5 min. Immunohistochemical and Western blot analysis of iba-1 were performed in the gerbil ischemic hippocampus. In the sham-operated group, iba-1-immunoreactive cells were detected in the CA1 region. Thirty minutes after ischemia/reperfusion, iba-1 immunoreactivity significantly increased, and its immunoreactive cells were well ramified. Three hours after ischemia/reperfusion, iba-1 immunoreactivity and level decreased, and thereafter they increased again with time after ischemia/reperfusion. Three days after ischemia/reperfusion, iba-1-immunoreactive cells had well-ramified processes, which projected to the stratum pyramidale of the CA1 region. Seven days after ischemia/reperfusion, iba-1 immunoreactivity and level were highest in the CA1 region, whereas they significantly decreased in the CA1 region 10 days after ischemia/reperfusion. Iba-1-immunoreactive cells in the ischemic CA1 region were co-localized with OX-42, a microglia marker. In brief, iba-1-immunoreactive cells change morphologically and iba-1 immunoreactivity alters in the CA1 region with time after ischemia/reperfusion. These may be associated with the delayed neuronal death of CA1 pyramidal cells in the gerbil ischemic hippocampus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transient cerebral ischemia induced by the temporary deprivation of blood flow to the brain results in the insidious degeneration of specific vulnerable neurons such as pyramidal cells in the hippocampal CA1 region [1–3]. Changes in the hippocampal CA1 pyramidal cells are slower, and this process is described as “delayed neuronal death”. Many researchers have studied on delayed neuronal death in the hippocampus after transient forebrain ischemia [4–6].
Neuronal death induced by the injury of the central nervous system causes the activation of microglia [7–9]. Microglia are resident macrophages in the brain and are thought to modulate degenerative and regenerative functions in brain. Microglia can contribute to the elimination of deleterious debris and may be involved in neuroprotection by producing neurotrophic factors [10–13]. On the other hand, microglia exert a cytotoxic function by releasing nitric oxide, tumor necrosis factor-α, and radicals [14–17].
Microglia response factor-1 (mrf-1) is expressed in microglia and macrophages following cerebral ischemia in the rat [18]. In addition, allograft inflammatory factor-1 (aif-1) is up-regulated in microglial cells in human cerebral infarctions [19]. Ionized calcium-binding adapter molecule 1 (iba-1) is a novel calcium-binding protein, and is specifically expressed in microglia in the brain, which suggests that iba-1 plays an important role in the regulation of the function of microglia [20–23]. In a view of the localization of iba-1 gene within major histocompatibility complex class III region [20], iba-1 up-regulation shown in activated microglia is of great interest, because in activated state, microglia may function in antigen presentation and lymphocyte activation for CNS immune responses [24].
Although iba-1 is an established marker of microglia, and microglial activation in the post-ischemic hippocampus has been extensively studied [21, 22, 25], no study on the morphological change of iba-1-immunoreactive cells in the hippocampus induced by a global cerebral ischemia has been performed. In the present study, therefore, we investigated the chronological change of iba-1-immunoreactive cells as well as iba-1 level in the gerbil hippocampal CA1 region after 5 min of transient forebrain ischemia.
Experimental procedure
Animals
The present study used the progeny of Sprague–Dawley (SD) rats, ICR mice and Mongolian gerbils (Meriones unguiculatus) obtained from the Experimental Animal Center, Hallym University, Chunchon, South Korea. Male SD rats, ICR mice and Mongolian gerbils were used at 6 months of age. The animals were housed in a conventional state under adequate temperature (23 °C) and humidity (60%) control with a 12-h light/12-h dark cycle, and provided with free access to water and food. Procedures involving animals and their care conformed with the guidelines, which are in compliance with current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985, revised 1996) and were approved by the Hallym’s Medical Center Institutional Animal Care and Use Committee. All experiments were conducted to minimize the number of animals used and suffering caused.
Confirmation of antibody specificity
Brains of rats (n = 5), mice (n = 5) and gerbils (n = 5) were removed and homogenized in a 10 mM phosphate buffer (PB) containing 0.1 mM ethylenediamine tetraacetic acid (EDTA), 1 mM 2-mercaptoethanol, and 1 mM phenylmethyl sulfonyl fluoride (PMSF). The individual 25% (w/v) homogenate was centrifuged at 10,000g for 1 h. Five microliters of each supernatant were mixed with an equal volume of a 2× SDS-sample buffer and boiled for 3 min. The cooled samples were applied to a SDS-PAGE and transferred to nitrocellulose membranes. The blots were processed by the procedure described in Western blot analysis.
