Abstract
Target population
These recommendations apply to adults with a newly diagnosed lesion with a suspected or histopathologically proven glioblastoma (GBM).
Question
What are the optimal imaging techniques to be used in the management of a suspected glioblastoma (GBM), specifically: which imaging sequences are critical for most accurately identifying or diagnosing a GBM and distinguishing this tumor from other tumor types?
Recommendations
Critical Imaging for the Identification and Diagnosis of Glioblastoma Level II: In patients with a suspected GBM, it is recommended that the minimum magnetic resonance imaging (MRI) exam should be an anatomic exam with both T2 weighted, FLAIR and pre- and post-gadolinium contrast enhanced T1 weighted imaging. The addition of diffusion and perfusion weighted MR imaging can assist in the assessment of suspected GBM for the purposes of distinguishing GBM from other tumor types. Computed tomography (CT) can provide additional information regarding calcification or hemorrhage and also can be useful for subjects who are unable to undergo MR imaging. At a minimum, these anatomic sequences can help identify a lesion as well as its location, and potential for surgical intervention.
Improvement of diagnostic specificity with the addition of non-anatomic (physiologic imaging) to anatomic imaging Level II: One blinded prospective study and a significant number of case series support the addition of diffusion and perfusion weighted MR imaging in the assessment of suspected GBM, for the purposes of distinguishing GBM from other tumor types (e.g., primary CNS lymphoma or metastases).
Level III: It is suggested that magnetic resonance spectroscopy (MRS) and nuclear medicine imaging (PET 18F-FDG and 11C-MET) be used to provide additional support for the diagnosis of GBM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Rationale
Glioblastomas are diffuse, rapidly growing, intra-axial, primary brain tumors that are classified as grade 4 tumors by the World Health Organization (WHO). Accurate diagnosis of these tumors (distinction of GBM from lower grade tumors and from other intrinsic brain tumors including brain metastases and primary central nervous system (CNS) lymphoma) and subsequent appropriate management is critically important. The contribution of imaging to both measures of diagnosis and prognosis is increasingly being recognized.
GBM is typically identified on an anatomic MRI study as an enhancing mass lesion, hypointense on T1 weighted precontrast imaging and hyperintense on T2 and FLAIR imaging sequences. There is often a presence of signal change within the tumor consistent with central necrosis. The relevance of imaging in the accurate diagnosis of GBM predates MR imaging technology, as tumor size, edema, and the presence or absence of contrast enhancement on CT have been recognized for decades as having relevance in predicting the histological classification of primary brain tumors [1, 2]. However, the advent of MR imaging has offered additional imaging sequences that can aid in the preoperative management of patients with suspected GBM. Nuclear medicine techniques have also been increasingly considered as potential markers for diagnosis and prognosis in GBM.
Objectives
The purpose of this guideline is to assess the ability of the most widely used imaging techniques, primarily MRI and PET/radiotracer techniques, to accurately diagnose a GBM (distinguishing this from other tumor types, and from less aggressive primary brain tumors) while simultaneously aiding in the identification of subtypes of tumors for assistance with prognosis and management decisions.
Methods
Writing group and question establishment
The evidence-based clinical practice guideline taskforce members, the Joint Tumor Section of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS) have prioritized an update of the guidelines for management of newly diagnosed glioblastoma (GBM). The writers represent a multi-disciplinary panel of clinical experts encompassing neurosurgery, neuro-oncology, and radiation oncology. Together, they were recruited to develop this update on the evidence-based practice guidelines for newly diagnosed glioblastoma (GBM) in adults. The methodology and findings of the previous guidelines were reviewed, and additional questions were developed to incorporate recent literature addressing practice patterns in the management of GBM patients.
Literature review and eligibility criteria
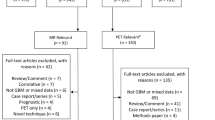
The following databases were searched from January 1st, 2005 thru October 31st, 2018 using glioblastoma and surgery relevant search MeSH and non-MeSH search terms: PubMed (National Library of Medicine, https://www.ncbi.nlm.nih.gov)) using “ABSTRACT/TITLE” and entering “GLIOBLASTOMA” AND “IMAGING” without date limits for a broad initial search. Additional subsequent searches were performed searching “GLIOBLASTOMA” and other more specific imaging based terms including “MRI”/“MAGNETIC RESONANCE IMAGING”, “CT”/“COMPUTED TOMOGRAPHY”, “PET”/“POSITRON EMISSION TOMOGRAPHY”, AND “DIFFUSION”, “PERFUSION”, “SPECTROSCOPY”, “FDG”, “FET”, “MET”, AND “SPECT. The results were then hand searched based on the titles and abstracts to exclude laboratory only studies and titles not on topic. To answer our questions related to prognosis, terms of “DIAGNOSIS” and “PROGNOSIS” were added to the search strategy. Similar search strategies were used to search additional databases including the Cochrane Database of Systematic Reviews, the DARE (Database of Abstracts of Reviews of Effect), and the Cochrane Central Register of Controlled Trials. This overall search strategy yielded a total of 4493 unique citations.
Data collection process
The 4493 citations were manually reviewed by the team with specific inclusion and exclusion criteria as outlined below. Three independent reviewers considered abstracted and/or full text data for each article and the two sets of data were compared for agreement by a third party. Inconsistencies were re-reviewed and disagreements were resolved by consensus. Citations that considered adult patients focusing on imaging in the diagnosis of GBM, prognosis of GBM, or correlation of imaging with molecular markers were considered. We allowed that manuscripts could focus on a comparison of imaging features of GBM with low grade glioma or other tumor types as long as the GBM data could be extracted separately from other tumor information. Abstracts that focused on a pediatric population, therapeutic studies, case reports noting imaging features of unusual tumor types, and articles focusing on brainstem gliomas or spinal cord tumors were not included for review. Those abstracts that met with the selection criteria mentioned above were retrieved in full text form. The adherence to the selection criteria were confirmed. Corresponding full-text PDFs were obtained for all citations meeting the criteria, and reviewed. This manual secondary review resulted in a list of 150 references that appeared best suited to answer the questions. Data was extracted by the first reviewer and verified by another, all of which were compiled into evidence tables. The tables and data were reviewed by all of the authors. Articles not meeting the selection criteria were removed from consideration for inclusion in the evidence tables.
scientific foundation
Classification of evidence and recommendation levels
Both the quality of the evidence and the eventual strength of the recommendations generated by this evidence were graded according to a three-tiered system for assessing studies addressing diagnostic testing as approved by the American Association of Neurological Surgeons (AANS)/ Congress of Neurological Surgeons (CNS) Joint Guidelines Review Committee criteria. Additional information on study classification and recommendation development can be found at https://www.cns.org/guidelines/guideline-procedures-policies/guideline-development-methodology. Imaging studies that considered markers of diagnostic specificity were reviewed using these guidelines, considering a histopathological diagnosis as a “gold standard.”
In order to have class I evidence and/or a level I recommendation regarding imaging, data must be from one or more well-designed clinical studies in a diverse population using a "gold standard" reference test. Well-designed clinical studies should include a blinded evaluation appropriate for the diagnostic applications, sensitivity, specificity, positive and negative predictive values, and where applicable, likelihood ratios. Class II evidence and level II recommendations require that evidence be provided by one or more well-designed clinical studies of a restricted population using a “gold standard” reference test in a blinded evaluation appropriate for the diagnostic applications and enabling the assessment of sensitivity, specificity, positive and negative predictive values, and, where applicable, likelihood ratios. For Class III evidence and/or a Level III recommendation, data is provided by expert opinion or studies that do not meet the criteria for the delineation of sensitivity, specificity, positive and negative predictive values, and, where applicable, likelihood ratios.
Imaging series that consider these same markers with respect to prognosis were reviewed considering five technical criteria. If all five of these criteria are satisfied, the evidence is classified as Class I. If four out of five are satisfied, the evidence is Class II, and if less than 4 are satisfied, it is Class III:
-
Was a well-defined representative sample of patients ( which includes adult patients where those with newly diagnosed glioblastoma can clearly be delineated and assessed within the reported population) assembled at a common (usually early) point in the course of their disease?
-
Was patient follow-up sufficiently long defined as (≥ 1 year) and complete (shorter periods acceptable if follow-up till death described).
-
Were objective outcome criteria applied in a “blinded” fashion?
-
If subgroups with different prognoses were identified, was there adjustment for important prognostic factors?
-
If specific prognostic factors were identified, was there validation in an independent “test set” group of patients?
Study selection and characteristics
Following broad screening for relevance, two independent reviewers evaluated citations and full text screening of potentially relevant papers using a priori criteria for data extraction on a standardized form. Disagreements were resolved with the involvement of a third reviewer, followed by primary re-review until agreement was achieved.
Overall, 27 publications met the eligibility criteria and are included in the evidentiary tables below. These papers were subdivided into publications that answered the specific imaging questions of diagnostic specificity (13), prognosis (8), and methods that suggest correlation between imaging and molecular tumor subtype (6) and are identified specifically in Tables 1, 2, and 3. The details of this evidence are described in detail below.
