Abstract
Purpose
The presence of necrosis or microvascular proliferation was previously the hallmark for glioblastoma (GBM) diagnosis. The 2021 WHO classification now considers IDH-wildtype diffuse astrocytic tumors without the histological features of glioblastoma (that would have otherwise been classified as grade 2 or 3) as molecular GBM (molGBM) if they harbor any of the following molecular abnormalities: TERT promoter mutation, EGFR amplification, or chromosomal + 7/−10 copy changes. We hypothesize that these tumors are early histological GBM and will eventually develop the classic histological features.
Methods
Medical records from 65 consecutive patients diagnosed with molGBM at three tertiary-care centers from our institution were retrospectively reviewed from November 2017-October 2021. Only patients who underwent reoperation for tumor recurrence and whose tissue at initial diagnosis and recurrence was available were included in this study. The detailed clinical, histopathological, and radiographic scenarios are presented.
Results
Five patients were included in our final cohort. Three (60%) patients underwent reoperation for recurrence in the primary site and 2 (40%) underwent reoperation for distal recurrence. Microvascular proliferation and pseudopalisading necrosis were absent at initial diagnosis but present at recurrence in 4 (80%) patients. Radiographically, all tumors showed contrast enhancement, however none of them showed the classic radiographic features of GBM at initial diagnosis.
Conclusions
In this manuscript we present preliminary data for a hypothesis that molGBMs are early histological GBMs diagnosed early in their natural history of disease and will eventually develop necrosis and microvascular proliferation. Further correlative studies are needed in support of this hypothesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the 2016 WHO classification for tumors of the central nervous system, the diagnosis of glioblastoma (GBM) WHO grade 4 was given by the histological presence of necrosis and microvascular proliferation [1]. However, researchers found a subgroup of IDH-wildtype (IDH-wt) grade 2 and grade 3 gliomas that showed a similar clinical behavior to grade 4 tumors [2,3,4]. In 2018, the cIMPACT-NOW consortium recommended to reclassify this subset of tumors into “diffuse astrocytic glioma, IDH-wt, with molecular features of GBM (WHO grade 4)” [5]. These IDH-wildtype tumors are characterized by the absence of microvascular proliferation and necrosis, but the presence of any of the following three molecular characteristics: TERT promoter (TERTp) mutation, EGFR gene amplification, and combined gain of chromosome 7 and loss of chromosome 10 (+ 7/−10) [5]. The most updated WHO classification now considers this molecular GBM (molGBM) within the diagnosis of glioblastoma, IDH-wt, WHO grade 4 [6].
Our group recently published our multi-institutional data comparing the survival outcomes of molGBM compared to histological (histGBM). We found that PFS is significantly longer in molGBM and that OS is mildly longer in this same subgroup [7]. While collecting the data, we encountered certain clinical and radiographic patterns. Hence, we hypothesize that molGBMs might be histGBMs at an early stage and being histopathologically diagnosed before they cause any alteration in the cytoarchitectural structure of the brain tissue and will eventually develop pseudopalisading necrosis and microvascular proliferation. To evaluate this hypothesis, we screened our multi-institutional data for those patients who underwent reoperation for tumor recurrence and present our experience in this manuscript.
Methods
Patient selection
We retrospectively reviewed the electronic medical records of our previously published 65 consecutive molGBM cases who underwent histological and molecular evaluation at our three tertiary-care centers located in different geographical areas of the USA (Arizona, Florida, and Minnesota) from November 2017 to October 2021. Only patients who underwent reintervention for recurrent disease at our institution or whose tumor tissue obtained elsewhere was available for our review were included in this manuscript. Patients with recurrent disease whose tumor tissue from the original diagnosis was unavailable or who did not undergo any molecular testing were excluded from our cohort. This study was approved by our institutional review board.