In order to establish the specificity of the immunostaining, a negative control test was carried out with pre-immune serum instead of the primary antibody. The negative control test was conducted in all groups.
Induction of transient forebrain ischemia
Male Mongolian gerbils were used at 6 months (B.W., 70–75 g) of age. The animals were anesthetizes with a mixture of 2.5% isoflurane in 33% oxygen and 67% nitrous oxide. A midline ventral incision was then made in the neck, and bilateral common carotid arteries were isolated, freed of nerve fibers, and occluded using non-traumatic aneurysm clips. The complete interruption of blood flow was confirmed by observing the central artery in retinae using an ophthalmoscope. After 5 min of occlusion, the aneurysm clips were removed from the common carotid arteries. The restoration of blood flow (reperfusion) was observed directly using the ophthalmoscope. We maintained the body (rectal) temperature under free regulating or normothermic (37 ± 0.5 °C) conditions with a rectal temperature probe (TR-100; YSI, USA) and a thermometric blanket before, during and after the surgery until the animals completely recovered from anesthesia. Sham-operated animals served as controls: these sham-operated animals were subjected to the same surgical procedures except that the common carotid arteries were not occluded.
Tissue processing for histology
For histology, sham-operated and ischemia-operated animals (n = 7 at each time point) at designated times (30 min, 3, 6, 12 h, 1, 2, 3, 4, 5, 7 and 10 days after reperfusion) were sacrificed. The animals were anesthetized with pentobarbital sodium and perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate-buffer (PB, pH 7.4). The brains were removed and post-fixed in the same fixative for 6 h. The brain tissues were cryoprotected by infiltration with 30% sucrose overnight. Thereafter the frozen tissues were serially and transversely cut to 30 μm thickness on a cryostat and then collected into six-well plates containing PBS.
NeuN immunohistochemistry for delayed neuronal death
To confirm the delayed neuronal death in the hippocampal CA1 region after transient forebrain ischemia, the sections were sequentially treated with 0.3% hydrogen peroxide (H2O2) in PBS for 30 min and 10% normal horse serum or normal rabbit serum in 0.05 M PBS for 30 min. The sections were next incubated with diluted mouse anti-NeuN (Chemicon International, diluted 1:1000) overnight at room temperature. Thereafter the tissues were exposed to biotinylated goat anti-mouse IgG and streptavidin peroxidase complex (Vector, USA). And they were visualized with 3,3'-diaminobenzidine in 0.1 M Tris–HCl (pH 7.2) buffer and mounted on the gelatin-coated slides.
Immunohistochemical staining of iba-1
To ensure that immunohistochemical data were comparable between groups, free-floating sections were carefully processed by immunohistochemistry under the same conditions. The sections were sequentially treated with 0.3% hydrogen peroxide (H2O2) in PBS for 30 min and 10% normal goat serum in 0.05 M PBS for 30 min. They were next incubated with diluted rabbit anti-iba-1 (Wako, 1:500, Japan) overnight at room temperature and subsequently exposed to biotinylated goat anti-rabbit IgG and streptavidin peroxidase complex (diluted 1:200, Vector, USA). Then, the sections were visualized by staining with 3,3'-diaminobenzidine in 0.1 M Tris–HCl buffer (pH 7.2) and mounted on the gelatin-coated slides.
To establish the specificity of primary antibody, procedure included the omission of the primary antibody, goat anti-rabbit, substitution of normal goat serum for the primary antibody. As a result, immunoreactivity disappeared completely in tissues.
Double immunofluorescence staining of iba-1/GFAP or iba-1/OX-42
To confirm the glial type containing iba-1 immunoreactivity, double immunofluorescence staining for rabbit anti-iba-1 (1:100)/mouse anti-glial fibrillary acidic protein (GFAP, 1:200, Chemicon International, USA) or rabbit anti-iba-1/mouse anti-OX-42 (1:100, Serotec, USA) was performed. Brain tissues were incubated in the mixture of antisera overnight at room temperature. After washing three times for 10 min with PBS, the sections were also incubated in a mixture of both FITC-conjugated goat anti-rabbit IgG (1:200; Jackson ImmunoResearch, USA) and Cy3 conjugated goat anti-mouse IgG (1:600; Jackson ImmunoResearch) for 2 h at room temperature. The immunoreactions were observed under the microscope (Axioscope, Carl Zeiss, Germany) attached HBO100.