Specifically, as regards levels of evidence, although numerous series compared imaging markers with histopathology (as a gold standard) to consider diagnostic specificity, none were prospective, continuous series in a large enough (~ 100 +) diverse population (various ages, pathologies, and stages of disease process) to classify the study as class I. In the case when a series was able to show statistically significant data with blinded comparisons, sample size (< 100) was the reason for downgrading. This is not uncommon in imaging studies, where technological limitations still prevent the large sample sizes that would be required to provide class I evidence. Many series provided interesting and useful consideration of imaging biomarkers but did not review data in a blinded fashion, or were not able to provide enough statistical analyses to provide sensitivity, specificity or predictive data and were thus downgraded to class III. In the studies that provided data regarding imaging markers and prognosis, several considered models for multiparametric image analysis and did have a test set for comparison, providing Class II data; in series considering individual imaging markers and clinical prognosis, only class III evidence was achieved, largely because of a lack of a test set for comparison, and again the relatively small sample size in these patient populations.
Assessment for risk of bias
Our search generated a list of abstracts, which were screened, and those articles that addressed our identified questions underwent full independent review by the authors. Reviewers were critical in their assessment, specifically in regard to trial design, such as randomization of treatment, blindedness, prospective character, etc., size of study population, baseline characteristics between study groups, which could account for survivorship bias, selection bias, and appropriate statistical analyses of reported data.
Results of individual studies
Imaging and diagnostic specificity—glioblastoma
Anatomic MR imaging
Most recent imaging studies do not specifically reference the diagnostic specificity of anatomic MR imaging sequences, although historically, studies have considered aspects of anatomic imaging, particularly focusing on the presence or absence of contrast enhancement on CT or MR imaging studies. However, a total of three studies provide evidence for the use of anatomic imaging features in considering GBM diagnostic specificity. Two of these provide Class II evidence and one provides Class III evidence.
The earliest of these studies, by Amundsen et al., described a prospective and blinded assessment of CT with and without contrast in the diagnosis of 46 brain tumors, 15 of which were glioblastoma [1]. 13 of these were diagnosed correctly by imaging without knowledge of histology. Another early study considered CT imaging and brain tumors as the technology was just coming into common practice. Ketonen et al. were able to collect over eighteen hundred cases and review all the diagnoses given retrospectively [2]. Ninety cases were tumors and all but one was confirmed histologically. The authors claim 21 of 22 GBMs were diagnosed correctively suggesting the utility of CT imaging in evaluating malignant primary brain tumors. However, no prospective assessment of these images by blinded observers was performed. While CT imaging is not used routinely in the analysis of patients with GBM, often a CT is the first study acquired in a patient with symptoms suggestive of a tumor, and at times, even today, patients may have implanted technology (pacemaker, stimulator) that precludes obtaining MR imaging studies, so these original studies remain valid.
In subsequent decades, as MR imaging became more common, Dean et al. considered MR imaging features alone in separating astrocytoma from anaplastic astrocytoma and glioblastoma by asking two blinded neuroradiologists to score each imaging features on a 0–2 scale [3]. They concluded that imaging review provides a valuable adjunct to clinical and neuropathologic assessments. The report provides the histologic diagnosis and the results of the blinded interpretation that allow calculation of standard predictive parameters. The authors emphasized that even neuropathology interpretation (at this point the gold standard for diagnostic assessment) is subject to variation due to sampling variation.
These preliminary studies alone established a standard anatomic MR imaging protocol for patients with suspected glioblastoma including sequences that include T1 and T2 weighted imaging sequences and, in particular, acquisition of imaging after the administration of gadolinium based contrast, and prior guidelines for the neuroradiologic assessment of newly diagnosed glioblastoma have established these recommendations [4]. The development of physiologically based MR imaging sequences including diffusion and perfusion weighted imaging (also termed dynamic susceptibility contrast imaging) as well as magnetic resonance spectroscopy, led to investigation of these sequences in the management of patients with brain tumors. In particular, Moller-Hartman et al. retrospectively considered 176 patients with intracranial mass lesions, evaluating the potential utility of MR spectroscopy in these patients [5]. This series included 23 low grade gliomas, 28 anaplastic astrocytomas and 39 glioblastomas. Choline/Creatine and NAA/Creatine ratios were considered. These parameters were not independent predictors of tumor histology, but they did show some degree of correlation with tumor grade. The authors concluded that MRS data assisted the interpreter of anatomic images in providing a correct diagnosis, increasing the rate of correct diagnoses by 15.4% and decreasing the number of incorrect diagnoses by 6.2%. These early studies suggested a potential role for MRS in the evaluation of GBM, although further studies have not yet established a clear role for the technology in the evaluation of suspected GBM.
Perfusion weighted imaging is another useful tool used to evaluate patients with brain tumors. Early studies such as one by Lev et al. prospectively considered perfusion weighted imaging parameters in a series of patients that included 9 low grade gliomas and 13 high grade tumors [6]. The authors considered whether cerebral blood volume, calculated as normal cerebral blood volume (nCBV) could discriminate between high and low grade gliomas and successfully discriminated between high and low grade tumors with a cutoff value of 1.5. Numerous additional studies have considered perfusion weighted imaging, diffusion weighted imaging, and other imaging sequences and their ability to discriminate GBM from lower grade primary brain tumors. These studies were extensively reviewed in the recent guidelines for the management of low grade glioma and are thus not repeatedly outlined here [7].
While there are studies (as noted above) that suggest a potential role for advanced imaging in delineation among primary tumor types, discrimination between low and high grade tumors can be established relatively well with anatomic imaging alone. Clinicians have long recognized, however, that an MRI showing a solitary contrast-enhancing tumor can be a glioblastoma, but also carries a differential diagnosis of metastasis as well as primary CNS lymphoma (PCNSL). Numerous studies over the past decade have established a role for physiologic imaging sequences in diagnosing GBM versus these other tumor types.
A total of seventeen studies specifically considered imaging parameters in distinguishing GBM from brain metastases and met the inclusion criteria for these guidelines. All but one of these studies provides Class III data, and one provided Class II data [8]. Eight studies focused on perfusion weighted imaging which has more recently been referred to as dynamic susceptibility contrast (DSC) imaging. Four studies considered diffusion weighted imaging, 3 utilized DTI imaging methods, and 2 used arterial spin MRI methods. The last 2 studies considered MR spectroscopy and amide proton transfer weighted MRI. Several studies considered more than one imaging method.
Cha et al. considered 43 subjects with a histopathologic diagnosis of GBM (27) or metastasis (18) who underwent DSC perfusion weighted imaging prior to surgery [9]. Peak signal height and average signal recovery offered measures that, in the enhancing tumor and peritumoral region offered a probability that a tumor is not GBM with a specificity of 100% and sensitivity of 69%. Subsequently Hakyemez et al. retrospectively considered 48 subjects with primary brain tumors including 20 GBM and 26 metastases to determine whether DSC imaging could differentiate gliomas from metastases [10]. In these subjects, comparison of relative cerebral blood volume (rCBV) from the peritumoral region offered a statistically significant way to distinguish High Grade Glioma (HGG) from metastasis. Using a cutoff value of 0.46 for the peritumoral region, rCBV measures differentiated high grade gliomas from metastasis with a sensitivity and specificity of 77.3 and 96.2%. While original studies considering physiologic imaging focused on the solid enhancing tumor when placing regions of interest (ROIs), these studies identified signal changes in the peritumoral non enhancing tissue that were different in infiltrating gliomas as compared with solid metastases with a discrete tumor margin [6].
Bette et al. reviewed GBM (n = 165) vs brain metastasis (n = 129) utilizing DTI to determine whether fractional anisotropy would help distinguish between GBM and brain metastasis [11]. They found GBMs to have significantly higher fractional anisotropy values in the contrast enhancing portion of the tumor. Yang et al. also utilized DTI images to create a 3D morphologic analysis of the tumor surface to differentiate between GBM (n = 30) and metastasis (n = 18) [12]. Their method resulted in a 95.8% accuracy rate. Skogen et al. performed texture analysis utilizing TexRAD software on DTI imaging to show this software has an 80% sensitivity and 90% specificity for differentiating between GBM and metastasis [13].
Arterial spin MRI (ASL MRI) is a technique that measures tissue perfusion using a freely diffusible intrinsic tracer, this method offers another way to assess tissue perfusion. ASL MRI was utilized by Sunwoo et al. and Ganbold et al. [14, 15]. Sunwoo et al. reviewed GBM (n = 89) and metastasis (n = 39) and found increased peritumoral perfusion to have the best discriminative power for differentiating between the two pathologies [14]. Ganbold et al. utilized ASL MRI to create a 3D analysis comparing high signal on ASL with contrast enhancing regions [15]. They found GBM to have a significantly higher difference and ratio between high signal on ASL and areas of contrast enhancement in comparison to metastasis.