Demographic and clinical characteristics
Demographic and clinical data were collected from the electronic medical records: age at diagnosis, gender, date of diagnosis, date of death or last follow up, race, and preoperative KPS. Histological and molecular data were obtained from the pathology studies performed at our institution and the following variables were extracted: TERT promoter mutation, EGFR amplification, + 7/−10, presence of necrosis or microvascular proliferation, IDH-mutation status, and MGMT promoter methylation status. All tumors would have been classified as WHO grade 2 or 3 according to the previous classification but are all now grade 4.
Overall survival (OS) was obtained from the date from initial pathologic diagnosis to the date of death. Progression-free survival (PFS) was defined as the date from initial pathologic diagnosis to the date of radiographic progression, as assessed by MRI as previously described.
Radiographic characteristics
Radiographic characteristics were obtained from available imaging and imaging reports. All imaging studies were reviewed and interpreted by a subspecialty certified neuroradiologist. The following variables were extracted from the imaging report: laterality, location, contact with the lateral ventricles (LV), presence of contrast enhancement, and extent of resection. Extent of resection was divided into biopsy (when tumor debulking was not performed), subtotal resection (when there was postoperative radiographic evidence of residual tumor), and gross total resection (when there was no postoperative radiographic evidence of residual tumor) as previously described.
Tumor volumes were manually obtained through the slicing method by defining the region of interest on each MRI slide and subsequent automatic 3D computerization and reconstruction on Horos software for Mac. Tumor diffusivity (proliferation/diffusion ratio [p/D]) was calculated as previously described by Swanson et al. using the following formula (4π/3)2/3 × (6.106/[VT21/3 − VT11/3])2 [8, 9]. VT2 and VT1 refer to the preoperative T2/FLAIR volume and contrast-enhancing volume, respectively. Tumors with a low p/D ratio (< 0.38 mm−2) are considered highly diffuse tumors, while tumors with a high p/D ratio (> 1.30 mm−2) [10].
Statistical analysis
Due to the limited available data, no comparative analysis was performed, and descriptive statistics were used to report our data. Measures of central tendency and percentages were calculated on Microsoft Excel for Mac (Microsoft Corporation 2022, Redmond, WA, USA).
Results
Patient selection
We screened our 65 consecutive patients diagnosed with molGBM at our institution between November 2017 and October 2021. The demographics, clinical characteristics, and outcomes of these 65 patients are published [7]. Eight patients met the inclusion criteria for this study as they underwent reoperation at our institution. From the eight patients, four underwent reoperation within the first month of diagnosis for a more extensive resection without reoperation for late recurrence and were further excluded from our study.
Demographic and clinical characteristics
The median age at diagnosis was 58.5 years, all patients were male, and all patients were of non-Hispanic white race. Out of the 4 patients, 2 (50%) underwent reoperation for recurrence at the primary site, 1 (20%) underwent reoperation for distal recurrence in the ipsilateral brain hemisphere, and 1 (20%) underwent reoperation for a distal recurrence to the spine. Necrosis and microvascular proliferation were absent at initial diagnosis but present at reoperation in all cases (100%), including both patients with distal recurrence. All patients underwent standard treatment for GBM with concomitant temozolomide plus radiation therapy and adjuvant temozolomide.
Radiographic characteristics and survival
The median PFS of these patients was 12 months and the median OS was 26 months. For their first resection, 1 (25%) patient underwent gross-total resection, 1 (25%) patient underwent subtotal resection, and 2 (50%) patients underwent a biopsy. A detailed description of imaging findings and representative imaging slides can be found in the clinical vignette section and Figs. 1, 2, 3, 4. The contrast-enhancing tumor volumes were 0.0829 cm3, 0.8986 cm3, 0.2668 cm3, 2.5808 cm3; for cases 1 through 4, respectively. The T2/FLAIR tumor volumes were 1.0792 cm3, 27.0526 cm3, 20.3813 cm3, 34.6695 cm3; for cases 1 through 4, respectively. The p/D ratios were 2.786 mm−2, 0.233 mm−2, 0.136 mm−2, 0.271 mm−2; for cases 1 through 4, respectively.
