During the early postnatal period the central nervous system (CNS) is extremely sensitive to external agents. The present study aims at the investigation of critical phases where methylmercury (MeHg) induces cerebellar toxicity during the suckling period in mice. Animals were treated with daily subcutaneous injections of MeHg (7 mg/kg of body weight) during four different periods (5 days each) at the early postnatal period: postnatal day (PND) 1–5, PND 6–10, PND 11–15, or PND 16–20. A control group was treated with daily subcutaneous injections of a 150 mM NaCl solution (10 ml/kg of body weight). Subjects exposed to MeHg at different postnatal periods were littermate. At PND 35, behavioral tests were performed to evaluate spontaneous locomotor activity in the open field and motor performance in the rotarod task. Biochemical parameters related to oxidative stress (levels of glutathione and thiobarbituric acid reactive substances, as well as glutathione peroxidase and glutathione reductase activity) were evaluated in cerebellum. Hyperlocomotor activity and high levels of cerebellar thiobarbituric acid reactive substances were observed in animals exposed to MeHg during the PND 11–15 or PND 16–20 periods. Cerebellar glutathione reductase activity decreased in MeHg-exposed animals. Cerebellar glutathione peroxidase activity was also decreased after MeHg exposure and the lowest enzymatic activity was found in animals exposed to MeHg during the later days of the suckling period. In addition, low levels of cerebellar glutathione were found in animals exposed to MeHg during the PND 16–20 period. The present results show that the postnatal exposure to MeHg during the second half of the suckling period causes hyperlocomotor activity in mice and point to this phase as a critical developmental stage where mouse cerebellum is a vulnerable target for the neurotoxic and pro-oxidative effects of MeHg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methylmercury (MeHg) is a highly neurotoxic compound leading to neurological and developmental deficits in both animals and humans [1]. Even thought MeHg-induced neurotoxicity is extensively reported, the molecular mechanisms underlying its toxicity are not fully understood. The major mechanisms involved in MeHg neurotoxicity currently being explored are the impairment of intracellular calcium homeostasis [2], oxidative stress [3] and the alteration of glutamate homeostasis [4–6].
Several studies have reported that the developing brain is extremely sensitive to external agents, including MeHg (for review, see Ref. 7). In this regard, experimental studies have showed behavioral/functional deficits in rodents exposed to MeHg during the prenatal and/or earlier postnatal periods [8–10]. It is noteworthy that developmental exposure to MeHg has behavioral effects that extend into adulthood and aging [11]. Of particular importance, during the early postnatal period, a substantial acceleration of the synthesis of cerebral RNA, DNA, protein, and myelin is observed [12]. This period is also characterized by intense gliogenesis—mainly astrocytes, a major cell type that accumulates MeHg [13]. It is important to state that these aforementioned phenomena are affected by developmental MeHg exposure [7].
Oxidative stress has been proposed as an important mechanism related to MeHg-induced neurotoxicity [3]. Of particular importance, some studies of our and other groups have pointed to the suckling period as a crucial phase where mouse central nervous system (CNS) is a vulnerable target for the pro-oxidative effects of MeHg [5–7]. MeHg exposure has been shown to decrease the cerebellar levels of glutathione [14], the most important antioxidant sulfhydryl-containing molecule, and to increase the presynaptic release of glutamate [5], which could be involved with excitotoxic events [15]. In addition, MeHg exposure during the suckling period has been shown to increase the levels of peroxides in mouse cerebellum [6]. Even though the aforementioned studies point to the suckling period as a critical stage where mouse CNS represents a vulnerable target for the pro-oxidative effects of MeHg, they do not delve into specific phases of the early postnatal period where MeHg exerts these effects.