Yu et al. explored the use of amide proton transfer weighted MRI to establish parameters to analyze the peritumoral zone in order to distinguish between GBM and metastasis [16].
Wang et al. considered DSC measures from the peritumoral region in 67 subjects with enhancing brain tumors [17]. While this study did not provide cutoff values or sensitivity and specificity, it further established the value of rCBV measures beyond the enhancing tumor margin in GBM diagnosis. This study also considered diffusion weighted imaging metrics as well as patients with PCNSL. Server et al. prospectively considered 61 subjects with enhancing tumors (40 GBM, 21 metastases) in a 2011 series that established that DSC measures, specifically rCBV from the peritumoral region could accurately discriminate GBM from metastasis [18]. While the reviewers in the study were not blinded to histology, downgrading this study to Class III, the data identified a cutoff value of 0.80 for the peritumoral region that differentiated GBM from metastases with a sensitivity, specificity, PPV and NPV of 95%, 92%, 86% and 97%, respectively.
Tsougos et al. considered 49 subjects with GBM (35) and brain metastasis (14) prospectively to determine whether DSC imaging was able to discriminate between these two diagnoses [8]. In addition, the reviewers considered diffusion weighted imaging (DWI) parameters as well as MR spectroscopy (MRS) for the same diagnostic distinctions. They found that peritumoral rCBV ratios as well as NAA/Cr, Cho/Cr and Cho/NAA ratios significantly differentiated GBM from metastasis. Apparent diffusion coefficient (ADC) and fractional anisotropy (FA) presented no significant difference between the groups. A cutoff of 1.7 for rCBV had a sensitivity/specificity of 80%/94% for identifying GBM. rCBV values were not corrected for leakage (no contrast preload). Using a cutoff of 1.5 for NAA/Cr the peritumoral ratio had a sensitivity/specificity of 78%/82%. A cutoff of 1.4 for Cho/Cr had a sensitivity/specificity of 89%/62%. A cutoff of 1.1 for Cho/NAA had a sensitivity/specificity of 78%/93%. This study provided class II data supporting the use of DSC and MRS imaging in differentiating GBM from brain metastases.
Lu et al. evaluated dynamic contrast-enhanced MRI to differentiate between GBM, PCNSL and Metastases [19]. Derived values included the volume transfer constant 9Ktrans), the flux rate constant between extravascular extracellular space and plasma (Kep), the extravascular extracellular volume (Ve) and the fractional plasma volume (Vp) using the Tofts model. Mean Ktrans value and Ve value were significantly higher in PCNSLs than in GBMs and MTs. GBM and MT had no significant differences between them. The AUC for Ktrans in PCNSL vs GBM was 0.847 with a sensitivity 81.2% and specificity 79%. Ve AUC was 0.785 with a sensitivity of 81.2% and specificity of 65.8%. The sensitivity and specificity for PCNSL vs MTs was 81.2% and 81% respectively for Ktrans and 87.5% and 81% for Ve.
Further studies confirmed the ability of rCBV to differentiate between GBM and metastasis. Goyal et al. evaluated the most enhancing regions of tumors (18 GBM, 15 lymphoma, 13 mets, 10 anaplastic gliomas) and compared this to co-registered DSC perfusion maps to assess cerebral blood volume (CBV) [20]. CNS lymphomas was found to have significantly lower mean rCBV compared to the other pathologies. Mean rCBV was found to have the best threshold for differentiating these malignant lesions. Neska-Matuszewska et al. looked at additional parameters and agreed that rCBV had the best ability to differentiate tumor type with max core rCBV much lower in PCNSL and max peritumoral rCBV much higher in GBM to distinguish from metastasis [21].
Four other class III studies also considered DWI in distinguishing between brain metastases and GBM. Wang et al. found that in addition to the peritumoral rCBV markers noted above, ADC and FA from the enhancing tumor region of interest differentiated between GBM and non-glioblastoma [17]. Kolakshyapati et al. found that nonenhancing peritumoral DWI is highly predictive of GBM when present [22]. Saini et al. reviewed 100 patients (70 GBM and 30 PCNSL) using DWI, T1 perfusion, and susceptibility-weighted imaging (SWI) [23]. They created a model with 90% sensitivity and 70% specificity in differentiating between GBM and PCNSL.
Byrne et al. also considered DWI in brain metastasis and GBM [24]. 28 patients with histologically proven GBM or metastasis were studied retrospectively and DTI metrics including MD and FA were considered to determine whether tumoral or peritumoral diffusion metrics could aid in diagnosis. When MD and FA from the tumor and peritumoral region were considered together, GBM could be reliably distinguished from metastasis, although no sensitivity or specificity data were calculated and cutoff values were not suggested.
All of these studies together suggest a potential role for DSC, DWI and MRS imaging in considering solitary enhancing tumors when a diagnosis is uncertain. In particular, a preponderance of Class III data suggests that consideration of the peritumoral region with these advanced imaging studies can be useful in yielding a non-invasive diagnosis. No individual study has been validated, however, as a reliable alternative to tissue diagnosis when available.
Not only did Wang et al. consider DSC and DWI for discrimination of GBM from brain metastasis, but they also considered PCNSL [17]. Importantly, in this study, while both DSC and DWI parameters could distinguish GBM from non-GBM (metastasis or PCNSL), neither sequence was, in this study, able to distinguish between metastasis and PCNSL. A total of 8 studies meeting our inclusion criteria more specifically addressed comparison of GBM and PCNSL. In 2012, Okada et al. retrospectively considered 22 patients with an enhancing brain tumor [25]. All patients had undergone dynamic PET studies prior to surgery with 18F-FDG and 11C-MET to determine whether either of these PET tracers could distinguish between GBM and PCNSL. In this study, the SUVmax value for FDG PET and the ΔSUVmax for C-MET PET measurements were useful in distinguishing between GBM and PCNSL. With a cutoff value of 1.17, the ΔSUVmax for 11C-MET distinguished GBM from PCNSL with 100% sensitivity and specificity. Using a cutoff point of 12 for SUVmax for FDG PET, the diagnostic accuracy was 92% sensitivity and 86% specificity. While many clinical centers may not have routine availability for dynamic PET studies and, in particular, 11C-MET tracers, this is a study that does suggest a potential benefit to PET tracers in the preoperative evaluation of patients with suspected glioblastoma. In addition, just as DSC imaging was found to be of value in discriminating GBM from brain metastases, Toh et al. considered DSC parameters in 35 subjects with GBM (20) and PCNSL (15) in a retrospective study [26]. GBM demonstrated significantly higher perfusion parameter values including CBV and CBV with leakage correction when data were acquired with contrast preload and lower K2 compared with PCNSL. A cutoff value of 1.88 for uncorrected CBV ratio (in enhancing tissue) discriminated GBM from PCNSL with a sensitivity and specificity of 100% and 87%, and a cutoff value of 3 for corrected CBV ratio (in enhancing tissue) discriminated GBM from PCNSL with a sensitivity and specificity of 90% and 93.3% respectively. Also using dynamic contrast imaging and DWI MRI, Lu et al. determined that mean ADC provided the best threshold to distinguish primary CNS lymphoma from GBM [27]. A rADCmean threshold of 1.7 indicated PCNSL with a specificity of 78% and sensitivity of 75%.
Choi et al. also utilized dynamic contrast MRI as well as ADC to differentiate between PCNSL and GBM [28]. Initial area under the curve (IAUC) and ADC values were higher in GBM than in PCNSL. By combining p90 of IAUC30 with p10 of ADC, the diagnostic performance was improved to an AUC of 0.886, compared to that of p10 of ADC alone (AUC = 0.744) and p90 of IAUC30 (AUC = 0.789). IAUC is a potentially useful imaging biomarker with added diagnostic value to ADC in the discrimination of PCNSL from atypical GBM.
Ko et al. evaluated ADC measurements in tumor necrosis, the most strongly-enhancing tumor area, and the peritumoral edema [29]. They were able to differentiate between GBM and PCNSL with 90% sensitivity and 86% specificity. Lin et al. also utilized ADC imaging which they coregistered to contrast enhancing regions and FLAIR hyperintense regions [30]. The enhancing region of PCNSL had significantly lower ADC mean, relative ADC mean and relative 90th percentile values for plasma volume. They found that mean ADC provided the best threshold to distinguish PCNSL from GBM. In their study, ADC alone was superior to dynamic contrast-enhanced MR perfusion in differentiating PCNSL from GBM. Xiao et al. performed 3D texture and shape analysis to assess GBM vs PCNSL [31]. Various selected parameters produced sensitivities ranging from 70 to 83.3% and specificities ranging from 71.4 to 90.5%. They found the support vector machine models produced the best discrimination potential between GBM and PCNSL.
Yamashita et al. used IVIM MR imaging data including perfusion fraction and diffusion coefficient to discriminate between GBM and PCNSL [32]. They found that fraction and diffusion coefficients were significantly higher for GBM patients while the standard uptake value with FDG-PET was significantly lower for GBM in comparison to PCNSL. Perfusion fraction max cutoff was 12.4% showing 79.3% sensitivity, 69.2% specificity, and 76.2% accuracy.