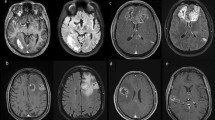
MRI of the brain with and without contrast from case 1. (left) study obtained at initial diagnosis demonstrating a cortical-based intra-axial T2 hyperintense lesion with nodular enhancement in the right middle temporal gyrus. (right) study obtained at disease progression demonstrating interval development of new cortical and subcortical enhancement with mild surrounding vasogenic edema
MRI of the brain with and without contrast from case 2. (left) Study obtained at initial diagnosis demonstrating an infiltrating T2 hyperintense lesion involving the cortex and white matter of the left inferior temporal lobe with mild associated enhancement. (right) Study obtained at disease progression demonstrating an increase in size of the T2 signal abnormality with an increase in contrast enhancement
MRI of the brain with and without contrast from case 3. (left) Study obtained at initial diagnosis demonstrating a perirolandic infiltrating T2 hyperintense lesion with very mild diffuse contrast enhancement. (right) Study with perfusion obtained at disease progression demonstrating a new rim-enhancing, centrally necrotic cortical lesion in the ipsilateral anterior left middle frontal gyrus with associated surrounding T2 hyperintensity with low perfusion
MRI of the CNS axis from case 4. (left) MRI of the brain with and without contrast obtained at initial diagnosis demonstrating a T2 hyperintense lesion in the right mesial temporal lobe and insula with associated contrast enhancement and increased perfusion. (right) MRI of the cervical spine with and without contrast obtained at disease progression demonstrating a contrast enhancing dural-based lesion most prominent at the level of C3 causing cord compression and edema concerning for dural metastases
From the whole initial cohort of 65 molGBM patients [7], the mean PFS was 13 months and the mean OS was 26 months. Initial imaging from 38 patients (58.46%) showed contrast enhancement.
Histomolecular characteristics
All tumors were IDH-wt gliomas. 3 (60%) tumors had a TERTp mutation, 3 (60%) had an EGFR amplification, and 1 (20%) had a + 7/−10. 3 (60%) tumors had a single molecular alteration and 2 (40%) had a combination of 2 molecular alterations. MGMT methylation was present in 1 (20%) tumor and absent in the rest.
Clinical vignette
Case 1
61-year-old man originally diagnosed with an anaplastic astrocytoma, IDH-wt, WHO grade 3 according to the previous classification with a TERTp mutation (case performed before the cIMPACT-NOW recommendation) due to the absence of microvascular proliferation and pseudopalisading necrosis. Patient underwent initial gross-total resection (GTR) and then was treated with concomitant temozolomide plus radiation to 60 Gy in 30 fractions and adjuvant temozolomide with tumor treating fields. Follow-up imaging at 21 months after diagnosis showed evidence of local recurrence (Fig. 1) and patient underwent reoperation revealing glioblastoma IDH-wt WHO grade 4 with the presence of pseudopalisading necrosis and microvascular proliferation. Patient passed 38 months after initial diagnosis.
Case 2
52-year-old man originally diagnosed with an anaplastic astrocytoma, IDH-wt, WHO grade 3 according to the previous classification with a + 7/−10 and an EGFR mutation (case performed before the cIMPACT-NOW recommendation) due to the absence of microvascular proliferation and pseudopalisading necrosis. Patient underwent initial stereotactic needle biopsy and then was treated with concomitant temozolomide plus radiation to 60 Gy in 30 fractions and adjuvant temozolomide for six cycles. At 10 months since diagnosis patient began to deteriorate clinically and imaging showed tumor progression vs. pseudoprogression and patient was started on bevacizumab (Fig. 2). Patient continued to deteriorate clinically and MRI with perfusion after 12 months since diagnosis showed new areas of enhancement and increased rCBV. Patient underwent reoperation revealing glioblastoma IDH-wt WHO grade 4 with the presence of pseudopalisading necrosis and microvascular proliferation. Patient passed 26 months after initial diagnosis.