Western blot analysis
To confirm changes in iba-1 level in the hippocampal CA1 region after transient forebrain ischemia, at designated times (30 min, 12 h, 4 and 10 days after the surgery), sham-operated and ischemia-operated animals (n = 7 at each time point) were sacrificed and used for the Western blot analysis. After sacrificing them and removing the hippocampus, the tissues were serially and transversely cut to 400 μm thickness on a vibratome (Leica, Germany) and the hippocampal CA1 region was dissected with surgical blade. The tissues were homogenized in 50 mM PBS (pH 7.4) containing ethylene glycol bis (2-aminoethyl ether)-N,N,N',N' tetraacetic acid (EGTA) (pH 8.0), 0.2% Nonidet P-40, 10 mM EDTA (pH 8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2 mM sodium orthvanadate, 1 mM PMSF and 1 mM dithiothreitol (DTT). After centrifugation, iba-1 level was determined in supernatants using a Micro BCA protein assay kit with bovine serum albumin as the standard (Pierce Chemical, USA). Aliquots containing 20 μg of total protein were boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM DTT, 6% sodium dodecyl sulfate (SDS), 0.3% bromophenol blue and 30% glycerol. Then, each aliquot was loaded onto a 10% polyacryamide gel. After electrophoresis, the gels were transferred to nitrocellulose transfer membranes (Schleicher and Schuell, USA). To reduce background staining, the membranes were incubated with 5% non-fat dry milk in PBS containing 0.1% Tween 20 for 45 min and then with rabbit anti-iba-1 antiserum (1:500), peroxidase-conjugated goat anti-rabbit IgG (Sigma, USA), and an ECL kit (Amersham, USA).
Quantification of data and statistical analysis
All measurements were performed in order to ensure objectivity in blind conditions, by two observers for each experiment, carrying out the measures of control and experimental samples under the same conditions.
For the quantitative analysis of iba-1 immunoreactivity in the hippocampal CA1 region, 15 sections in each animal were randomly selected within hippocampal CA1 region. The mid-point areas of the CA1 region were measured on the monitor at a magnification of 25–50×. Images of all iba-1-immunoreactive structures taken from three layers (strata oriens, pyramidale and radiatum in the hippocampal CA1 region) were obtained through an Axiophot light microscope (Carl Zeiss, Germany) equipped with a CCD (Charge Coupled Device) camera connected to a PC monitor. Video images were digitized into an array of 512 × 512 pixels corresponding to a tissue area of 140 × 140 μm (40× primary magnification). Each pixel resolution was 256 gray levels. The intensity of all iba-1 immunoreactive structures was evaluated on the basis of a relative optical density (ROD), which was obtained after the transformation of the mean gray level using the formula: ROD = log (256/mean gray level). We measured the ROD of the complete field, and the level of background staining was subtracted from the ROD level of immunoreactive structure before statistically processing. The relative % of control level was demonstrated in the graph. The results of the Western blot analysis were also scanned, and ROD was obtained using Scion Image software (Scion Corp., USA).
The interanimal differences in each group, as well as the interexperimental differences, were not statistically significant. The data shown here represent the means of experiments performed for each hippocampal area. Differences among the means were statistically analyzed by one-way analysis of variance followed by Duncan’s new multiple range method or the Newman–Keuls test to elucidate ischemia-associated changes in iba-1 to determine differences between sham-operated and ischemia-operated groups.
Results
Specificity of iba-1 antibody in the rat, mouse and gerbil brain
In order to examine the specificity of iba-1 antibody, brain homogenates from rat, mouse and gerbil were immunoblotted with iba-1 antibody. This antibody reacted with a single-protein band of 17 kDa (Fig. 1). In a pre-serum treated negative study, iba-1 immunoreactivity was not detected.