While the number of included studies considering GBM and PCNSL are smaller than those considering GBM and metastases, all of these studies, taken together, affirm the potential for physiologic imaging, in particular DWI and DSC sequences, and also potentially MRS and PET studies, in the discrimination of GBM from nonglial enhancing brain tumors.
Synthesis of results: diagnostic specificity
MRI remains a mainstay in the initial evaluation and diagnosis of patients with suspected GBM. A minimum of T2 weighted, FLAIR and pre- and post-gadolinium contrast enhanced T1 weighted imaging is recommended. CT provides baseline information regarding location and may provide additional information such as the presence of calcification or hemorrhage. Recent studies have shown that the addition of diffusion and perfusion weighted MRI can help to distinguish GBM from other tumor types (e.g., primary CNS lymphoma or metastases). Several series offer Class III evidence to support the use of magnetic resonance spectroscopy (MRS), diffusion tensor imaging (DTI) and nuclear medicine modalities (PET 18F-FDG and 11C-MET) to offer additional diagnostic certainty.
Further study into current diagnostic studies have been completed since previous guidelines as well as technological advances in evaluating current imaging. Advances in computer learning and analysis of texture and shape have also started to emerge as a possibility for future use in diagnosis.
Imaging and prognosis—glioblastoma
A total of eight studies met the inclusion criteria suggesting evidence to support the use of imaging in predicting the clinical behavior, or prognosis, for patients with GBM. Only one of these studies was classified as Class II [33]. The others were all downgraded to class III, because of a lack of blinding and a lack of a test and validation data set, with individual studies including Hirai et al. and Shankar et al. additionally downgraded due to small sample size (< 100) and short follow up (< 1 year), respectively [34, 35]. These imaging studies considered a range of imaging data types, including anatomic imaging features, DSC, DWI, and PET markers. In addition, a single 2016 study (the sole class II study) considered modeling methods to integrate multiparametric imaging data for prediction of clinical outcome. These studies suggest that imaging may play a role in prediction of clinical outcome that supplements traditional clinical and histopathological markers.
Pope et al. considered 15 anatomic imaging variables in 153 patients with HGG (n = 43) and GBM (n = 110) to determine whether any of these imaging variables was predictive of clinical outcome [36]. The presence of edema (HR = 1.6), satellite tumors (HR = 1.7) and multifocality (HR = 4.3) associated with shorter survival and the presence of non-contrast enhancing tumor (HR = 0.55) associated with longer survival. The authors identify non-contrast enhancing tumor as discrete from cerebral edema and as a novel, independent predictor of outcome. McGarry et al. also showed that contrast enhancement was significantly indicative of OS [37]. They created radiomic profiles maps looking at 4 areas: (1) within the contrast-enhancing lesion only, (2) within the FLAIR hyperintensity only, (3) within the union of the FLAIR hyperintensity and contrast-enhancing lesion, and (4) within the FLAIR hyperintensity excluding contrast enhancement. Five profiles were found to be predictive of prognosis. Four of the five of these were contrast enhancing and hyperintense on FLAIR imaging. Additional investigators have considered anatomic features and clinical prognosis including Baldock et al., who prospectively considered 243 patients with GBM to determine whether a mathematical model for tumor invasiveness (based upon T1 and T2 weighted imaging) could be predictive of survival [38]. Reviewers were not blinded to survival data. The authors’ mathematical model defines tumors as diffuse or nodular based upon imaging features that define tumor invasiveness. Patients with more diffuse tumors showed no survival benefit even from gross total resection, while those with nodular tumors showed a significant benefit with a median survival benefit of over 8 months compared to subtotally resected tumors in the same cohort. Traditionally, extent of resection has been used as a strong predictor of survival. This novel imaging method suggests that there may be a patient cohort for whom, even if a complete resection of enhancing tumor were possible, it might not provide a survival benefit.
Anatomic imaging features have been increasingly studied. Wangaryattawanich et al. considered 94 patients with GBM [39]. From this data set, univariate analysis suggested 10 imaging features that might be associated with overall survival, and multivariate analysis showed that only tumor enhancing volume was independently predictive of overall survival. On the other hand, Kickingereder et al. found 11 radiographic features to be useful in dividing patients into low- or high-risk for PFS and OS groups [33]. Chaddad et al. and Wangaryattawanich et al. both utilized TCIA (The Cancer Imaging Archive) in order to assess data from multiple institutions [39, 40]. Chaddad et al. utilized TCIA data to compare post-contrast T1W imaging and T2 weighted FLAIR in 40 GBM patients [40]. They defined three GBM phenotypes: necrosis, active tumor and edema/invasion, which were then segmented. A set of shape features were extracted slice wise from each phenotype region and combined to describe the three-dimensional shape of the phenotypes. The survival analysis based on the Kaplan–Meier estimator identified three features derived from necrotic regions (i.e. eccentricity, extent and solidity) that were significantly correlated with overall survival.
Lasocki et al. looked specifically at multifocal and multicentric GBM and found patients with multiple lesions had a nonsignificant worse survival (median 176 days, compared to 346 days p = 0.253) [41]. However, these tumors more frequently involved deep structures (p < 0.001) and the posterior fossa (p = 0.045), both of which were associated with significantly worse survival.
Another study considering measures of tumor volume and clinical prognosis was that of Suchorska et al. In this study, the authors prospectively considered 79 patients with newly diagnosed GBM and evaluated them with 18F-FET PET prior to and after surgical intervention [42]. The authors identified an imaging marker of biological tumor volume (BTV), defined as the region of tissue with evidence of FET uptake. Regions of BTV measured for all subjects prior to radio-chemotherapy (irrespective of extent of surgical resection) were independently predictive of overall survival. Patients with smaller BTV had significantly longer PFS and OS independent of MGMT methylation status and clinical variables. A cutoff volume value of 9.5 cc had a sensitivity and specificity of 64% and 70%, respectively, to predict improved survival in the group with smaller BTV. Median OS (PFS) was 17.5 (8.8) months for patients with smaller BTV versus 10.7 (3.9) months for patients with larger BTV. The authors suggested that using FET PET to identify a biological tumor volume could justify a return to surgery for repeat resection prior to radiochemotherapy if it was felt that residual BTV could be brought below 9.5 cc of residual tumor. Bekaert et43 al utilized PET imaging ([18F]-FMISO PET) and MRI to calculate maximum standardized uptake values and hypoxia volume.[18F]-FMISO PET was closely linked to tumor grade—higher uptake was observed in GBM. The expression of biomarkers for hypoxia and angiogenesis were significantly higher in [18F]-FMISO PET uptake groups. Patients without [18F]-FMSIO uptake had longer survival times.
In contrast with diagnostic and prognostic studies in LGG, where numerous studies have considered the role of DSC imaging, in glioblastoma, only one study met the inclusion criteria for consideration of DSC imaging as a marker of clinical prognosis [7]. Hirai et al. considered a total of 49 subjects with GBM including 31 with GBM, evaluating pretreatment DSC imaging [34]. In patients with GBM, rCBV was independently predictive of clinical outcome. With a cutoff value of 2.3, low rCBV was associated with a 67% 2-year survival rate, while elevated rCBV was associated with a 9% 2-year survival. Perfusion measures were calculated without contrast preloading in this 2008 study. It is important to recognize that more recent studies considering DSC imaging often derive rCBV values after contrast preloading, so for clinicians giving consideration to specific cutoff values, this must be taken into account.
Three studies considered DWI metrics and clinical outcome in GBM. Pope et al. retrospectively analyzed data from 121 patients with GBM to determine whether diffusion markers corresponded to clinical outcome [44]. In this cohort, 59 patients were treated up front with Bevacizumab (Bev) and 62 with standard radiochemotherapy. Imaging analyses were stratified by treatment groups. In the control group there was no association between ADC histogram analyses and clinical outcome. In patients treated with Bev, however, ADC(L) measures corresponded to PFS and OS. The ADC(L) measure was taken from the mean ADC of the lower curve of the histogram, and a cutoff value of 1200 gave a median PFS of 459 days, versus 315 days. Subjects with lower ADC(L) had a longer PFS in patients, though only in the subgroup treated with Bev. The specific method used for diffusion histogram analysis and the unusual subgroup of patients treated up front with Bev makes it more challenging to extrapolate these results to a more general GBM patient population.
Shankar et al. considered another cohort of GBM subjects, identifying 84 patients with newly diagnosed GBM who had preoperative DWI sequences [35]. In this patient population, the median normalized apparent diffusion coefficient (nADC)within the enhancing tumor volume correlated with clinical outcome, measured as survival at 90 days after diagnosis. With a cutoff value of 0.75, patients whose tumors had elevated ADC values had shorter overall survival. The relatively short follow up of this study is a limitation, however the study does suggest a rationale for consideration of nADC as a potential marker for prognosis. Boonzaier et al. performed a retrospective review of 43 patients who underwent conventional, diffusion-weighted, perfusion-weighted, and spectroscopic MRI [45]. Overlapping low-ADC and high rCBV, regions of interest (ROI) were generated using pre- and post-contrast scans. The volume of ADC-rCBV ROI in nonenahncing regions significantly contributed to a multivariant model for progression free (hazard ratio 1.454; P = 0.017) and overall survival (hazard ratio 1.132; P = 0.026). Volumetric analysis of ADC-rCBV ROIs in nonenhancing regions can thus be used to identify patients with poor prognosis after accounting for known confounders of GBM outcome.