Case 3
71-year-old man originally diagnosed with a right-sided fronto-parietal diffuse astrocytic glioma, IDH-wt, with molecular features of GBM, WHO grade 4. Patient underwent initial stereotactic needle biopsy and then was treated with 60 Gy radiation in 30 fractions, concomitant and adjuvant temozolomide for six cycles. Follow-up imaging at 20 months after diagnosis showed a new area of contrast enhancement distal to the original location in the ipsilateral frontal lobe (Fig. 3). Patient underwent reoperation revealing glioblastoma IDH-wt WHO grade 4 with the presence of necrosis and microvascular proliferation. Patient is still alive 28 months after initial diagnosis.
Case 4
62-year-old man originally diagnosed with diffuse astrocytic glioma, IDH-wt, with molecular features of GBM, WHO grade 4. Patient underwent initial sub-total resection and was then treated with concomitant temozolomide plus radiation to 60 Gy in 30 fractions and started on adjuvant temozolomide. 6 months after initial diagnosis the patient presented to the emergency department with progressive neck pain and bilateral upper extremity weakness. Cervical MRI revealed a contrast enhancing lesion at C3 with spinal cord compression (Fig. 4). Brain MRI revealed leptomeningeal spread suggesting progressive disease. Patient underwent emergent cervical surgery for decompression revealing glioblastoma IDH-wt, WHO grade 4 with the presence of necrosis and microvascular proliferation. Patient passed 9 months after initial diagnosis.
Discussion
Recently, the WHO classification of CNS tumors introduced tumors lacking the histological characteristics of GBM but harboring any three specific molecular abnormalities (TERTp mutation, EGFR amplification, or + 7/−10) to be classified as glioblastoma, IDH-wt, WHO grade 4 [6]. In this manuscript we propose that the natural history of GBM includes an early stage where the classic histological characteristics (microvascular proliferation and pseudopalisading necrosis) are not present but will eventually develop in these tumors. Although statistically similar, the mean PFS and OS of patients with molGBM tends to be around 5 months longer than in patients with histological GBM [7, 9, 11,12,13,14]. Our hypothesis might suggest that this slight difference in survival outcomes could be due to a slightly earlier diagnosis.
All of our reoperated patients underwent standard of care treatment with chemotherapy and radiation, which raises the question whether the histological changes seen at reoperation represent pseudo progression or real progression. Even though pseudo progression and real progression are histologically distinct, in some cases pathology may report a mix of viable tumor with non-viable necrotic tumor, hence making it difficult to attribute the histological characteristics to pseudo or real progression. Due to the previous aggressive treatment of these tumors, studying the natural history of disease is complex as it would be unethical to withhold treatment on these patients to study the natural history of these. However, human-derived animal models might provide some insight into the histomolecular behavior of these tumors [15, 16]. On the contrary, two of the reoperated patients recurred distally, one on the ipsilateral frontal lobe and another had drop metastases to the cervical spine. These zones were most likely spared from the high doses of radiation, which suggests that the histological changes may be related to tumor progression instead of the treatment itself. Nevertheless, it is also possible that the cells involved in the new areas of enhancement originated from the first tumor and the treatment affected and increased their invasiveness, allowing them to migrate and form a new area of distal enhancement [17]. Although unlikely, there’s also a possibility that these distal recurrences may be two different de novo lesions.
It is well known that GBM is an infiltrative tumor where the core of the tumor has the majority of the tumor burden while the periphery has a lower tumor burden, although more invasive [8, 9, 18]. On the other hand, molGBM appears to lack a clear core with the whole tumor burden diffusely distributed. If we are diagnosing these tumors at an early stage, perhaps GBM has an initial infiltrative stage before rapid proliferation and development of a tumor core. The presence of contrast enhancement on MRI has been previously correlated with long-term outcomes [7, 19,20,21]. Where certain patterns of enhancement including location, size of enhancing and non-enhancing portions, edema size, among others; have been shown to accurately predict long-term survival. Additionally, imaging bio-markers have also been shown to predict the molecular characteristics of tumors [21].
