Abstract
Introduction
Temozolomide (TMZ) is a life prolonging DNA alkylating agent active against glioblastomas (GBM) in which the O6-methylguanine-DNA methyltransferase (MGMT) gene is silenced by promoter methylation. Unfortunately acquired TMZ resistance severely undermines its clinical efficacy. Using an in vitro model, we tested whether poly (ADP-ribose) polymerase-1 and -2 (PARP) inhibition could suppress the emergence of resistance to enhance the effectiveness of TMZ.
Methods
Using the MGMT-methylated GBM line U251N, in which TMZ resistance can be induced, we developed a method to rapidly recreate mechanisms of TMZ resistance seen in GBMs, including MMR mutations and MGMT re-expression. We then assessed whether TMZ resistant U251N sub-clones could be re-sensitized to TMZ by co-treatment with the PARP inhibitor ABT-888, and also whether the emergence of resistance could be suppressed by PARP inhibition.
Results
U251N cultures chronically exposed to TMZ developed discrete colonies that expanded during TMZ treatment. These colonies were isolated, expanded further as sub-clones, and assessed for mechanisms of TMZ resistance. Most resistant sub-clones had detectable mutations in one or more mismatch repair (MMR) genes, frequently MSH6, and displayed infrequent re-expression of MGMT. TMZ resistance was associated with isolated poly(ADP-ribose) (pADPr) up-regulation in one sub-clone and was unexplained in several others. TMZ resistant sub-clones regressed during co-treatment with TMZ and ABT-888, and early co-treatment of U251N parental cultures suppressed the emergence of TMZ resistant colonies.
Conclusion
In a model of acquired resistance, co-treatment with TMZ and a PARP inhibitor had two important benefits: re-sensitization of TMZ resistant cells and suppression of TMZ resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since its discovery 25 years ago, Temozolomide (TMZ) has been the chemotherapy of choice for Glioblastoma (GBM). The unequivocal life-prolonging benefits of TMZ for GBM patients are enhanced by its favourable safety profile and oral bioavailability [1,2,3]. Unfortunately, nearly all TMZ responsive GBMs become resistant to TMZ through prolonged drug exposure [4]. The most common mechanism of acquired resistance is loss of DNA mismatch repair (MMR), either by gene mutation or loss of protein expression. Indeed, Johnson et al. showed that half of recurrent low-grade gliomas exposed to TMZ developed one or more MMR mutations in MSH6, MSH2, MSH3, MLH1, PMS1, or MLH3 [5], while others have shown similarly high rates of mutation (up to 60%) after TMZ exposure, predominantly of MSH6 [6,7,8,9]. TMZ resistance and recurrence of GBM have also been associated with loss of expression of MSH6, MSH2, MLH1 and PMS2. By comparing 43 newly diagnosed GBMs with their matched recurrences, Felsberg et al. observed reductions in MSH6, MSH2, or PMS2 protein expression in over 50% of tumours compared to their pre-TMZ treated counterparts, and reductions in MLH1 expression were seen in 33% [10]. Other glioma studies have also shown TMZ resistance is associated with loss of MMR proteins [11,12,13]. Although less common than MMR loss, resistance has also been associated with O6-methylguanine-DNA methyltransferase (MGMT) re-expression [14, 15] and with intact base excision repair (BER); the latter of which corrects secondary cytotoxic N7-methylguanine (N7-MeG) and N3-methyladenine (N3-MeA) DNA adducts induced by TMZ [16, 17].
One strategy to overcome TMZ resistance involves blocking BER through inhibition of poly (ADP) ribose polymerase-1 and -2 (collectively referred to as ‘PARP’). Indeed, we demonstrated that co-treatment with the PARP-inhibitor ABT-888 restored TMZ sensitivity in brain tumor initiating cells (BTICs) and xenografts that had acquired resistance through TMZ exposure; re-sensitization by PARP inhibition was seen in the setting of MSH6 mutation and MGMT expression [18], an observation corroborated recently by Higuchi et al. [19]. To further explore the role of PARP inhibition in the treatment of GBM and its potential to mitigate acquired TMZ resistance, we sought a pre-clinical model for rapid drug screening, and turned to a published in vitro system in which mutations of MSH6 occurred in TMZ treated GBM cells [8]. Herein, using this model we show that persistent exposure of U251N cells to TMZ promotes the emergence of resistant sub-clones which harbour the exact mechanisms of resistance seen in recurrent GBMs. We then demonstrate that PARP inhibition restores TMZ sensitivity to resistant colonies, and reveal for the first time that inhibition suppresses the emergence of acquired resistance. Finally, we show that prolonged exposure to TMZ may undermine the beneficial effect of PARP inhibition.