Each of these studies suggests a potential utility to both anatomic and advanced imaging methods in stratifying patients with GBM based upon imaging markers with regards to their expected clinical response to treatment. The studies have considered, typically, imaging markers on an individual basis and then performed secondary multivariate analyses. Increasingly, imaging researchers are recognizing the potential value for more complex modeling methods, including application of machine learning techniques to studies of imaging and neuro-oncology. Kickingereder et al. have presented a study considering whether a multiparametric imaging analysis method could establish a radiomic profile that allows prediction of clinical outcome in patients with newly diagnosed GBM [33]. In a series of 119 patients evaluated retrospectively, a total of 12,190 imaging features were extracted from anatomic and physiologic MR imaging sequences and considered, using machine learning techniques, to determine which features were, collectively, most predictive of clinical outcome. While the researchers were not blinded to clinical outcome, patients were divided into a "discovery" cohort and a "validation" cohort, providing a multiparametric imaging marker for clinical prognosis in patients with GBM. Ultimately, 11 imaging features were identified that, together, were predictive of PFS and OS. The performance of this model was further improved when combined with clinical data variables. This class II study is an example of the potential for the application of complex computational analyses to imaging studies in neuro-oncology. This study, combined with the other studies showing potential benefit to anatomic and advanced imaging methods in prognostication for patients with GBM, supports the continued acquisition of these advanced imaging sequences, at least as a part of organized clinical trials. While it may not, yet, be clear how to apply these imaging data to individual clinical cases, there is clear evidence to support a potential benefit to these imaging sequences in the clinical management of patients with GBM in years to come.
Synthesis of results: prognosis
A total of eight studies met the inclusion criteria suggesting evidence to support the use of imaging in predicting the clinical behavior, or prognosis, for patients with glioblastoma. However, only one of these studies was classified as class II [33]. The others were all downgraded to class III because of a lack of blinding and a lack of a test and validation data set, with individual studies including Hirai et al. and Shankar et al. additionally downgraded due to small sample size and short follow up, respectively [34, 35]. These imaging studies considered a range of imaging data types. While they suggest that imaging may play a role in prediction of clinical outcome that supplements traditional clinical and histopathological markers, these imaging modalities are not validated well enough, or in common enough use, to support a practice guideline recommendation regarding imaging and prognosis in primary glioblastoma.
Imaging and molecular tumor markers—glioblastoma
Despite many advances in the understanding of glioblastoma imaging, histopathology, and clinical treatment, the prognosis for patients with this tumor remains dismal. Progress in genetic profiling of these tumors has, however, increased our understanding of the heterogeneous nature of this tumor, even at the molecular level. As both imaging and genetic profiles are recognized to be heterogeneous, even within a single tumor, increasingly researchers are recognizing the potential for correlation of imaging with genomics and molecular tumor behavior [46]. Whether imaging can be used to predict the molecular profile of GBMs is currently under investigation, although there is still insufficient data to recommend their widespread use in clinical practice. A total of fourteen studies met the inclusion criteria for our analysis offering preliminary data suggesting a potential correlation between imaging markers and biological tumor behavior (with correlations made between both individual molecular markers as well as defined molecular subtypes). All of the included studies were class III.
Just as DSC imaging showed relevance in clinical diagnostic specificity for GBM, Jain et al. considered whether addition of rCBV data could further clarify correspondence of molecular tumor subtype with clinical survival [47]. In this series of 50 subjects with GBM taken from the Cancer Genome Atlas (TCGA), rCBV (max) measurements were found to be independently predictive of overall survival. The combination of rCBV data with previously identified genomic data, specifically the Verhaak molecular GBM subtype, further clarified the correspondence of rCBV with overall survival. This early study integrates advanced imaging data analysis and molecular analysis, but does not directly correlate the two.
By contrast, Romano et al. reviewed 47 patients with histologically proven GBM, all of whom had undergone DWI within one week prior to surgery [48]. MGMT promoter methylation status was also identified. ADC measures were correlated with MGMT methylation status as well as clinical outcome. Various ADC measurements were evaluated. Only rADC(min) was found to correlate to molecular subtype (MGMT methylation status) and clinical outcome. A cutoff value of 0.8 for ADC(min) distinguished MGMT methylation status with a sensitivity and specificity of 84% and 91%. Overall survival was 14.8 vs 9.8 months in patients with high versus low ADC(min) values. DWI metrics were also considered by Young et al. in combination with anatomic imaging measures [49]. In 147 consecutive patients with GBM, these imaging measures were correlated with epidermal growth factor receptor (EGFR) amplification status. ADC measurements were statistically significantly different between patients with EGF receptor amplification and those without. Cutoff values and sensitivity/specificity were not provided. Aside from restricted diffusion, no anatomic imaging features corresponded with EGF receptor status. Korfiatis et al. reviewed co-occurrence and run length texture features to predict MGMT methylation status [50]. A support vector machine (SVM) based classifier using four texture features (correlation, energy, entropy and local intensity) originating from T2-weighted images have a sensitivity of 0.803 and a specificity of 0.813 at predicting MGMT methylation status.
In another series, Naeini et al. reviewed MRI studies from 46 patients with histologically confirmed GBMs retrospectively [51]. Anatomic imaging measures including the volume of contrast enhancement and volume of T2/FLAIR signal were measured and correlated to molecular subtypes of GBM. A volume ratio comparing T2 hyperintensity to contrast enhancement was found to be significantly lower in the mesenchymal (MES) subtype of GBM. This could be used to stratify clinical prognosis as well. Using a cutoff ratio of 1.0, the T2/FLAIR: CE ratio had a sensitivity/specificity of 100%/60% for identifying the mesenchymal subtype. With a cutoff value of 2.3, the sensitivity/specificity was 83%/87%.
Macyszyn et al. also considered correlation of imaging markers with molecular GBM subtype in a cohort of 105 subjects with histologically proven GBM [52]. In this series, however, a machine-learning algorithm was employed to evaluate 60 imaging features and demonstrate imaging patterns that would be predictive of molecular subtype and/or clinical prognosis. A machine learning technique was employed to evaluate imaging features from anatomic and physiologic imaging sequences and evaluate a training set and then a test set of patients. This machine-learning model was able to adequately predict survival 80% of the time and identify one of four molecular subtypes with 76% accuracy.
Recognizing that molecular tumor subtypes can be predictive of outcome, Yamashita et al. retrospectively identified 66 Patients with GBM to determine whether MR imaging markers corresponded with the molecular imaging diagnosis of IDH1 wild type (55) or mutant (11) status [32]. Anatomic and physiologic imaging parameters were correlated with histologic subtype. Absolute and relative CBV within the enhancing tumor as well as necrosis volumes were found to be significantly higher in patients with wild type IDH1 compared with those with mutant IDH1. No difference was found in ADC values between the two groups.
Grossman et al. evaluated 141 GBMs assessing MRI volumetric features including necrotic core(NE), contrast enhancement (CE), tumor volume on post-contrast scans (TB), and tumor volume based on edema with T2-FLAIR [53]. They then correlated this to molecular pathways and found NE and TB were enriched with immune response pathways and apoptosis. CE was increased with signal transduction and protein folding. ED was increased for homeostasis and cell cycling. CE was the strongest predictor of overall survival.
Heiland et al. reviewed 20 GBMs to identify correlations between metabolites from proton MR spectroscopy and genetic pathway activity in GBM [54]. The metabolites nNAA, nCr, and nGlx correlate with a specific gene expression pattern reflecting the previously described subtypes of GBM. Also, high nNAA was associated with better clinical prognosis, whereas patients with lower nNAA revealed a shorter progression-free survival (PFS).
Zinn et al. utilized diffusion-weighted sequences as well as post contrast T1WI and FLAIR to define proliferative vs necrotic regions and edema/invasion respectively and correlated this to miRNA expression profiles [55]. Patients with restricted diffusion phenotypes within peritumoral FLAIR regions were found to have distinct gene expression and miRNA profiles compared to patients with facilitated diffusion. These genomic networks were found to be associated with increased cell migration, invasion, chemotaxis and cell movement.
Bosnyak et al. evaluated 21 newly diagnosed GBM patients who underwent presurgical MRI and PET scanning with alpha[C-11]-L-methyl-tryptophan (AMT) [56]. They were assessed for EGFR amplification and MGMT methylation. EGRF amplification was associated with lower T1 postcontrast volume, lower T1 postcontrast/T2 volume ratio and lower T1 postcontrast/PET volume ratio. MGMT methylation correlated to lower metabolic volume on PET and lower tumor/cortex AMP uptake ratios.