Strengths and limitations
This study carries inherent limitations due to its retrospective nature such as the risk for inaccurate medical records, short follow up period due to the recent introduction of this diagnosis into the classification, and relatively small group size. Two of our cases underwent stereotactic needle biopsy only at diagnosis, which poses the risk of undersampling due to tumor heterogeneity. However, to reduce this risk, we included radiographic data to show that these tumors did not resemble the classical GBM at diagnosis. As a recently described entity, data comparing the precise histological and radiographic characteristics of molGBM to the different astrocytomas are limited; however future studies may provide insight into the similarities/differences of these tumors. On the other hand, a major strength for this study is the inclusion of multicenter data from three distinct geographical regions of the United States.
Conclusion
In this manuscript we show our institutional experience on molGBM to present a hypothesis were these tumors are early histological GBM diagnosed early in their natural history of disease and will eventually develop necrosis and microvascular proliferation. Further correlative studies are needed in support of this hypothesis.
Data availability
Data obtained and analyzed in this manuscript is patient confidential. However, non-identified data is available from the corresponding author upon reasonable request.
Abbreviations
- + 7/−10:
-
Combined gain of chromosome 7 and loss of chromosome 10
- cIMPACT-NOW:
-
Consortium to inform molecular and practical approaches to CNS tumor taxonomy
- EGFR:
-
Epidermal growth factor receptor
- GBM:
-
Glioblastoma
- IDH:
-
Isocitrate DEHYDROGENASE
- KPS:
-
Karnofsky performance score
- MGMT:
-
O6-methylguanine-DNA-methyltransferase
- molGBM:
-
Molecular glioblastoma
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- TERT:
-
Telomerase Reverse transcriptase
References
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Aoki K, Nakamura H, Suzuki H, Matsuo K, Kataoka K, Shimamura T, Motomura K, Ohka F, Shiina S, Yamamoto T, Nagata Y, Yoshizato T, Mizoguchi M, Abe T, Momii Y, Muragaki Y, Watanabe R, Ito I, Sanada M, Yajima H, Morita N, Takeuchi I, Miyano S, Wakabayashi T, Ogawa S, Natsume A (2018) Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro Oncol 20:66–77. https://doi.org/10.1093/neuonc/nox132
Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML, Smirnov IV, Sarkar G, Caron AA, Kollmeyer TM, Praska CE, Chada AR, Halder C, Hansen HM, McCoy LS, Bracci PM, Marshall R, Zheng S, Reis GF, Pico AR, O’Neill BP, Buckner JC, Giannini C, Huse JT, Perry A, Tihan T, Berger MS, Chang SM, Prados MD, Wiemels J, Wiencke JK, Wrensch MR, Jenkins RB (2015) Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 372:2499–2508. https://doi.org/10.1056/NEJMoa1407279
Reuss DE, Kratz A, Sahm F, Capper D, Schrimpf D, Koelsche C, Hovestadt V, Bewerunge-Hudler M, Jones DTW, Schittenhelm J, Mittelbronn M, Rushing E, Simon M, Westphal M, Unterberg A, Platten M, Paulus W, Reifenberger G, Tonn J-C, Aldape K, Pfister SM, Korshunov A, Weller M, Herold-Mende C, Wick W, Brandner S, von Deimling A (2015) Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol 130:407–417. https://doi.org/10.1007/s00401-015-1454-8
Brat DJ, Aldape K, Colman H, Holland EC, Louis DN, Jenkins RB, Kleinschmidt-DeMasters BK, Perry A, Reifenberger G, Stupp R, von Deimling A, Weller M (2018) cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV.” Acta Neuropathol 136:805–810. https://doi.org/10.1007/s00401-018-1913-0