Results
A clinically relevant model of acquired TMZ resistance using MGMT-methylated U251N cells
U251N cultures were expanded in flasks and treated with TMZ for 3 weeks using the TMZ-resistance protocol described by Yip et al. [8]. Within 14 days widespread cell death and the emergence of discrete cell colonies was observed. These colonies had become resistant to TMZ and continued expanding in the face of continuous TMZ exposure (Fig. 1). To investigate the biological basis of TMZ resistance 24 colonies were isolated from the TMZ-treated parental culture and expanded in media containing TMZ to establish 18 independent TMZ-resistant sub-clones.
Chronic TMZ exposure in the MGMT-methylated GBM cell line U251N leads to Temozolomide-resistant cell colonies that harbor known mechanisms of TMZ resistance. Bright-field photomicrographs (2 × magnification) showing the emergence of drug-resistant colonies in a U251N culture treated with TMZ (100 µM) for 3 weeks. No colonies emerged in a parallel culture treated with TMZ delivery vehicle. Arrows denote the number of TMZ doses administered. “P” indicates that the culture was passaged at a 1:4 dilution between photomicrographs
Western blotting on these sub-clones was performed to assess expression of MMR proteins. Sub-clones from colonies 6, 9, 12, 13, 20, 23 and 24 had a decrease in MSH6 expression ranging from 70–93%, while those from 1, 2, 16 and 18 had no detectable expression (Fig. S1A). Sub-clones from colonies 2, 6, 9, 12, 16 and 20 had a 49–64% reduction in MSH2 levels, while sub-clones from 1 and 18 had no detectable protein (Fig. S1B). All sub-clones retained levels of expression of MLH1 similar to the parental line (Fig. S1C), while sub-clones from colonies 2 and 23 had a 48–68% decrease in PMS2 (Fig. S1D). MGMT expression was assessed in all sub-clones; only one displayed MGMT re-expression (Fig. S1E).
Based on patterns of protein expression we then selected a subset of TMZ-resistant sub-clones to assess the mutational status of MMR genes frequently mutated in TMZ-treated, recurrent GBMs (Table S1) [5,6,7,8,9]. As seen clinically, MSH6 was the most commonly mutated gene: sub-clones from colonies 6, 7, 9 and 12 displayed de novo missense mutations in MSH6, while sub-clones 6 and 9 had multiple mutations in MSH6. The mutation in sub-clone 9 (amino acid residue 864) has been reported in recurrent methylated GBM [20]. Mutations in MLH1 were found in sub-clones 12 and 15. A deletion of exon 1 of MSH2 was found in sub-clone 1. Most mutations were guanine-to-adenine transition-type nucleotide substitutions often observed in the hyper-mutation phenotype exhibited by TMZ treated GBMs [21]. For all missense mutations, both the mutant and wild type nucleotides were detected suggesting allelic heterozygosity or multiple populations of cells within each sub-clone (Table. S2). All sub-clones harboured a silent mutation and two single-nucleotide polymorphisms (SNPs) in PMS2 (Table S1), an alteration common in the general population and not associated with cancer susceptibility or other known pathologies [22, 23].
Concomitant PARP inhibition restores TMZ sensitivity in emerging U251N resistant colonies
Previously we observed that brain tumour initiating cell (BTIC) cultures derived from TMZ-treated patients could be re-sensitized to TMZ by co-treatment with a PARP inhibitor [18]. We revisited this observation in the U251N model system using colonies which had acquired resistance to TMZ. First, we induced resistant colonies in U251N parental cultures as described above. When colonies emerged, cultures were exposed to TMZ alone (100 µM) or TMZ plus ABT-888 (10 µM). TMZ-treated colonies continued to expand (Fig. 2A), whereas co-treated colonies regressed (Fig. 2B). Over the ensuing 3 weeks, co-treated colonies did not resume expanding despite being maintained in in TMZ-free media. These observations reveal PARP inhibition restores sensitivity to TMZ and can have a sustained effect.