Cho et al. evaluated for branched-chain amino acid transaminase 1(BCAT1) levels [57]. Increased BCAT1 has been associated with tumor growth and disease progression. BCAT1 significantly correlated with the mean and 95th percentile normalized cerebral blood volume (nCBV) as well as the mean ADC based on FLAIR images. Normalized CBV from contrast enhanced T1WI also had a significant correlation with BCAT1 levels. Lower levels for BCAT1 correlated to longer progression free survival times. High BCAT1 correlates to high CBV and low ADC as well as poor prognosis.
Kanas et al. used segmentation and registration software to perform quantitative analysis of 86 treatment-naïve GBM [58]. Using machine learning to build multivariate prediction models, the MGMT promoter methylation status was able to be predicted with 73.6% accuracy. The edema/necrosis volume ratio, tumor/necrosis volume ratio, edema ratio, tumor location, and enhancement were all significant variables with respect to MGMT promotor methylation status. Han et al. also evaluated predicting MGMT promotor methylation status and found that increased ADC (p < 0.001) and decreased rCBF (p < 0.001) were associated with MGMT promoter methylation.
Han et al. investigated the value of features from structural and advanced imaging to predict methylation of MGMT promotor status [59]. Significantly increased ADC value (P < 0.001) and decreased rCBF (P < 0.001) were associated with MGMT promoter methylation in primary GBM. The ADC achieved the better predicting efficacy with a sensitivity of 81.1% and specificity of 82.5%. Relative CBF had a sensitivity of 75.0% and specificity of 78.4%. The combination of tumor location, necrosis, ADC and rCBF resulted in the highest AUC of 0.914.
Synthesis of results: molecular markers
The role of various MRI sequences including DWI and perfusion studies have been explored for their ability to predict molecular tumor subtypes. Correlation with MGMT mutation status, EGFR amplification and IDH 1 mutation were explored. This area of focus was not previously discussed in the former guidelines. While these studies are all preliminary and none offer evidence above the class III level, each offers evidence demonstrating correlations between imaging markers and tumor molecular biology. Collectively, the studies support the potential utility for imaging markers in the clinical management of glioblastoma. Further large scale studies will be required to collect enough data in order to extrapolate these methods to clinical practice. Thus at this time no specific recommendations regarding the use of imaging to predict molecule markers can be made. The committee will continue to monitor progress in this area and address this subject in future updates. The integration of machine learning methods for data analysis is almost certain to allow more complex analyses and present relevant imaging markers in the future.
Discussion
The introduction of first CT, and subsequently MR imaging, has transformed the way we diagnose and treat GBM. It has allowed earlier identification of asymptomatic lesions and is critical for pre-surgical diagnosis, intraoperative management, and ultimately follow-up after treatment with surgery, radiation and chemotherapy. Anatomic imaging remains critical to the identification of these tumors, but increasingly, advanced imaging methods, including physiologic imaging, have impacted the way we manage this tumor type.
With respect to anatomic imaging, if a lesion is suspected to be GBM, an MRI that includes anatomic T2, FLAIR and T1 weighted images, along with T1 weighted images acquired after the administration of gadolinium based contrast is necessary to begin to characterize a lesion as a possible GBM. In further evaluation of a suspected GBM, significant class III evidence and one series with class II evidence suggest a benefit to the addition of diffusion and perfusion weighted sequences to aid in discriminating GBM from other tumor types, most commonly primary CNS lymphoma and brain metastases. In the unusual situation where surgical intervention is not recommended, these imaging sequences can be considered as surrogate markers. In preparation for surgery, having diagnostic information that allows clarification of a differential diagnosis can be useful, and in the unusual situation where surgical intervention is not possible, these imaging sequences can support a clinical diagnosis.
Performance of MR spectroscopy and PET imaging has been suggested to have relevance in some preliminary pilot studies, but there is not enough evidence to recommend the inclusion of either in standard diagnostic imaging protocols at this time as further investigation as to best metabolite ratios for consideration as well as best PET tracers and their role need to be further investigated.
With respect to consideration of advanced imaging as a prognostic imaging marker in GBM, the literature has examples of class III evidence that suggest a role for DWI and PWI in distinguishing different classes of GBM in terms of prognosis, but these imaging parameters, at this time, cannot be relied upon in isolation, as markers of prognosis for patients. In settings where PWI and DWI are obtained as a part of a routine tumor imaging protocol, increasingly investigators are considering methods where complex imaging data can be considered mathematically to offer prognostic information that further clarifies histological data. If these sequences are not available preoperatively, this does not, however, impact clinicians’ ability to adequately manage patients with GBM.
Overall conclusions
In conclusion, for patients with a suspected GBM, it is a class II recommendation that the minimum magnetic resonance imaging (MRI) exam should be an anatomic exam with both T2 weighted, FLAIR and pre- and post-gadolinium contrast enhanced T1 weighted imaging. With respect to prognostication, several class III series support the addition of diffusion and perfusion weighted MR imaging in the assessment of suspected GBM, for the purposes of distinguishing GBM from other tumor types (e.g., primary CNS lymphoma or metastases). There is also class III evidence to support magnetic resonance spectroscopy (MRS) and nuclear medicine (PET 18F-FDG and 11C-MET) may provide additional support for the diagnosis of GBM. Likewise, several class III studies have evaluated the use of imaging modalities to predict the molecular profile of GBM. At this time there is still insufficient data to make clear recommendations regarding the role of imaging for the prognostic stratification or prediction of molecular characteristics of tumors.
Limitations and bias
Importantly, with consideration of all of these imaging sequences, differences in methods for image acquisition, processing and analysis limit the ability to synthesize data across studies. Differences in scanner type, in image acquisition protocols, and in analysis methodologies may impact the quantitative measures obtained for different imaging parameters; this may account for some of the differences seen in threshold values, particularly in DWI and PWI measures. Larger, multi-site studies considering these markers as indicators of diagnosis and of prognosis will be beneficial (and are critical) to the standardization and integration of advanced imaging parameters into clinical practice. Many of the included manuscripts were limited by small sample size and retrospective nature of review. We also recognize the risk of selection bias upon initial review of the 4493 articles.
Key issues for future investigation
Ultimately, it is reasonable to consider perfusion and diffusion weighted imaging markers in larger, multi-site studies of prognosis and diagnostic specificity in GBM. Prospective studies considering these sequences in larger populations may lead to series that allow for class I diagnostic or prognostic evidence for this population. Similarly, in these larger series, consideration of multiparametric imaging models as methods to allow diagnostic specificity, prognostication, and correlations with molecular tumor subtype offer the potential for innovative methods for the integration of advanced imaging methods into glioblastoma management. Ultimately, this approach may also be reasonable for PET and MRS parameters, although additional studies still need to be done in smaller cohorts to identify the best PET tracers or specific MRS parameters to be chosen for integration into larger scale GBM trials.
Continued evaluation of imaging characteristics using DSC, DWI and PET are needed to further clarify their role in determining prognosis. Larger multi-center studies would help to delineate this. Certainly the use of computer learning models that are able to perform complex analysis of large data sets will likely advance this area of interest.