Delayed neuronal death in the CA1 region
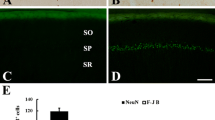
In this study, delayed neuronal death in the ischemic gerbil hippocampal CA1 region was confirmed using NeuN immunohistochemistry. In the sham-operated group, neurons in the gerbil hippocampus were positive to NeuN (Fig. 2A, B). One and 2 days after ischemia/reperfusion, neurons in the CA1 region were positive to NeuN (Fig. 2C–F). Four days after ischemia/reperfusion, a few NeuN-immunoreactive CA1 pyramidal cells were detected in the stratum pyramidale of the CA1 region (Fig. 2G, H). Almost all of the CA1 pyramidal cells in the stratum pyramidale were lost due to the delayed neuronal death
Immunohistochemical staining of NeuN in the hippocampus of the sham-operated (A, B) and ischemia-operated (C–H) animals. B, D, F and H is the high magnification of the CA1 region (CA1) in A, C, E and G, respectively. One and 2 days after ischemia/reperfusion, CA1 pyramidal cells in the stratum pyramidale (SP) show NeuN immunoreaction (C–F). Four days after ischemia/reperfusion, NeuN immunoreaction in the SP (asterisk) nearly disappears due to the delayed neuronal death of CA1 pyramidal cells (G, H). SO, stratum oriens; SR, stratum radiatum. Bar = 800 μm (A, C, E and G), 50 μm (B, D, F and H)
Change in iba-1 immunoreactivity
In the sham-operated group, iba-1-immunoreactive cells were detected in all the layers of the CA1 region (Fig. 3A). In this group, iba-1 immunoreactivity in the cells was weak. Thirty minutes after ischemia/reperfusion, iba-1 immunoreactivity in cells in the CA1 region significantly increased and iba-1-immunoreactive cells became ramified (Figs. 3B and 4).
Immunohistochemical staining for iba-1 in the CA1 region in sham-operated (A) and ischemia-operated (B–L) groups. In the sham-operated group, weak iba-1 immunoreactivity is shown in cells in the CA1 region (A). Iba-1 immunoreactivity significantly increases at 30 min after ischemia/reperfusion (B). Note that 3 days after ischemia/reperfusion, processes (arrows) of iba-1 immunoreactive cells extend to the stratum pyramidale (SP) (H). From 4 days after ischemia/reperfusion, iba-1-immunoreactive cells aggregate in the SP (I–K). Bar = 50 μm. SO, stratum oriens; SR, stratum radiatum. Bar = 50 μm
The relative optical density (ROD) as % of iba-1 immunoreactivity in the CA1 region after transient forebrain ischemia. Differences among the means are statistically analyzed by one-way analysis of variance followed by the Duncan’s new multiple range method or Newman–Keuls test (n = 7 per group; a P < 0.05, significant differences from sham-operated group, b P < 0.05, significant differences from pre-adjacent group). The bars indicate the means ± SD
Three hours after ischemia/reperfusion, iba-1 immunoreactivity decreased in the CA1 region compared to that in the 30 min post-ischemic group (Figs. 3C and 4). Thereafter, iba-1 immunoreactivity in cells in the CA1 region began to increase again with time after ischemia/reperfusion (Figs. 3D–K, 4). Two days after ischemia/reperfusion, the cytoplasm of iba-1-immunoreactive cells was bulky (Fig. 3G). Three days after ischemia/reperfusion, significant morphological change in iba-1-immunoreactive cells was shown: the processes of iba-1-immunoreactive cells in the strata oriens and radiatum of the CA1 region projected to the stratum pyramidale (Fig. 3H).
Four to 7 days after ischemia/reperfusion, iba-1-immunoreactive cells aggregated in the stratum pyramidale, and their iba-1 immunoreactivity was very strong (Fig. 3I–K). The iba-1 immunoreactivity peaked at 7 days after ischemia/reperfusion (Fig. 4). Ten days after ischemia/reperfusion, the size of iba-1-immunoreactive cells and their iba-1 immunoreactivity significantly decreased in the CA1 region (Figs. 3L, 4).
Co-localization of iba-1 and OX-42
We performed double immunofluorescence staining in the CA1 region for iba-1/GFAP or iba-1/OX-42 after ischemia/reperfusion. Many iba-1-immunoreactive cells in the CA1 region were co-localized with OX-42, a microglia marker (Fig. 5). In this study, iba-1-immunoreactive cells were not co-localized with GFAP, an astrocytes marker, in the CA1 region after ischemia/reperfusion (data not shown).
Change in iba-1 level
The result of Western bolt analysis showed that the change pattern of iba-1 level in the hippocampal CA1 region after ischemia/reperfusion was similar to the immunohistochemical data in ischemic groups (Fig. 6). Iba-1 level increased 30 min and decreased 12 h after ischemia/reperfusion, respectively. Thereafter, iba-1 level increased with time after ischemia/reperfusion. Iba-1 level in the CA1 region was highest 7 days after ischemia/reperfusion and significantly decreased again 10 days after ischemia/reperfusion.