There is evidence of important changes in protein levels and enzyme activities of the antioxidant defense system in mouse brain during the early postnatal period [16]. In fact, gradual increases in the amount of the antioxidant enzyme glutathione peroxidase (GPx) and in the glutathione levels were observed in mouse brain throughout the early postnatal period and this phenomenon was proposed to represent a physiologic mechanism by which the brain protects itself from the surge in oxygen concentration encountered after delivery, which results in an increase in oxidative metabolism and, consequently, increase in free radical generation [16]. Since MeHg exposure decreases GPx activity [17] and cerebellar glutathione content in mouse CNS [14], one could suggest that the harmful neurotoxic effects of MeHg exposure during the early postnatal period is related, at least in part, to an imbalance in the physiological changes that occur in the mouse brain antioxidant defense system during the early postnatal period. However, to the best of our knowledge, such issues were never investigated.
Taking into account the high vulnerability of the CNS to toxicants during the early postnatal period and the harmful effects of MeHg on glutathione and glutathione-related enzymes in the CNS, the aim of this study was to investigate specific phases where MeHg induces cerebellar toxicity during the suckling period in mice. MeHg-induced neurotoxicity was evaluated based on biochemical parameters related to oxidative stress [levels of glutathione and thiobarbituric acid reactive substances (TBARS), as well as GPx and glutathione reductase (GR) activity] in mouse cerebellum. Moreover, considering the relationship between cerebellar function and motor activity, the presence of signs of motor impairment was evaluated either.
Experimental procedure
Chemicals
Methylmercury (II) chloride was from Aldrich Chemical Co. (Milwaukee, WI). β-nicotinamide adenine dinucleotide phosphate sodium salt—reduced form, 5-5′-dithio-bis (2-nitrobenzoic) acid, GR from baker’s yeast, and reduced glutathione were obtained from Sigma (St Louis, MO, USA). All other chemicals were of the highest grade available commercially.
Animals
Adult Swiss Albino mice (male and female), 90 days old, from our own breeding colony were maintained at 22±2°C, on a 12:12 h light/dark cycle, with free access to food (Nuvital, PR, Brazil) and water. The breeding regimen consisted of grouping three virgin females with one male for 5 days. Eight pregnant mice were selected and housed individually in opaque plastic cages.
Treatment
In the first day after parturition (PND 1), the litters’ size was standardized (eight animals per litter) and pups were maintained with their respective mothers in separated opaque plastic cages until the end of the suckling period (PND 21). Five pups of each litter were submitted to five different treatments: control, PND 1–5, PND 6–10, PND 11–15, and PND 16–20. Pups of both sexes were employed. Animals from the control group received daily subcutaneous injections of a 150 mM NaCl solution (10 ml/kg of body weight) during the PND 1–5 period. Animals from the groups PND 1–5, PND 6–10, PND 11–15, and PND 16–20 were treated with daily subcutaneous injections of MeHg (7 mg/kg of body weight). The moderately high dose of MeHg was based on previous study from our group [17] and on the fact that the period of exposure was very short (5 days). Several studies [10, 18], including some from our group [5, 6], use MeHg doses lower than 7 mg/kg, but during all fetal and/or early postnatal periods. Although oral exposure represents the most common route for MeHg intoxication, subcutaneous route was chosen in our experimental protocol because it appears to be a convenient way to intoxicate one-day suckling mice. Subjects exposed to MeHg at different postnatal periods were littermate. All experiments were conducted in accordance with the Guiding Principles in the Use of Animals in Toxicology, adopted by the Society of Toxicology in July 1989, and all experiments were approved by our ethics committee for animal use (313/CEUA; 23080.026023/2004-39/UFSC).
Behavioral tests
At PND 35, animals were submitted to behavioral/functional tests that evaluate locomotor activity and motor coordination (open field and rotarod tasks, respectively). Initially, pups were subjected to the open field according to previously described [19], with minor modifications. Open-field was performed in a separated room with no interference of noise. The locomotor activity was assessed in a session of 3 min using an open-field box measuring 56 cm×42 cm×40 cm (high) with the floor divided into 12 squares. The number of squares crossed with the four paws was used as measure of locomotor activity. After the open field, pups were subjected to the rotarod task and this procedure was based on the study of Duham and Miya [20], with minor modifications. In short, the homemade apparatus consisted of a bar with a diameter of 2.5 cm, subdivided into four compartments by disks, 25 cm in diameter. The bar rotated at a constant speed of 17 r.p.m. and the time of the permanence of pups on the apparatus was recorded. Each pup was subjected to two training trials and a third trial was used in the statistical analysis as actual value.