Finally, both anatomic and advanced imaging methods are increasingly being considered as investigators evaluate radiogenomic profiling of glioblastomas. While there is only preliminary evidence linking individual and multiparametric imaging markers to molecular GBM subtypes, as both imaging technology and methods for complex data analysis expand, such as computer learning, this will further develop the understanding of the use of imaging to classify GBM molecular subtypes and mutations. The integration of imaging markers as secondary endpoints in larger clinical trials will foster their ultimate translation to standard aspects of the management of patients with GBM.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- FA:
-
Fractional Anisotropy
- GBM:
-
Glioblastoma multiforme
- PCNL:
-
Primary central nervous system lymphoma
- ROI:
-
Region of interest
- DSC:
-
Dynamic susceptibility contrast
- DTI:
-
Diffusion tensor imaging
References
Amundsen P, Dugstad G, Syvertsen AH (1978) The reliability of computer tomography for the diagnosis and differential diagnosis of meningiomas, gliomas, and brain metastases. Acta Neurochir 41(1–3):177–190
Ketonen L (1978) Computerized tomography for diagnosis of supratentorial tumors. Acta Neurol Scand Suppl 67:153–164
Dean BL, Drayer BP, Bird CR et al (1990) Gliomas: classification with MR imaging. Radiology 174(2):411–415
Mukundan S, Holder C, Olson JJ (2008) Neuroradiological assessment of newly diagnosed glioblastoma. J Neurooncol 89(3):259–269
Moller-Hartmann W, Herminghaus S, Krings T et al (2002) Clinical application of proton magnetic resonance spectroscopy in the diagnosis of intracranial mass lesions. Neuroradiology 44(5):371–381
Lev MH, Ozsunar Y, Henson JW et al (2004) Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas [corrected]. AJNR Am J Neuroradiol 25(2):214–221
Fouke SJ, Benzinger T, Gibson D, Ryken TC, Kalkanis SN, Olson JJ (2015) The role of imaging in the management of adults with diffuse low grade glioma: a systematic review and evidence-based clinical practice guideline. J Neurooncol 125(3):457–479
Tsougos I, Svolos P, Kousi E et al (2012) Differentiation of glioblastoma multiforme from metastatic brain tumor using proton magnetic resonance spectroscopy, diffusion and perfusion metrics at 3 T. Cancer Imaging 12:423–436
Cha S, Lupo JM, Chen MH et al (2007) Differentiation of glioblastoma multiforme and single brain metastasis by peak height and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 28(6):1078–1084
Hakyemez B, Erdogan C, Gokalp G, Dusak A, Parlak M (2010) Solitary metastases and high-grade gliomas: radiological differentiation by morphometric analysis and perfusion-weighted MRI. Clin Radiol 65(1):15–20
Bette S, Huber T, Wiestler B, Boeckh-Behrens T, Gempt J, Ringel F, Meyer B, Zimmer C, Kirschke JS (2016) Analysis of fractional anisotropy facilitates differentiation of glioblastoma and brain metastases in a clinical setting. Eur J Radiol 85:2182–2187. https://doi.org/10.1016/j.ejrad.2016.10.002
Yang G, Jones TL, Howe FA, Barrick TR (2016) Morphometric model for discrimination between glioblastoma multiforme and solitary metastasis using three-dimensional shape analysis. Magn Reson Med 75:2505–2516. https://doi.org/10.1002/mrm.25845
Skogen K, Schulz A, Helseth E, Ganeshan B, Dormagen JB, Server A (2018) Texture analysis on diffusion tensor imaging: discriminating glioblastoma from single brain metastasis. Acta Radiol (Stockholm, Sweden: 1987): 284185118780889 https://doi.org/10.1177/0284185118780889
Sunwoo L, Yun TJ, You SH, Yoo RE, Kang KM, Choi SH, Kim JH, Sohn CH, Park SW, Jung C, Park CK (2016) Differentiation of Glioblastoma from brain metastasis: qualitative and quantitative analysis using arterial spin labeling MR imaging. PLoS ONE 11:e0166662. https://doi.org/10.1371/journal.pone.0166662
Ganbold M, Harada M, Khashbat D, Abe T, Kageji T, Nagahiro S (2017) Differences in high-intensity signal volume between arterial spin labeling and contrast-enhanced T1-weighted imaging may be useful for differentiating Glioblastoma from brain metastasis. J Med Invest JMI 64:58–63. https://doi.org/10.2152/jmi.64.58
Yu H, Lou H, Zou T, Wang X, Jiang S, Huang Z, Du Y, Jiang C, Ma L, Zhu J, He W, Rui Q, Zhou J, Wen Z (2017) Applying protein-based amide proton transfer MR imaging to distinguish solitary brain metastases from glioblastoma. Eur Radiol 27:4516–4524. https://doi.org/10.1007/s00330-017-4867-z
Wang S, Kim S, Chawla S et al (2011) Differentiation between glioblastomas, solitary brain metastases, and primary cerebral lymphomas using diffusion tensor and dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol 32(3):507–514
Server A, Orheim TE, Graff BA, Josefsen R, Kumar T, Nakstad PH (2011) Diagnostic examination performance by using microvascular leakage, cerebral blood volume, and blood flow derived from 3-T dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in the differentiation of glioblastoma multiforme and brain metastasis. Neuroradiology 53(5):319–330
Lu S, Gao Q, Yu J, Li Y, Cao P, Shi H, Hong X (2016) Utility of dynamic contrast-enhanced magnetic resonance imaging for differentiating glioblastoma, primary central nervous system lymphoma and brain metastatic tumor. Eur J Radiol 85:1722–1727. https://doi.org/10.1016/j.ejrad.2016.07.005
Goyal P, Kumar Y, Gupta N, Malhotra A, Gupta S, Gupta S, Mangla M, Mangla R (2017) Usefulness of enhancement-perfusion mismatch in differentiation of CNS lymphomas from other enhancing malignant tumors of the brain. Quant Imaging Med Surg 7:511–519. https://doi.org/10.21037/qims.2017.09.03
Neska-Matuszewska M, Bladowska J, Sasiadek M, Zimny A (2018) Differentiation of glioblastoma multiforme, metastases and primary central nervous system lymphomas using multiparametric perfusion and diffusion MR imaging of a tumor core and a peritumoral zone-Searching for a practical approach. PLoS ONE 13:e0191341. https://doi.org/10.1371/journal.pone.0191341
Kolakshyapati M, Adhikari RB, Karlowee V, Takayasu T, Nosaka R, Amatya VJ, Takeshima Y, Akiyama Y, Sugiyama K, Kurisu K, Yamasaki F (2018) Nonenhancing peritumoral hyperintense lesion on diffusion-weighted imaging in glioblastoma: a novel diagnostic and specific prognostic indicator. J Neurosurg 128:667–678. https://doi.org/10.3171/2016.10.jns161694
Saini J, Kumar Gupta P, Awasthi A, Pandey CM, Singh A, Patir R, Ahlawat S, Sadashiva N, Mahadevan A, Kumar Gupta R (2018) Multiparametric imaging-based differentiation of lymphoma and glioblastoma: using T1-perfusion, diffusion, and susceptibility-weighted MRI. Clin Radiol 73:986.e987–986.e915. https://doi.org/10.1016/j.crad.2018.07.107
Byrnes TJ, Barrick TR, Bell BA, Clark CA (2011) Diffusion tensor imaging discriminates between glioblastoma and cerebral metastases in vivo. NMR Biomed 24(1):54–60
Okada Y, Nihashi T, Fujii M et al (2012) Differentiation of newly diagnosed glioblastoma multiforme and intracranial diffuse large B-cell Lymphoma using (11)C-methionine and (18)F-FDG PET. Clin Nucl Med 37(9):843–849
Toh CH, Wei KC, Chang CN, Ng SH, Wong HF (2013) Differentiation of primary central nervous system lymphomas and glioblastomas: comparisons of diagnostic performance of dynamic susceptibility contrast-enhanced perfusion MR imaging without and with contrast-leakage correction. AJNR Am J Neuroradiol 34(6):1145–1149
Lu S, Wang S, Gao Q, Zhou M, Li Y, Cao P, Hong X, Shi H (2017) Quantitative evaluation of diffusion and dynamic contrast-enhanced magnetic resonance imaging for differentiation between primary central nervous system Lymphoma and Glioblastoma. J Comput Assist Tomogr 41:898–903. https://doi.org/10.1097/rct.0000000000000622
Choi YS, Lee HJ, Ahn SS, Chang JH, Kang SG, Kim EH, Kim SH, Lee SK (2017) Primary central nervous system lymphoma and atypical glioblastoma: differentiation using the initial area under the curve derived from dynamic contrast-enhanced MR and the apparent diffusion coefficient. Eur Radiol 27:1344–1351. https://doi.org/10.1007/s00330-016-4484-2
Ko CC, Tai MH, Li CF, Chen TY, Chen JH, Shu G, Kuo YT, Lee YC (2016) Differentiation between Glioblastoma multiforme and primary cerebral Lymphoma: additional benefits of quantitative diffusion-weighted MR Imaging. PLoS ONE 11:e0162565. https://doi.org/10.1371/journal.pone.0162565
Lin X, Lee M, Buck O, Woo KM, Zhang Z, Hatzoglou V, Omuro A, Arevalo-Perez J, Thomas AA, Huse J, Peck K, Holodny AI, Young RJ (2017) Diagnostic accuracy of T1-weighted dynamic contrast-enhanced-MRI and DWI-ADC for differentiation of Glioblastoma and primary CNS Lymphoma. AJNR Am J Neuroradiol 38:485–491. https://doi.org/10.3174/ajnr.A5023
Xiao DD, Yan PF, Wang YX, Osman MS, Zhao HY (2018) Glioblastoma and primary central nervous system lymphoma: preoperative differentiation by using MRI-based 3D texture analysis. Clin Neurol Neurosurg 173:84–90. https://doi.org/10.1016/j.clineuro.2018.08.004