Western blot analysis of iba-1 in the hippocampal CA1 region derived from sham-operated and ischemia-operated groups. The relative optical density (ROD) as % of immunoblot band is also represented (n = 7 per group; a P < 0.05, significant differences from sham-operated group, b P < 0.05, significant differences from pre-adjacent group). The bars indicate the means ± SD
Discussion
Iba-1 mRNA and protein is clearly expressed in monocyte cell lines, and iba-1 protein functions as an adapter molecule that mediates calcium signals in the monocytic lineage, including microglia [20, 23].
There are several studies on the expression of iba-1, aif-1 and mrf-1 by microglia/macrophages under brain damages. These include, for example, experimental studies on expression of these proteins following cerebral ischemia [18, 26–29] and following cerebral hemorrhages [30]. Furthermore, aif-1 expresses in microglia in normal human brains and in human ischemic brain [19, 31].
In the present study, we observed the chronological change of iba-1-immunoreactive cells and in iba-1 level in the hippocampal CA1 region after 5 min of transient forebrain ischemia in gerbils. Iba-1 immunoreactivity in CA1 cells and iba-1 level increased significantly in the hippocampal CA1 region at 30 min after ischemia/reperfusion. This result indicates that the activation of microglia is shown at very early time after ischemia/reperfusion. This is supported by previous reports that one of the characteristics of microglia is their activation at a very early stage in response to injury [32, 33]. In a transient focal cerebral ischemia model, a slight increase in iba-1 after 3.5 h after 90 min of transient focal cerebral ischemia in rat brain [22].
We also observed in this study that the morphological change of iba-1-immunoreactive cells was noted in the CA1 region 2–3 days after ischemia/reperfusion. When the brain is injured or affected by an insult, such as transient ischemia, ramified microglia at the affected site morphologically transform into cells with retracted processes and enlarged cell bodies, and they increase in number [33]. From 4 days after ischemia/reperfusion, we observed that iba-1-immunoreactive cells aggregated in the stratum pyramidale in the CA1 region, in which most pyramidal cells were lost via delayed neuronal death in this time period after ischemia/reperfusion. Recently, Tanaka et al. [27] reported on the distribution of microglia/macrophages in cerebral ischemia using bone marrow chimera mice known to express enhanced green fluorescent protein (EGFP). EGFP/iba-1-positive cells began to increase at 48 h after middle cerebral artery occlusion (MCAO), and the distribution of EGFP/Iba 1-positive cells was morphologically variable up to 14 days from MCAO.
In the present study, iba-1 immunoreactivity and level in the ischemic CA1 region were highest 7 days after transient ischemia in gerbils. In a focal ischemia induced by MCAO in rats, iba-1-immunoreactive cells rapidly appear at 3–5 h after reperfusion, and its immunoreactivity further increased, and peaks at 7 days after reperfusion [22]. In conclusion, iba-1 immunoreactive cells alter in morphology with time after ischemia/reperfusion. In addition, iba-1 in microglia significantly increases in the hippocampal CA1 region at every early and later time after ischemia/reperfusion.
References
Kirino T, Sano K (1984) Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol (Berl) 62:201–208
Kondo Y, Kondo F, Asanuma M, Tanaka K, Ogawa N (2000) Protective effect of oren-gedoku-to against induction of neuronal death by transient cerebral ischemia in the C57BL/6 mouse. Neurochem Res 25:205–209
Candelario-Jalil E, Mhadu NH, Al-Dalain SM, Martinez G, Leon OS (2001) Time course of oxidative damage in different brain regions following transient cerebral ischemia in gerbils. Neurosci Res 41:233–241
Kirino T (1982) Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 239:57–69
Hwang IK, Yoo KY, Kim DS, et al (2004) Expression and changes of galanin in neurons and microglia in the hippocampus after transient forebrain ischemia in gerbils. Brain Res 1023:193–199
Hwang IK, Eum WS, Yoo KY, et al (2005) Copper chaperone for Cu, Zn-SOD supplement potentiates the Cu, Zn-SOD function of neuroprotective effects against ischemic neuronal damage in the gerbil hippocampus. Free Radic Biol Med 39:392–402
Abraham H, Losonczy A, Czeh G, Lazar G (2001) Rapid activation of microglial cells by hypoxia, kainic acid, and potassium ions in slice preparations of the rat hippocampus. Brain Res 906:115–126