Tissue preparation
After the behavioral tests, animals were killed by decapitation. Cerebellar tissue was homogenized (1:5 w/v) in HEPES buffer 25 mM, pH 7.4 and the homogenate was rapidly centrifuged at 20,000 × g, at 4°C for 30 min. The supernatants obtained were used for the determinations of enzymatic activities, as well as for the quantifications of the levels of glutathione and TBARS. It is important to state that the cerebellum was the chosen encephalic structure due to the high MeHg affinity by cerebellar granular cells [21, 22] and because MeHg affects the motor performance in humans [23].
Biochemical determinations
GR and GPx activities were determined by the procedures of Carlberg and Mannervik [24] and that of Wendel [25], respectively. Enzymatic assays were performed at room temperature (approximately 25°C). For GPx assay, an aliquot of the tissue supernatant (200–250 μg protein) was added to the assay mixture (total volume=0.5 ml) and the reaction started by the addition of 0.1 ml of 4 mM tert-butylhydroperoxide to give a final concentration of 0.4 mM. Conversion of NADPH to NADP+ was monitored continuously at 340 nm for 2 min. For GR assay, an aliquot of the tissue supernatant (200–250 μg protein) was added to the assay mixture (total volume=0.5 ml) and the reaction started by the addition of 25 μl of 20 mM oxidized glutathione (GSSG) to give a final concentration of 1 mM. Conversion of NADPH to NADP+ was monitored continuously at 340 nm for 2 min. In the GR assay, oxidized glutathione (GSSG) is reduced by GR at the expense of NADPH consumption, which is followed at 340 nm. GR activity is proportional to NADPH decay [24]. In the GPx assay, the enzyme activity is measured indirectly by means of NADPH decay. Tert-butyl peroxide is decomposed, generating GSSG from GSH. GSSG is regenerated back to GSH by the GR present in the assay media, at the expense of NADPH [25]. Glutathione was measured as nonprotein thiols by the method of Ellman [26] with minor modifications [27]. In short, 500 μl of 10% trichloroacetic acid were added to 500 μl tissue supernatant. After centrifugation (4000 × g at 4°C for 10 min), the protein pellet was discarded and free sulfhydryl groups were determined in the clear supernatant (which was neutralized with 0.1 M NaOH) by the method of Ellman [26]. TBARS levels were determined by the method of Ohkawa et al. [28], in which malondialdehyde (MDA), an end-product of fatty acid peroxidation, reacts with thiobarbituric acid (TBA) to form a colored complex. Protein concentration was determined by the method of Bradford [29], using a bovine serum albumin as standard.
Statistical analysis
Differences between groups were evaluated by one-way ANOVA, followed by Duncan’s multiple range tests when appropriate.
Results
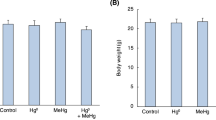
Figure 1 depicts biochemical parameters related to oxidative stress in the cerebellum of animals exposed to MeHg during different phases of the early postnatal period. MeHg treatment caused a significant increase in cerebellar levels of TBARS (Fig. 1A) in animals exposed during the postnatal days (PND) 11–15 or PND 16–20 when compared to corresponding values in controls (F 4,34=4.03, P=0.009). In addition, MeHg-exposure decreased the cerebellar glutathione levels in animals exposed during the PND 16–20 when compared to corresponding values in controls (Fig. 1B; F 4,35=2.99, P=0.032). Cerebellar GPx activity decreased in MeHg-exposed animals in a time period-dependent way (Fig. 1C; F 4,34=17.57, P<0.001). Cerebellar GR activity also decreased in MeHg-exposed animals, independent on the exposure period (Fig. 1D; F 4,33=4.07, P=0.009).