Co-treatment with TMZ and ABT-888 restores sensitivity to TMZ-resistant U251N cells. Bright-field photomicrographs (2 × magnification) showing the regression of emerging U251N drug-resistant colonies following the addition of ABT-888 (10 µM) to TMZ (100 µM) (B). In contrast, colonies continued to grow when TMZ monotherapy was continued (A). Arrows indicate the number of drug doses administered
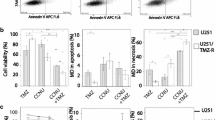
To document extent of cell death Annexin V staining was performed five days following treatment on three sub-clones derived from resistant colonies 1, 5 and 15 (Fig. 3). Lower drug concentrations produced variable responses with sub-line 15 having the greatest sensitivity to co-treatment: 10 µM TMZ induced significant apoptosis (p < 0.01) when combined with 10 µM ABT-888, and as little as 1 µM ABT-888 induced apoptosis in a significant proportion of cells (p < 0.05) when combined with 100 µM TMZ. At higher concentrations of TMZ (100 µM) and ABT-888 (10 µM) apoptosis was induced in 54–61% of resistant cells in all sub-clones. These results suggest that apoptosis is the mechanism of cell death in TMZ resistant cells that have responded to the combination of TMZ and ABT-888, and that the degree of apoptosis is dose and sub-clone dependent.
Assessment of cell death in ABT-888 and TMZ treated cells. Annexin V-FITC and PI analysis by flow cytometry suggests that apoptosis was induced by co-treatment with TMZ (1, 10 or 100 µM) and ABT-888 (0.1, 1 or 10 µM) in TMZ-resistant sub-clones. Control samples were treated with drug delivery vehicles (Veh), ABT-888 alone or TMZ alone. The One-Way ANOVA and Tukey multiple comparisons tests were applied to assess differences (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). *Comparisons between the proportion of early apoptotic cells. #Comparisons between the proportion of viable cells
Concomitant PARP inhibition and TMZ treatment suppress the emergence of resistant colonies
Having shown that co-treatment with ABT-888 was cytotoxic to TMZ resistant cells, we then asked if the emergence of TMZ resistance could be prevented. To test this possibility, we assessed parental U251N cultures during exposure to TMZ (100 µM) alone or co-treated with TMZ and ABT-888 (10 µM). TMZ resistant colonies emerged as expected in the cultures exposed to TMZ alone. However, TMZ resistant colonies did not appear in the cultures co-treated with ABT-888; these cultures displayed extensive cell death (Fig. 3). This effect of co-treatment was sustained; U251N parental cultures did not recover from co-treatment over a 3-week period of post-treatment while maintained in TMZ-free media. The suppression of the formation of resistant colonies suggests that PARP inhibition either enhances the cytotoxic effect of TMZ, or selectively prunes resistant cells when they first arise in parental U251N cultures.
MMR mutant and MGMT expressing resistant sub-clones are sensitive to co-treatment
Next, we asked whether benefit from co-treatment was seen in both MMR-mutant and MGMT-expressing sub-clones. Co-treatment of a sub-clone harbouring a deletion in exon 1 of MSH2 with 10 µM ABT-888 led to a decrease in cell viability ranging from 78% in cultures treated with 1 µM TMZ (p < 0.0001) to 97% in cultures treated with 100 µM TMZ (p < 0.0001; Fig. 4A). Concomitant treatment with 1 µM ABT-888 and 100 µM TMZ resulted in a 94% decrease in viability (p < 0.0001). Co-treatment of the sub-line re-expressing MGMT with ABT-888 led to a significant decrease in culture viability; a dose of 10 µM ABT-888 led to a decrease in viability that ranged from 60% in the presence of 1 µM TMZ (p < 0.0001) to 97% when treated with 100 µM TMZ (p < 0.0001; Fig. 4B), and co-treatment with as little as 1 µM ABT-888 lead to a 96% decrease in viability in the presence of 100 µM TMZ (p < 0.0001). These results demonstrate that co-treatment can be effective in both MMR-mutant and MGMT expressing resistant GBM cells. We have made similar observations in TMZ resistant BTICs derived from recurrent GBMs [18].