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Ramos-Fresnedo A, Pullen MW, Perez-Vega C, Domingo RA, Akinduro OO, Almeida JP, Suarez-Meade P, Marenco-Hillembrand L, Jentoft ME, Bendok BR, Trifiletti DM, Chaichana KL, Porter AB, Quiñones-Hinojosa A, Burns TC, Kizilbash SH, Middlebrooks EH, Sherman WJ (2022) The survival outcomes of molecular glioblastoma IDH-wildtype: a multicenter study. J Neurooncol. https://doi.org/10.1007/s11060-022-03960-6
Swanson KR, Bridge C, Murray JD, Alvord EC Jr (2003) Virtual and real brain tumors: using mathematical modeling to quantify glioma growth and invasion. J Neurol Sci 216:1–10. https://doi.org/10.1016/j.jns.2003.06.001
Tripathi S, Vivas-Buitrago T, Domingo RA, Biase G, Brown D, Akinduro OO, Ramos-Fresnedo A, Sherman W, Gupta V, Middlebrooks EH, Sabsevitz DS, Porter AB, Uhm JH, Bendok BR, Parney I, Meyer FB, Chaichana KL, Swanson KR, Quiñones-Hinojosa A (2021) IDH-wild-type glioblastoma cell density and infiltration distribution influence on supramarginal resection and its impact on overall survival: a mathematical model. J Neurosurg. https://doi.org/10.3171/2021.6.Jns21925
Baldock A, Ahn S, Rockne R, Johnston S, Neal M, Corwin D (2014) Patient-specific metrics of invasiveness reveal significant prognostic benefit of resection in a predictable subset of gliomas. PLoS ONE 9:e99057. https://doi.org/10.1371/journal.pone.0099057
Vivas-Buitrago T, Domingo RA, Tripathi S, De Biase G, Brown D, Akinduro OO, Ramos-Fresnedo A, Sabsevitz DS, Bendok BR, Sherman W, Parney IF, Jentoft ME, Middlebrooks EH, Meyer FB, Chaichana KL, Quinones-Hinojosa A (2021) Influence of supramarginal resection on survival outcomes after gross-total resection of IDH-wild-type glioblastoma. J Neurosurg. https://doi.org/10.3171/2020.10.Jns203366
Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, Brandes AA, Taal W, Domont J, Idbaih A, Campone M, Clement PM, Stupp R, Fabbro M, Le Rhun E, Dubois F, Weller M, von Deimling A, Golfinopoulos V, Bromberg JC, Platten M, Klein M, van den Bent MJ (2017) Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med 377:1954–1963. https://doi.org/10.1056/NEJMoa1707358
McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, Laterra J, Kleinberg LR, Grossman SA, Brem H, Quiñones-Hinojosa A (2009) Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg 110:583–588. https://doi.org/10.3171/2008.5.17557
Stupp R, Taillibert S, Kanner A, Read W, Steinberg DM, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K, Di Meco F, Lieberman F, Zhu J-J, Stragliotto G, Tran DD, Brem S, Hottinger AF, Kirson ED, Lavy-Shahaf G, Weinberg U, Kim C-Y, Paek S-H, Nicholas G, Bruna J, Hirte H, Weller M, Palti Y, Hegi ME, Ram Z (2017) Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318:2306–2316. https://doi.org/10.1001/jama.2017.18718
Garcia CA, Bhargav AG, Brooks M, Suárez-Meade P, Mondal SK, Zarco N, ReFaey K, Jentoft M, Middlebrooks EH, Snuderl M, Carrano A, Guerrero-Cazares H, Schiapparelli P, Sarabia-Estrada R, Quiñones-Hinojosa A (2021) Functional characterization of brain tumor-initiating cells and establishment of GBM preclinical models that incorporate heterogeneity, therapy, and sex differences. Mol Cancer Ther. https://doi.org/10.1158/1535-7163.MCT-20-0547
Chaichana KL, Guerrero-Cazares H, Capilla-Gonzalez V, Zamora-Berridi G, Achanta P, Gonzalez-Perez O, Jallo GI, Garcia-Verdugo JM, Quiñones-Hinojosa A (2009) Intra-operatively obtained human tissue: protocols and techniques for the study of neural stem cells. J Neurosci Methods 180:116–125. https://doi.org/10.1016/j.jneumeth.2009.02.014