Effects of MeHg exposure during different early postnatal periods on TBARS and glutathione levels and on the activities of glutathione peroxidase and glutathione reductase in mouse cerebellum. TBARS levels (A) are expressed as nmol of malondialdehyde (MDA)/mg protein. Glutathione content (B) was measured as nonprotein thiols and is expressed as nmol/mg protein. Glutathione peroxidase (C) and glutathione reductase (D) activities are expressed as nmol of oxidized NADPH mg protein/min. All data are represented as mean±SEM (n=8 animals per group). *Significantly different from control by one-way ANOVA (P<0.05). #Significantly different from control and from (*) by one-way ANOVA (P<0.05). PND=postnatal day.
Figure 2 depicts the effects of MeHg exposure during different periods of the early postnatal period on the spontaneous locomotor activity in an open field task (Fig. 2A) and on the motor performance in a rotarod task (Fig. 2B). MeHg treatment caused a significant increase in the locomotor activity in a time period-dependent way (F 4,34=3.33, P=0.021). However, the motor performance of animals in the rotarod task was not affected by MeHg-exposure (F 4,34=1.09, P=0.376).
Effects of MeHg exposure during different early postnatal periods on the spontaneous locomotor activity in an open field and motor performance in the rotarod task. Details about the methodologies used on the behavioral tests are described in the Experimental procedure section. In the open field test (A), the number of squares crossed with the four paws was used as measuring of locomotor activity. In the rotarod test (B), each animal was subjected to two training trials and a third trial was used in the statistical analysis as actual value. Data are expressed as squared crossed for the open field test and as time of permanence on the apparatus for the rotarod test. All data are represented as mean±SEM (n=8 animals per group). *Significantly different from control by one-way ANOVA (P<0.05). PND=postnatal day.
Discussion
Several studies, including some from our group [5, 6], have pointed to the suckling period as a crucial phase where mouse CNS is a vulnerable target for the pro-oxidative effects of MeHg. However, those studies do not delve into specific phases of the early postnatal period where MeHg exerts neurotoxic effects. Here, we showed that MeHg caused significant neurotoxic effects related to oxidative stress in mouse cerebellum mainly from the second half of the suckling period (PND 11–20). In fact, MeHg exposure increased the cerebellar lipid peroxidation (measured by the TBARS levels) and decreased the cerebellar glutathione levels and the activities of the glutathione-related enzymes GPx and GR. Although cerebellar GPx activity was also decreased in mice exposed to MeHg during the first half of the suckling period, the higher effect of MeHg toward cerebellar GPx were observed in animals exposed to MeHg during the PND 16–20 period. In addition, animals from this same group showed decreased cerebellar glutathione levels. Based on these findings, it is reasonable to suggest that the second half of the suckling period represent a critical phase where MeHg induces alterations on the oxidant/antioxidant status in mouse cerebellum. Such phenomenon might be related, at least in part, to the well reported neurological deficits observed in mice exposed to this neurotoxicant during this crucial phase of the early postnatal period [6, 18].
It has been noted that the antioxidant system plays a protective role during ontogeny, especially in the perinatal and neonatal periods when there is increased intracellular generation of ROS in response to changes in oxygen tension, leading to compensatory up-regulation of antioxidant protein levels and enzyme activities [16]. In this regard, these same authors showed that GPx exhibits a steady increase from late fetal life to adulthood, with a significant increase noted around PND 14. We showed that MeHg exposure decreased the activity of cerebellar GPx. GPx belongs to a class of enzymes that catalyze the reduction of hydrogen peroxide, phospholipid-hydroperoxide and other organic hydroxyperoxides by glutathione [30]. Taking into account the importance of GPx in protecting the cerebellar tissue from the oxidative properties of endogenous peroxides, one could assume that the MeHg-induced inhibition of cerebellar GPx could lead to increased lipid peroxidation. Consistent with this conclusion is our finding of increased cerebellar levels of TBARS in the animals exposed to MeHg during the PND 11–15 or PND 16–20 periods. Since glutathione is a crucial molecule for the peroxidase activity of GPx and GR is an important enzyme that catalyzes the conversion of oxidized glutathione to its reduced form [31], it is important to mention that cerebellar GR activity was also decreased in all MeHg-exposed animals and the cerebellar glutathione levels decreased in animals exposed to MeHg at the PND 16–20 period.