Co-treatment with TMZ and ABT-888 prevents the emergence of TMZ-resistant U251N cells and is independent of MMR mutations or MGMT re-expression. Bright-field photomicrographs (2 × magnification) showing that co-treatment with TMZ (100 µM) and ABT-888 (10 µM) suppresses the emergence of drug-resistant colonies and causes widespread cell death in a parental U251N culture. In contrast, resistant colonies emerged in a parallel culture treated with TMZ alone. Treatment with ABT-888 alone or drug delivery vehicles did not affect U251N growth. Arrows indicate the number of drug doses administered. “P \(\times\) #” indicates that cultures were passaged the indicated number of times at a 1:4 dilution between photomicrographs (A). Average viability (n = 3, ± SD) inferred using the Alamarblue assay of an MMR-mutant (B) and an MGMT re-expressing (C) U251N subline on Day 10 of treatment with TMZ (1, 10 or 100 µM), ABT-888 (0.1, 1 or 10 µM) or the combination of TMZ and ABT-888. Both MMR-mutant and MGMT re-expressing cells displayed sensitivity to various combinations of TMZ and ABT-888. Control cultures were treated with drug-delivery vehicles. The One-Way ANOVA and Tukey multiple comparisons tests were applied to assess differences (****p < 0.0001)
Early co-treatment with a PARP inhibitor may be an important strategy
Although all resistant sub-clones responded favourably to co-treatment, this beneficial effect became progressively less apparent for the sub-line from colony 5. After 80 days in TMZ-containing media, sensitivity to 10 µM TMZ was restored by co-treatment with 10 µM ABT-888; compared to TMZ alone, there was an 81% decrease in viability with co-treatment (p < 0.0001; Fig. 5A). This effect waned after extended culturing, however. After 160 days in TMZ-containing media this sub-line became less sensitive to co-treatment; 10 µM ABT-888 with 10 µM TMZ was less cytotoxic (22%, p < 0.0001; Fig. 5B). The extent of re-sensitization declined further after sub-line 5 had been in TMZ for 180 days. At this point, no re-sensitization by ABT-888 was detected except at the highest doses of both drugs; a significant decrease in cell viability was only seen when 100 µM TMZ was combined with 10 µM ABT-888 (97%; p < 0.0001; Fig. 5C). Potential mechanisms for loss of sensitization in sub-line 5 were then considered further. Sub-line 5 did not have a mutation in MSH6, MSH2, MLH1, or PMS2, and did not re-express MGMT. However, sub-line 5 expressed a high level of the poly(ADP-ribose) (pADPr) protein, the enzymatic by-product of activated PARP, compared to parental U251N cells (Fig. 5D). Taken together, these additional findings raise the possibility that prolonged TMZ exposure may diminish the beneficial effect of co-treatment with a PARP inhibitor.
Prolonged exposure to TMZ is associated with increased resistance to the co-treatment strategy and may be explained by pADPr upregulation in U251N sub-line 5. Average viability (n = 3 \(\pm\) SD), inferred by the Alamarblue assay, of sub-line 5 on Day 10 of co-treatment with TMZ (1, 10 or 100 µM) and ABT-888 (0.1, 1 or 10 µM) following 80 (A), 160 (B) or 180 (C) days of pre-exposure to TMZ. Greater pre-exposure to TMZ was observed with diminished sensitivity to lower doses of TMZ and ABT-888. Assessments and statistical comparisons were performed as described in Fig. 4 (****p < 0.0001). Western blot analysis reveals that sub-line 5 demonstrated induction of pADPr expression, the enzymatic by-product of activated PARP (D). “+” denotes the positive control. “P” denotes the parental U251N sample
Discussion
TMZ is an effective chemotherapy for GBM but acquired resistance compromises its effectiveness. Here, we tested the hypothesis that acquired resistance to TMZ could be prevented or delayed by co-treatment with a PARP inhibitor using a model first described by Yip et al. [8] in which resistance to TMZ and mutations of MSH6 could be induced in the MGMT-methylated, TMZ sensitive cell line, U251N. In our hands, exposure of U251N cultures to TMZ resulted in the emergence of discrete sub-clones which continued to expand despite continuous treatment with TMZ. Furthermore, TMZ-resistant sub-clones had a spectrum and frequency of mutations in DNA repair genes identical to those found in recurrent GBMs. Indeed, similar to TMZ refractory recurrent GBMs, more than half of the sub-clones displayed loss of expression in at least one of MSH6, MSH2, or PMS2, all of which are crucial for the initiation of MMR signalling in TMZ-induced apoptosis [10,11,12,13]. Additionally, six of nine sequenced colonies had one or more mutations in MSH6, MSH2, or MLH1 [5,6,7,8,9], with alterations consisting of guanine to adenine transition-type nucleotide substitutions, typical of post-TMZ recurrent GBMs [21]. Moreover, we observed MGMT re-expression, another mechanism of TMZ resistance in GBMs [14, 15]. Together these findings spoke to the authenticity of the U251N model system of inducible TMZ resistance.