Gupta K, Burns TC (2018) Radiation-induced alterations in the recurrent glioblastoma microenvironment: therapeutic implications. Front Oncol 8:503–503. https://doi.org/10.3389/fonc.2018.00503
Swanson KR, Alvord EC Jr, Murray JD (2000) A quantitative model for differential motility of gliomas in grey and white matter. Cell Prolif 33:317–329. https://doi.org/10.1046/j.1365-2184.2000.00177.x
Carrillo JA, Lai A, Nghiemphu PL, Kim HJ, Phillips HS, Kharbanda S, Moftakhar P, Lalaezari S, Yong W, Ellingson BM, Cloughesy TF, Pope WB (2012) Relationship between tumor enhancement, edema, IDH mutational status, MGMT promoter methylation, and survival in glioblastoma. Am J Neuroradiol 33:1349. https://doi.org/10.3174/ajnr.A2950
Pope WB, Chen JH, Dong J, Carlson MRJ, Perlina A, Cloughesy TF, Liau LM, Mischel PS, Nghiemphu P, Lai A, Nelson SF (2008) Relationship between gene expression and enhancement in glioblastoma multiforme: exploratory DNA microarray analysis. Radiology 249:268–277. https://doi.org/10.1148/radiol.2491072000
Macyszyn L, Akbari H, Pisapia JM, Da X, Attiah M, Pigrish V, Bi Y, Pal S, Davuluri RV, Roccograndi L, Dahmane N, Martinez-Lage M, Biros G, Wolf RL, Bilello M, O’Rourke DM, Davatzikos C (2015) Imaging patterns predict patient survival and molecular subtype in glioblastoma via machine learning techniques. Neuro Oncol 18:417–425. https://doi.org/10.1093/neuonc/nov127
Funding
AQH was supported by the Mayo Clinic Professorship and a Clinician Investigator award, and Florida State Department of Health Research Grant, and the Mayo Clinic Graduate School, as well as the NIH (R43CA221490, R01CA200399, R01CA195503, and R01CA216855). SHK was supported by the FDA (R01 FD-R-07288). EHM receives unrelated research support from Boston Scientific Corp. and Varian Medical Systems, Inc.
Author information
Authors and Affiliations
Contributions
Conceptualization: ARF, MWP, CPV, RAD, OOA, JPA, EHM, WJS. Methodology: ARF, MWP, CPV, RAD, OOA, JPA, DMT, ABP, TCB, SHK, EHM, WJS. Software: ARF, MWP. Validation: ARF, MWP. Formal analysis: ARF. Investigation: ARF, MWP, CPV, RAD, OOA, JPA. Resources: MEJ, BRB, DMT, KLC, ABP, AQH, TCB, SHK, EHM, WJS. Data Curation: ARF, MWP, CPV, RAD, OOA, JPA. Writing—original draft: ARF, MWP, CPV, RAD, OOA, JPA. Writing—review and editing: ARF, MWP, CPV, RAD, OOA, JPA, MEJ, BRB, DMT, KLC, ABP, AQH, TCB, SHK, EHM, WJS. Visualization: ARF, MWP, CPV, RAD, OOA, JPA, MEJ, BRB, DMT, KLC, ABP, AQH, TCB, SHK, EHM, WJS. Supervision: AQH, EHM, WJS. Project administration: EHM, WJS. Funding acquisition: AQH, EHM, WJS.
Corresponding authors
Ethics declarations
Conflict of interest
EHM serves on an advisory board and receives consulting fees from Boston Scientific Corp. All other authors have no conflict of interest to disclose. The authors declare no competing interests.
Ethical approval
This study was approved by the institutional IRB.
Consent to participate
Written informed consent was obtained from all patients involved in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramos-Fresnedo, A., Domingo, R.A., Perez-Vega, C. et al. The early infiltrative phase of GBM hypothesis: are molecular glioblastomas histological glioblastomas in the making? A preliminary multicenter study. J Neurooncol 158, 497–506 (2022). https://doi.org/10.1007/s11060-022-04040-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04040-5