Astrocytes display important physiological roles in many aspects of CNS function during the early postnatal period. Particularly important: they fulfill crucial roles in developmental neurotoxicity induced by metal exposure [32, 33]. The beneficial roles of astrocytes in metal-induced neurotoxicity are related, at least in part, to their higher antioxidant capabilities compared to neurons. In fact, primary cultures of astrocytes contain higher levels of GSH and antioxidant enzymes (GPx and GR) than those in primary cultures of neurons—a phenomenon that appears to reflect the situation in vivo [34, 35]. Taking into account the preferential localization of GSH and antioxidant enzymes in astrocytes and that MeHg selectively accumulates in these cells [13], one could assume that the changes found in GSH levels and in GPx and GR activity in the cerebellum of MeHg treated animals may be related to those localized in astrocytes.
It has previously been shown that the aerobic metabolism increases as the brain maturation proceeds, and the parallel increment of some enzymes related to the cellular activation of oxygen, such as succinate dehydrogenase, citrate synthase and pyruvate dehydrogenase, has been reported [36]. In addition, increased oxygen free radicals are produced during the developmental process, especially during the period of myelination and synaptogenesis, which occurs at approximately PND 10–14 in the mouse brain. As consequence, the brain displays a compensatory increase in the antioxidant enzymatic capacity at this period to meet the metabolic demands that result in free radical generation [16]. Taking into account that the detoxification of oxygen free radicals is especially important for the developing brain and that these highly reactive molecules have been shown to be involved in several neurological disorders, the presence of behavioral alterations (presented herein as hyperlocomotor activity) in MeHg-exposed animals was an expected phenomenon. It is noteworthy that this alteration was significant only in animals exposed to MeHg from the second half of the suckling period (PND 11–20), which correspond to the above-mentioned period where oxygen free radicals production increases.
There is evidence that cerebellar cells are targeted selectively by mercury compounds in vivo [22] and that MeHg neurotoxicity affects the motor system [23]. In fact, the relationship between MeHg-induced motor deficits and MeHg-induced cerebellar damage is a well-described phenomenon [37]. In this regard, we have reported decreased locomotor activity in animals exposed to MeHg during adulthood [38, 39] and the early postnatal period [6]. Here, in contrast, we observed increased locomotor activity in MeHg-exposed animals in an open field test. The molecular mechanism by which the postnatal exposure to MeHg induces hyperlocomotor activity is still not known; however, our data are in agreement with previous findings that show increased locomotion and total activity in rats exposed to MeHg plus elemental mercury [40]. In addition, Daré and collaborators [41], showed a stimulatory effect of the pre- and postnatal MeHg exposures on dopamine-mediated locomotor activity in rats, proposing that changes in the dopaminergic transmission are induced by exposure to MeHg in early life. In this regard, the presence of increased hyperactivity index in Seychelles children exposed to MeHg has been reported [42], pointing to the relevance of the present phenomenon in terms of human health concerns.
In conclusion, the present results show that the postnatal exposure to MeHg from the second half of the suckling period (PND 11–20) caused behavioral changes (hyperlocomotor activity) in mice and point to this phase as a critical stage of the early postnatal period where mouse cerebellum is a vulnerable target for the neurotoxic effects of MeHg. The diminished activity of crucial cerebellar antioxidant enzymes in animals exposed to MeHg in these phases might lead to the decreased detoxification of reactive/oxidant molecules, causing oxidative stress and behavioral alterations.