Armed with a clinically relevant model of acquired TMZ resistance, we asked whether inhibition of PARP during treatment with TMZ had the potential to suppress the emergence of TMZ resistance. Indeed, we observed that while treatment of U251N with TMZ led to the rapid appearance of drug-resistant colonies, co-treatment with ABT-888 suppressed their emergence. Further, ABT-888 co-treatment led to the regression of expanding resistant colonies, suggesting ABT-888 may be preventing resistance by eliminating resistant subpopulations that pre-exist within the parental line or which develop de novo during TMZ exposure. Although we were unable to distinguish between these possibilities, both are consistent with the experimental observation that preventing emergence and resistance to TMZ is mitigated by PARP inhibition.
Recent work by Touat et al. [24] reinforces the association between MMR mutation, resistance to TMZ, and hypermutation in GBM, and further underscore the tragedy of acquired drug resistance because hypermutated tumours do not respond to immunotherapies, as many had hoped. Solutions to acquired resistance to TMZ are badly needed and the subject of active investigation. For example, Stritzelberger et al. [25] showed that the combination of TMZ and Lomustine (CCNU) was toxic to TMZ-resistant U251N cells, and hypothesized that this drug combination might be an effective therapy for recurrent MGMT-methylated GBM that had acquired resistance to TMZ. This concept was tested in a small phase 3 trial of newly diagnosed methylated GBMs, and as predicted, TMZ/CCNU was superior to single agent TMZ [26], These results and our findings give hope that resistance to TMZ can be circumvented by drug combinations that are easy and safe to prescribe.
Materials and methods
Cell culture
U251N was originally obtained from the American Type Culture Collection (ATCC). While this line is no longer available from ATCC, it may be obtained from other suppliers. U251N was maintained as previously described [8], and authenticated by our group to be U251N using short tandem repeat analysis.
Induction of TMZ resistance
A TMZ resistance strategy was adapted from Yip et al. [8]. At the end of the induction regimen, discrete TMZ resistant colonies became visible. These colonies were treated with 1 × Trypsin–EDTA and transferred into fresh culture dishes. Cultures were then re-treated with the resistance-inducing regimen. To ensure TMZ resistance was retained lines were exposed to TMZ (100 µM; Sigma Cat#T2577) after each passage.
Viability of TMZ resistant sub-clones following co-treatment
Cells were dispersed in 12-well plates (25,000 cells per well) and 24 h later treated for 10 consecutive days. On each treatment day, cells were given TMZ (1, 10 or 100 µM), ABT-888 (Santa Cruz Cat#sc-202901; 0.1, 1 or 10 µM), or TMZ (1, 10 or 100 µM) plus ABT-888 (0.1, 1 or 10 µM). For the co-treated group, TMZ and ABT-888 were given concurrently with 0.1% DMSO and 0.1% 1 × PBS applied as controls. On exposure day 10, cell viability was inferred using the AlamarBlue™ assay. Experiments were completed in triplicate, with three independent replicates. Differences were assessed using the One-Way ANOVA and Tukey multiple comparisons tests.
Assessment of the response of emerging TMZ resistant colonies to co-treatment
On every third day starting on Day 17 after TMZ resistance had been established resistant colonies were co-treated with 100 µM TMZ and 10 µM ABT-888 or 100 µM TMZ alone. Experiments were completed in triplicate, with three independent replicates.
Assessing emergence of resistant colonies with early co-treatment
To test whether co-treatment would prevent the emergence of TMZ resistant colonies, U251N parental cells were co-treated with 100 µM TMZ and 10 µM ABT-888 with the dosing schedule used to induce resistance. ABT-888 and TMZ were given concurrently. Experiments were completed in triplicate, with three independent replicates.
Western blotting of U251N and sub-clones
Western blotting was executed as previously described [27]. Primary antibodies: Abcam Cat #ab39253, MGMT; BD Transduction Laboratories Cat #610918 MSH6; Abcam Cat#ab70270, MSH2; Abcam Cat MLH1; Abcam Cat#ab110638, PMS2; Santa Cruz Cat#sc-56198, pADPr; and Cell Signaling Technologies Cat#8457S or Cat#3700S, \(\beta\)-actin. Secondary antibodies: Bio-Rad Cat#170-6516, and Bio-Rad Cat#170-6515. Western blots were cropped for presentation in Supplementary Fig. 1. Uncropped versions of these blots are displayed in Supplementary Fig. 4.