Kostulas N, Li HL, Xiao BG, Huang YM, Kostulas V, Link H (2002). Dendritic cells are present in ischemic brain after permanent middle cerebral artery occlusion in the rat. Stroke 33:1129–1134
Morino T, Ogata T, Horiuchi H, et al (2003) Delayed neuronal damage related to microglia proliferation after mild spinal cord compression injury. Neurosci Res 46:309–318
Hermann DM, Kilic E, Kugler S, Isenmann S, Bahr M (2001) Adenovirus-mediated glial cell line-derived neurotrophic factor (GDNF) expression protects against subsequent cortical cold injury in rats. Neurobiol Dis 8:964–973
Laurenzi MA, Arcuri C, Rossi R, Marconi P, Bocchini V (2001) Effects of microenvironment on morphology and function of the microglial cellline BV-2. Neurochem Res 26:1209–1216
Hashimoto M, Nitta A, Fukumitsu H, Nomoto H, Shen L, Furukawa S (2005) Involvement of glial cell line-derived neurotrophic factor in activation processes of rodent macrophages. J Neurosci Res 79:476–487
Lu YZ, Lin CH, Cheng FC, Hsueh CM (2005) Molecular mechanisms responsible for microglia-derived protection of Sprague–Dawley rat brain cells during in vitro ischemia. Neurosci Lett 373:159–164
Meda L, Cassatella MA, Szendrei GI, et al (1995) Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature 374:647–650
Brown DR, Schmidt B, Kretzschmar HA (1996) Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 380:345–347
Barger SW, Harmon AD (1997) Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature 388:878–881
McDonald DR, Brunden KR, Landreth GE (1997) Amyloid fibrils activate tyrosine kinase-dependent signaling and superoxide production in microglia. J Neurosci 17:2284–2294
Kato H, Tanaka S, Oikawa T, Koike T, Takahashi A, Itoyama Y (2000) Expression of microglial response factor-1 in microglia and macrophages following cerebral ischemia in the rat. Brain Res 882:206–211
Postler E, Rimner A, Beschorner R, Schluesener HJ, Meyermann R (2000) Allograft-inflammatory-factor-1 is upregulated in microglial cells in human cerebral infarctions. J Neuroimmunol 104:85–91
Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S (1996) A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun 224:855–862
Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S (1998) Microglia-specific localization of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res 57:1–9
Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y (2001) Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke 32:1208–1215
Hirasawa T, Ohsawa K, Imai Y, et al (2005) Visualization of microglia in living tissues using Iba1-EGFP transgenic mice. J Neurosci Res 81:357–362
Thomas WE (1992) Brain macrophages: evaluation of microglia and their functions. Brain Res Rev 17:61–74
Zhang N, Komine-Kobayashi M, Tanaka R, Liu M, Mizuno Y, Urabe T (2005) Edaravone reduces early accumulation of oxidative products and sequential inflammatory responses after transient focal ischemia in mice brain. Stroke 36:2220–2225
Imai H, Harland J, McCulloch J, Graham DI, Brown SM, Macrae IM (2002) Specific expression of the cell cycle regulation proteins, GADD34 and PCNA, in the peri-infarct zone after focal cerebral ischaemia in the rat. Eur J Neurosci 15:1929–1936
Tanaka R, Komine-Kobayashi M, Mochizuki H, et al (2003) Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience 117:531–539
Komine Kobayashi M, Chou N, Mochizuki H, Nakao A, Mizuno Y, Urabe T (2004) Dual role of Fcgamma receptor in transient focal cerebral ischemia in mice. Stroke 35:958–963
Schilling M, Besselmann M, Muller M, Strecker JK, Ringelstein EB, Kiefer R (2005) Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol 196:290–297
Wells JE, Biernaskie J, Szymanska A, Larsen PH, Yong VW, Corbett D (2005) Matrix metalloproteinase (MMP)-12 expression has a negative impact on sensorimotor function following intracerebral haemorrhage in mice. Eur J Neurosci 21:187–196
Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R (2001) Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol (Berl) 101:249–255
Gehrmann J, Matsumoto Y, Kreutzberg GW (1995) Microglia: intrinsic immuneffector cell of the brain. Brain Res Rev 20:269–287
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312–318
Acknowledgments
The authors would like to thank Mr. Seok Han, Mr. Seung Uk Lee and Ms. Hyun Sook Kim for technical help in this study. This work was supported by the MRC program of MOST/KOSEF (R13-2005-022-01002-0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, I.K., Yoo, KY., Kim, D.W. et al. Ionized Calcium-binding Adapter Molecule 1 Immunoreactive Cells Change in the Gerbil Hippocampal CA1 Region after Ischemia/Reperfusion. Neurochem Res 31, 957–965 (2006). https://doi.org/10.1007/s11064-006-9101-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9101-3