References
Clarkson TW, Magos L, Myers GJ (2003) The toxicology of mercury–current exposures and clinical manifestations. N Engl J Med 349:1731–1737
Sirois JE, Atchison WD (2000) Methylmercury affects multiple subtypes of calcium channels in rat cerebellar granule cells. Toxicol Appl Pharmacol 167:1–11
Ou YC, White CC, Krejsa CM, Ponce RA, Kavanagh TJ, Faustman EM (1999) The role of intracellular glutathione in methylmercury-induced toxicity in embryonic neuronal cells. Neurotoxicology 20:793–804
Aschner M, Yao CP, Allen JW, Tan KH (2000) Methylmercury alters glutamate transport in astrocytes. Neurochem Int 37:199–206
Farina M, Dahm KC, Schwalm FD, Brusque AM, Frizzo ME, Zeni G, Souza DO, Rocha JB (2003a) Methylmercury increases glutamate release from brain synaptosomes and glutamate uptake by cortical slices from suckling rat pups: modulatory effect of ebselen. Toxicol Sci 73:135–140
Manfroi CB, Schwalm FD, Cereser V, Abreu F, Oliveira A, Bizarro L, Rocha JB, Frizzo ME, Souza DO, Farina M (2004) Maternal milk as methylmercury source for suckling mice: neurotoxic effects involved with the cerebellar glutamatergic system. Toxicol Sci 81:172–178
Costa LG, Aschner M, Vitalone A, Syversen T, Soldin OP (2004) Developmental neuropathology of environmental agents. Annu Rev Pharmacol Toxicol 44:87–110
Rasmussen EB, Newland MC (2001) Developmental exposure to methylmercury alters behavioral sensitivity to d-amphetamine and pentobarbital in adult rats. Neurotoxicol Teratol 23:45–55
Newland MC, Reile PA, Langston JL (2004) Gestational exposure to methylmercury retards choice in transition in aging rats. Neurotoxicol Teratol 26:179–194
Weiss B, Stern S, Cox C, Balys M (2005) Perinatal and lifetime exposure to methylmercury in the mouse: behavioral effects. Neurotoxicology 26:675–690
Stern S, Cox C, Cernichiari E, Balys M, Weiss B (2001) Perinatal and lifetime exposure to methylmercury in the mouse: blood and brain concentrations of mercury to 26 months of age. Neurotoxicology 22:467–477
Gottlieb A, Keydar I, Epstein HT (1977) Rodent brain growth stages: an analytical review Biol Neonate 32:166–176
Aschner M (1996) Methylmercury in astrocytes – what possible significance? Neurotoxicology 17:93–106
Choi BH, Yee S, Robles M (1996) The effects of glutathione glycoside in methyl mercury poisoning. Toxicol Appl Pharmacol 141:357–364
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
Khan JY, Black SM (2003) Developmental changes in murine brain antioxidant enzymes. Pediatr Res 54:77–82
Farina M, Frizzo ME, Soares FA, Schwalm FD, Dietrich MO, Zeni G, Rocha JB, Souza DO (2003b) Ebselen protects against methylmercury-induced inhibition of glutamate uptake by cortical slices from adult mice. Toxicol Lett 144:351–357
Goulet S, Dore FY, Mirault ME (2003) Neurobehavioral changes in mice chronically exposed to methylmercury during fetal and early postnatal development. Neurotoxicol Teratol 25:335–347
Farina M, Franco JL, Ribas CM, Meotti FC, Pizzolatti MG, Dafre AL, Santos ARS (2005) Protective effects of Polygala paniculata extract against methylmercury-induced neurotoxicity in mice. J Pharm Pharmacol 57:1503–1508
Duhan NW, Miya TS (1957) A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc 46:208–209
Klein R, Herman SP, Brubaker PE, Lucier GW, Krigman MR (1972) A model of acute methyl mercury intoxication in rats. Arch Pathol 93:408–418
Sanfeliu C, Sebastia J, Cristofol R, Rodriguez-Farre E (2003) Neurotoxicity of organomercurial compounds. Neurotox Res 5:283–305
Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorensen N, Dahl R, Jorgensen PJ (1997) Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 19:417–428
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Farina M, Brandão R, de Lara FS, Pagliosa LB, Soares FA, Souza DO, Rocha JB (2003c) Profile of nonprotein thiols, lipid peroxidation and delta-aminolevulinate dehydratase activity in mouse kidney and liver in response to acute exposure to mercuric chloride and sodium selenite. Toxicology 184:179–187
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Flohe L (1971) Glutathione peroxidase: enzymology and biological aspects. Klin Wochenschr 49:669–683
Gul M, Kutay FZ, Temocin S, Hanninen O (2000) Cellular and clinical implications of glutathione. Indian J Exp Biol 38:625–634
Peuchen S, Bolanos JP, Heales SJ, Almeida A, Duchen MR, Clark JB (1997) Interrelationships between astrocyte function, oxidative stress and antioxidant status within the central nervous system. Prog Neurobiol 52:261–281
Tiffany-Castiglioni E, Guerri C, Aschner M, Matsushima GK, O’Callaghan JP, Streit WJ (2001) Roles of glia in developmental neurotoxicity: session VI summary and research needs. Neurotoxicology 22:567–573
Raps SP, Lai JC, Hertz L, Cooper AJ (1989) Glutathione is present in high concentrations in cultured astrocytes but not in cultured neurons. Brain Res 493:398–401
Makar TK, Nedergaard M, Preuss A, Gelbard AS, Perumal AS, Cooper AJ (1994) Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurons: evidence that astrocytes play an important role in antioxidative processes in the brain. J Neurochem 62:45–53
Booth RF, Patel TB, Clark JB (1980) The development of enzymes of energy metabolism in the brain of a precocial (guinea pig) and non-precocial (rat) species. J Neurochem 34:17–25
Sakamoto M, Nakano A, Kajiwara Y, Naruse I, Fujisaki T (1993) Effects of methyl mercury in postnatal developing rats. Environ Res 61:43–50
Dietrich MO, Mantese CE, Anjos GD, Souza DO, Farina M (2005) Motor impairment induced by oral exposure to methylmercury in adult mice. Environ Toxicol Pharmacol 19:169–175
Farina M, Cereser V, Portela LV, Mendez A, Porciúncula LO, Fornaguera J, Gonçalves CA, Wofchuk ST, Rocha JBT, Souza DO (2005c) Methylmercury increases S100B content in rat cerebrospinal fluid. Environ Toxicol Pharmacol 19:249–253
Fredriksson A, Dencker L, Archer T, Danielsson BR (1977) Prenatal coexposure to metallic mercury vapour and methylmercury produce interactive behavioural changes in adult rats. Neurotoxicol Teratol 18:129–134
Dare E, Fetissov S, Hokfelt T, Hall H, Ogren SO, Ceccatelli S (2003) Effects of prenatal exposure to methylmercury on dopamine-mediated locomotor activity and dopamine D2 receptor binding. Naunyn Schmiedebergs Arch Pharmacol 367:500–508
Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, Sloane-Reeves J, Wilding GE, Kost J, Huang LS, Clarkson TW (2003) Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet 361:1686–1692
Acknowledgements
This study was supported by grants from CNPq to M. Farina (475329/2004–0). J. Strigari was a recipient of a CNPq/PIBIC fellowship.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stringari, J., Meotti, F.C., Souza, D.O. et al. Postnatal Methylmercury Exposure Induces Hyperlocomotor Activity and Cerebellar Oxidative Stress in Mice: Dependence on the Neurodevelopmental Period. Neurochem Res 31, 563–569 (2006). https://doi.org/10.1007/s11064-006-9051-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9051-9