Sanger sequencing of U251N parental cells and TMZ resistant sub-clones
RNA was extracted using the Qiagen RNeasy Mini-Kit (Qiagen Cat#74104). Reverse transcription was performed using Takara PrimeScript™ High Fidelity RT-PCR Kit (Takara Cat#R022A), and transcripts amplified using the FastStart™ High Fidelity PCR Kit (Roche Cat#03-553-400-001) with gene-specific primers (Supplementary Table 1). Sequences were aligned with NCBI references by Clustal Omega to find genetic variants (MSH2: NM_000251.2/NP_000242.1; MSH6: NM_000179.2/NP_000170.1; MLH1: NM_000249.3/NP_000240.1; PMS2: NM_000535.7/NP_000526.2).
Apoptosis assay
Apoptosis was assessed using flow cytometry on Day 5 after co-treatment with TMZ and ABT-888 as per manufacturer’s protocol (Abcam, ab14085). Experiments were completed in triplicate, with three independent replicates. Different proportions of early apoptotic and viable cells were assessed using the One-Way ANOVA and Tukey multiple comparisons tests.
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Hegi ME, Diserens A, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Omuro A, DeAngelis LM (2013) Glioblastoma and other malignant gliomas: a clinical review. JAMA 310:1842–1850
Johnson BE, Mazor T, Hong C et al (2014) Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 343:189–193
van Thuijl HF, Mazor T, Johnson BE et al (2015) Evolution of DNA repair defects during malignant progression of low-grade gliomas after temozolomide treatment. Acta Neuropathol 129:597–607
Wang J, Cazzato E, Ladewig E et al (2016) Clonal evolution of glioblastoma under therapy. Nat Genet 48:768–776
Yip S, Miao J, Cahill DP et al (2009) MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res 15:4622–4629. [Correction in (2013) Clin Cancer Res 19:4543–4544]
Maxwell JA, Johnson SP, McLendon RE et al (2008) Mismatch repair deficiency does not mediate clinical resistance to temozolomide in malignant glioma. Clin Cancer Res 14:4859–4868
Felsberg J, Thon N, Eigenbrod S et al (2011) Promoter methylation and expression of MGMT and the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2 in paired primary and recurrent glioblastomas. Int J Cancer 129:659–670
Cahill DP, Levine KK, Betensky RA et al (2007) Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clin Cancer Res 13:2038–2045
Shinsato Y, Furukawa T, Yunoue S et al (2013) Reduction of MLH1 and PMS2 confers temozolomide resistance and is associated with recurrence of glioblastoma. Oncotarget 4:2261–2270
McFaline-Figueroa JL, Braun CJ, Stanciu M et al (2015) Minor changes in expression of the mismatch repair protein MSH2 exert a major impact on glioblastoma response to temozolomide. Cancer Res 75:3127–3138
Jung TY, Jung S, Moon KS et al (2010) Changes of the O6-methylguanine-DNA methyltransferase promoter methylation and MGMT protein expression after adjuvant treatment in glioblastoma. Oncol Rep 23:1269–1276
Brandes AA, Franceschi E, Tosoni A et al (2010) O(6)-methylguanine DNA-methyltransferase methylation status can change between first surgery for newly diagnosed glioblastoma and second surgery for recurrence: clinical implications. Neuro Oncology 12:283–288
Bobola MS, Kolstoe DD, Blank A et al (2012) Repair of 3-methyladenine and abasic sites by base excision repair mediates glioblastoma resistance to temozolomide. Front Oncol 2:176
Kaur S, Ramdzan ZM, Guiot MC et al (2018) CUX1 stimulates APE1 enzymatic activity and increases the resistance of glioblastoma cells to the mono-alkylating agent temozolomide. Neuro Oncology 20:484–493
Yuan AL, Ricks CB, Bohm AK et al (2018) ABT-888 restores sensitivity in temozolomide resistant glioma cells and xenografts. PLoS ONE 13:e0202860
Higuchi F, Nagashima H, Ning J et al (2020) Restoration of temozolomide sensitivity by PARP inhibitors in mismatch repair deficient glioblastoma is independent of base excision repair. Clin Cancer Res 26:1690–1699