Yamashita K, Hiwatashi A, Togao O, Kikuchi K, Kitamura Y, Mizoguchi M, Yoshimoto K, Kuga D, Suzuki SO, Baba S, Isoda T, Iwaki T, Iihara K, Honda H (2016) Diagnostic utility of intravoxel incoherent motion mr imaging in differentiating primary central nervous system lymphoma from glioblastoma multiforme. J Magn Reson Imaging JMRI 44:1256–1261. https://doi.org/10.1002/jmri.25261
Kickingereder P, Bonekamp D, Nowosielski M, Kratz A, Sill M, Burth S, Wick A, Eidel O, Schlemmer HP, Radbruch A, Debus J, Herold-Mende C, Unterberg A, Jones D, Pfister S, Wick W, von Deimling A, Bendszus M, Capper D (2016) Radiogenomics of Glioblastoma: machine learning-based classification of molecular characteristics by using multiparametric and multiregional MR imaging features. Radiology 281:907–918. https://doi.org/10.1148/radiol.2016161382
Hirai T, Murakami R, Nakamura H et al (2008) Prognostic value of perfusion MR imaging of high-grade astrocytomas: long-term follow-up study. AJNR Am J Neuroradiol 29(8):1505–1510
Shankar JJ, Bata A, Ritchie K, Hebb A, Walling S (2016) Normalized apparent diffusion coefficient in the prognostication of patients with Glioblastoma multiforme. Can J Neurol Sci 43(1):127–133
Pope WB, Sayre J, Perlina A, Villablanca JP, Mischel PS, Cloughesy TF (2005) MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol 26(10):2466–2474
McGarry SD, Hurrell SL, Kaczmarowski AL, Cochran EJ, Connelly J, Rand SD, Schmainda KM, LaViolette PS (2016) Magnetic resonance imaging-based radiomic profiles predict patient prognosis in newly diagnosed Glioblastoma before therapy. Tomography (Ann Arbor, Mich) 2:223–228. https://doi.org/10.18383/j.tom.2016.00250
Baldock AL, Ahn S, Rockne R et al (2014) Patient-specific metrics of invasiveness reveal significant prognostic benefit of resection in a predictable subset of gliomas. PLoS ONE 9(10):e99057
Wangaryattawanich P, Hatami M, Wang J et al (2015) Multicenter imaging outcomes study of The Cancer Genome Atlas glioblastoma patient cohort: imaging predictors of overall and progression-free survival. NeuroOncology 17(11):1525–1537
Chaddad A, Desrosiers C, Hassan L, Tanougast C (2016) A quantitative study of shape descriptors from glioblastoma multiforme phenotypes for predicting survival outcome. Br J Radiol 89:20160575. https://doi.org/10.1259/bjr.20160575
Lasocki A, Gaillard F, Tacey M, Drummond K, Stuckey S (2016) Multifocal and multicentric glioblastoma: improved characterisation with FLAIR imaging and prognostic implications. J Clin Neurosci 31:92–98. https://doi.org/10.1016/j.jocn.2016.02.022
Suchorska B, Jansen NL, Linn J et al (2015) Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology 84(7):710–719
Bekaert L, Valable S, Lechapt-Zalcman E, Ponte K, Collet S, Constans JM, Levallet G, Bordji K, Petit E, Branger P, Emery E, Manrique A, Barre L, Bernaudin M, Guillamo JS (2017) [18F]-FMISO PET study of hypoxia in gliomas before surgery: correlation with molecular markers of hypoxia and angiogenesis. Eur J Nucl Med Mol Imaging 44:1383–1392. https://doi.org/10.1007/s00259-017-3677-5
Pope WB, Lai A, Mehta R et al (2011) Apparent diffusion coefficient histogram analysis stratifies progression-free survival in newly diagnosed bevacizumab-treated glioblastoma. AJNR Am J Neuroradiol 32(5):882–889
Boonzaier NR, Larkin TJ, Matys T, van der Hoorn A, Yan JL, Price SJ (2017) Multiparametric MR imaging of diffusion and perfusion in contrast-enhancing and nonenhancing components in patients with glioblastoma. Radiology 284:180–190. https://doi.org/10.1148/radiol.2017160150
Moton S, Elbanan M, Zinn PO, Colen RR (2015) Imaging genomics of Glioblastoma: biology, biomarkers, and breakthroughs. Top Magn Reson Imaging 24(3):155–163
Jain R, Poisson L, Narang J et al (2012) Genomic mapping and survival prediction in glioblastoma: molecular subclassification strengthened by hemodynamic imaging biomarkers. Radiology 267(1):212–220
Romano A, Calabria LF, Tavanti F et al (2013) Apparent diffusion coefficient obtained by magnetic resonance imaging as a prognostic marker in glioblastomas: correlation with MGMT promoter methylation status. Eur Radiol 23(2):513–520
Young RJ, Gupta A, Shah AD et al (2013) Potential role of preoperative conventional MRI including diffusion measurements in assessing epidermal growth factor receptor gene amplification status in patients with glioblastoma. AJNR Am J Neuroradiol 34(12):2271–2277
Korfiatis P, Kline TL, Coufalova L, Lachance DH, Parney IF, Carter RE, Buckner JC, Erickson BJ (2016) MRI texture features as biomarkers to predict MGMT methylation status in glioblastomas. Med Phys 43:2835–2844. https://doi.org/10.1118/1.4948668
Naeini KM, Pope WB, Cloughesy TF et al (2013) Identifying the mesenchymal molecular subtype of glioblastoma using quantitative volumetric analysis of anatomic magnetic resonance images. NeuroOncology 15(5):626–634
Macyszyn L, Akbari H, Pisapia JM et al (2016) Imaging patterns predict patient survival and molecular subtype in glioblastoma via machine learning techniques. NeuroOncology 18(3):417–425
Grossmann P, Gutman DA, Dunn WD Jr, Holder CA, Aerts HJ (2016) Imaging-genomics reveals driving pathways of MRI derived volumetric tumor phenotype features in Glioblastoma. BMC Cancer 16:611. https://doi.org/10.1186/s12885-016-2659-5
Heiland DH, Mader I, Schlosser P, Pfeifer D, Carro MS, Lange T, Schwarzwald R, Vasilikos I, Urbach H, Weyerbrock A (2016) Integrative network-based analysis of magnetic resonance spectroscopy and genome wide expression in glioblastoma multiforme. Sci Rep 6:29052. https://doi.org/10.1038/srep29052
Zinn PO, Hatami M, Youssef E, Thomas GA, Luedi MM, Singh SK, Colen RR (2016) Diffusion weighted magnetic resonance imaging radiophenotypes and associated molecular pathways in glioblastoma. Neurosurgery 63(Suppl 1):127–135. https://doi.org/10.1227/neu.0000000000001302
Bosnyak E, Michelhaugh SK, Klinger NV, Kamson DO, Barger GR, Mittal S, Juhasz C (2017) Prognostic molecular and imaging biomarkers in primary glioblastoma. Clin Nucl Med 42:341–347. https://doi.org/10.1097/rlu.0000000000001577
Cho HR, Jeon H, Park CK, Park SH, Kang KM, Choi SH (2017) BCAT1 is a new MR imaging-related biomarker for prognosis prediction in IDH1-wildtype glioblastoma patients. Sci Rep 7:17740. https://doi.org/10.1038/s41598-017-17062-1
Kanas VG, Zacharaki EI, Thomas GA, Zinn PO, Megalooikonomou V, Colen RR (2017) Learning MRI-based classification models for MGMT methylation status prediction in glioblastoma. Comput Methods Programs Biomed 140:249–257. https://doi.org/10.1016/j.cmpb.2016.12.018
Han Y, Yan LF, Wang XB, Sun YZ, Zhang X, Liu ZC, Nan HY, Hu YC, Yang Y, Zhang J, Yu Y, Sun Q, Tian Q, Hu B, Xiao G, Wang W, Cui GB (2018) Structural and advanced imaging in predicting MGMT promoter methylation of primary glioblastoma: a region of interest based analysis. BMC Cancer 18:215. https://doi.org/10.1186/s12885-018-4114-2
Acknowledgements
The authors would like to acknowledge the CNS Guidelines Committee for their contributions and the AANS/CNS Joint Guidelines Review Committee for their review, comments and suggestions, as well as Kirsten Aquino, JGRC Consultant Administrator, for her assistance. We also acknowledge the following individual peer reviewers their contributions to the development process: John O’Toole, MD (lead reviewer), David Bauer, MD, Kimon Bekelis, MD, Andrew Carlson, Isabelle Germano, MD, MD, Catherine McClung Smith, MD, Jonathan Sherman, MD.
Funding
These guidelines were funded exclusively by the CNS Guidelines Committee and the AANS/CNS Joint Tumor Section Executive Committee with no funding from any outside commercial sources to support the development of this document. Dr. Ormond has research funding from Agios, Medtronic, American Heart Association and the American Cancer Society. Dr. Olson is an editorial consultant for the American Cancer Society. There are no other disclosures to report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Update on Newly Diagnosed Glioblastoma Task Force members were required to report all possible COIs prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair are given latitude to approve nominations of Task Force Members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The authors declare that they have no conflict of interest. This research did not involve human or animal subjects and thus no informed consent was required.
Disclaimer of liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sponsored by the American Association of Neurological Surgeons and Congress of Neurological Surgeons Joint Section on Tumors.
Reviewed for evidence-based integrity and endorsed by the American Association of Neurological Surgeons and Congress of Neurological Surgeons.
Rights and permissions
About this article
Cite this article
Lundy, P., Domino, J., Ryken, T. et al. The role of imaging for the management of newly diagnosed glioblastoma in adults: a systematic review and evidence-based clinical practice guideline update. J Neurooncol 150, 95–120 (2020). https://doi.org/10.1007/s11060-020-03597-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03597-3