Nguyen SA, Stechishin ODM, Luchman HA et al (2014) Novel MSH6 mutations in treatment-naïve glioblastoma and anaplastic oligodendroglioma contribute to temozolomide resistance independently of MGMT promoter methylation. Clin Cancer Res 20:4894–4903
Choi S, Yu Y, Grimmer MR et al (2018) Temozolomide-associated hypermutation in gliomas. Neuro Oncology 20:1300–1309
National Library of Medicine, National Centre for Biotechnology Information (2018) Reference SNP(rs) report rs2228006. NIH. https://www.ncbi.nlm.nih.gov/snp/rs2228006. Accessed 8 Oct 2018
National Library of Medicine, National Centre for Biotechnology Information (2018) Reference SNP(rs) report rs1802683. NIH. https://www.ncbi.nlm.nih.gov/snp/rs1802683. Accessed 8 Oct 2018
Touat M, Li YY, Ligon KL (2020) Mechanisms and therapeutic implications of hypermutation in gliomas. Nature 580:517–523
Stritzelberger J, Distel L, Buslei R et al (2018) Acquired temozolomide resistance in human glioblastoma cell line U251 is caused by mismatch repair deficiency and can be overcome by lomustine. Clin Transl Oncol 20:508–516
Herrlinger U, Tzaridis T, Mack F et al (2019) Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet 393:678–688
Blough MD, Westgate MR, Beauchamp D et al (2010) Sensitivity to temozolomide in brain tumor initiating cells. Neuro Oncology 12:756–760
Funding
The Terry Fox Research Institute and Foundation, Alberta Cancer Foundation, Genome Canada, Alberta Innovates Health Solutions, and the family of Clark H. Smith supported this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2020_3561_MOESM1_ESM.tif
Supplementary file1 (TIF 24678 kb) Figure S1. Western blot analysis of MLH1, MSH6, MSH2, PMS2 and MGMT protein expression in the parental U251N line and 18 TMZ- resistant sub-clones. Compared to parental sample levels, expression of MSH6, MSH2 and PMS2 was decreased in some sub-clones, while MLH1 expression remained similar. MGMT induction was observed in one resistant sub-line. “+” denotes the positive control. “P” denotes the parental U251N sample.
11060_2020_3561_MOESM2_ESM.tif
Supplementary file2 (TIF 24677 kb) Figure S2. Cropped chromatograms showing MMR mutations observed in U251N TMZ-resistant sub-clones as outlined in Table: (A) Colony 15 MLH1 mutation (B) Colony 6 MSH6 mutation #1 (C) Colony 6 MSH6 mutation #2 (D) Colony 6 MSH6 mutation #3 (E) Colony 7 MSH6 mutation (F) Colony 9 MSH6 mutation #1 (G) Colony 9 MSH6 mutation #2 (H) Colony 12 MSH6 mutation (I) Colony 12 MLH1 mutation and (J) Colony 1 MSH2 exon 1 deletion. All missense mutations presented with the wild-type and mutant nucleotide, suggesting allelic heterozygosity or heterogeneity among sampled cells.
11060_2020_3561_MOESM3_ESM.tif
Supplementary file3 (TIF 24677 kb) Figure S3. Uncropped versions of the Western blots Data presented in Figure S1 of (A) MLH1 (B) PMS2 (C) MSH6 (D) MGMT (E) MSH2 and (F) pADPr protein expression in TMZ-resistant U251N sub-clones. Molecular weight ladders could not be pictured on indicated (*) blots.
11060_2020_3561_MOESM4_ESM.tif
Supplementary file4 (TIF 24676 kb) Table S1. Mutational status of MMR repair genes in TMZ resistant colonies. Mutation status of MSH2, MSH6, MLH1 and PMS2 in the parental U251N line and a subset of TMZ-resistant sub-clones introduced above. Most sub-clones harbored one or more mutations in at least one MMR gene. One silent mutation and 2 single-nucleotide polymorphisms were observed in the PMS2 gene of the parental U251N line and all derivative sub-clones. WT = wild type. All amino acids and nucleotides are denoted by standard symbols.
Rights and permissions
About this article
Cite this article
Yuan, A.L., Meode, M., Tan, M. et al. PARP inhibition suppresses the emergence of temozolomide resistance in a model system. J Neurooncol 148, 463–472 (2020). https://doi.org/10.1007/s11060-020-03561-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03561-1