Abstract
Background
Plexiform neurofibromas (PN) are the most frequent tumors associated with Neurofibromatosis type 1 (NF-1). PN can cause significant complications, including pain, functional impairment, and disfigurement. There is no efficient medical treatment and, surgical resection of large PN is frequently infeasible. Selumetinib (AZD6244/ARRY-142886) is a mitogen-activated protein kinase enzyme (MEK1/2) inhibitor and works by targeting the MAPK pathway. It is an investigational treatment option for inoperable symptomatic PN associated with NF-1. Herein, we describe a single institutional experience with selumetinib for inoperable PN in NF-1.
Methods
Case series study of demographics, clinical, baseline characteristics, treatment effect, and follow-up of consecutive genetically confirmed NF1 patients with inoperable PN associated with significant or potential significant morbidity treated with selumetinib (April 2018 to April 2019).
Results
Nineteen patients were treated with selumetinib. Predominant target locations were head and neck (31.6%, 6/19), chest (26.3%, 5/19) and pelvis (21%, 4/19) and the most important comorbidities were disfigurement (47.4%, 9/19) and pain (26.3%, 5/19). The mean follow-up time was 223 days (range 35–420 days). All but one had sustained clinical improvement, mainly in the first 60–90 days of treatment. In one patient, the treatment was suspended after 168 days (lack of clear benefit and left ventricular ejection fraction drop). There were no adverse effects leading to treatment suspension.
Conclusions
In the first observational study of selumetinib for NF-1 associated PN we showed that the drug was associated with clinical and radiological improvement. Our study also confirms the safety described in the clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurofibromatosis type 1 (NF1) is a rare tumor predisposition disorder with an estimated birth incidence of 1 in every 2500–3500 individuals [1]. Plexiform neurofibromas (PN) are the most frequent tumors associated with NF1 [2]. PN can cause significant complications, including pain, functional impairment, and disfigurement [2, 3]. The lifetime risk of developing a malignant peripheral nerve sheath tumor (MPNST) in NF1 is 8–13% [4]. Most PN are detected in early childhood and grow during that period [5]. Complete surgical resection of PN is technically challenging and frequently infeasible [2, 3].
Selumetinib (AZD6244/ARRY-142886) is an investigational treatment option for inoperable symptomatic PN associated with NF1. In children with inoperable PN, treatment with selumetinib was able to stop disease progression in all and decreased the tumor volume ≥ 20% from baseline in 71% of cases (17/24) [6]. In the long-term, in the same cohort of children, treatment with selumetinib induced a persistent favorable radiological response and also improved PN-related pain and motor impairment [3].
Real-world data, including long-term side effects and optimal treatment duration, is extremely sparse. We describe the experience with selumetinib used under a national "early access drug program" in a comprehensive and referral cancer center.
Methods
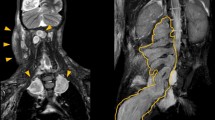
Case series study performed to describe the demographics, clinical, baseline characteristics, treatment effect, and follow-up of consecutive genetically confirmed NF1 patients with inoperable PN associated with significant or potentially significant morbidity who started treatment with selumetinib from April 2018 to April 2019 in a single reference oncology center. Approval for the study was obtained through the local institutional review board. The pharmacy and therapeutics committee approved the use of selumetinib for each patient. Magnetic resonance imaging (MRI) focused on the target PN, electrocardiogram, echocardiogram, eye exam, complete blood count, renal and hepatic tests, creatine phosphokinase (CPK) and positron emission tomography with FluoroDeoxyglucose (FDG-PET/CT) in patients older than 5 years were the initial exams performed in all patients. Treatment consisted of selumetinib 25 mg/m2 BID administered during the whole study period or until unacceptable toxicities, based on a previously published protocol [6]. Dose adjustment was required for the 10 mg and 25 mg available capsules and was based on the provided dosing nomogram. Patient follow-up included monthly complete physical examination, evaluation of treatment adherence, blood analysis, echocardiogram every 3 months, and MRI every 6 months. Response evaluation criteria in solid tumors (RECIST) was used to evaluate imaging improvement [7]. In brief, favorable response or PN shrinkage was consider when at least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters was present. For clinical evaluation, we considered the single most trouble symptom in each patient and used a qualitative all or nothing response (visual inspection for disfigurement), and self-reported benefits (any improvement: yes/no, improvement of specific symptoms: yes/no).
Adverse events were reported to the National Authority of Medicines and Health Products (INFARMED) and the drug sponsor and followed the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Results
Of 83 NF1 patients treated during the study period in our institution, 45 had PN. Of these, 42% (19/45) fulfilled the criteria for selumetinib treatment adapted from Dombi et al. [6]: inoperable PN associated with significant or potentially significant morbidity, at least 6 months of follow up, MPNST exclusion after FDG-PET/CT scan, and normal laboratory results and cardiac function. Twenty-six patients (n = 26) did not receive treatment for the following reasons: asymptomatic PN (n = 14); incomplete workup study (n = 8); surgery candidate (n = 1); MPNST (n = 1); low performance status (n = 1) and treatment refusal (n = 1).
Baseline characteristics
Table 1 summarizes the demographics, clinical, and baseline characteristics of the nineteen patients treated with selumetinib. The first patient received treatment in April 2018. The majority of patients had one (47.4%, 9/19) or two (31.6%, 6/19) PNs; predominant target locations were head and neck (31.6%, 6/19), chest (26.3%, 5/19) and pelvis (26.3%, 5/19); the most important comorbidities were disfigurement (42.1%, 8/19) and pain (21.1%, 4/19) (Table 2). By the time of selumetinib treatment initiation, patients had been suffering from PN related symptoms, on average, for 58 months (range 5–134 months). Ineffective previous medical treatment (either pegylated interferon alfa-2b and/or imatinib) occurred in five patients (Table 2).
Follow up
The mean follow-up time was 223 days (range 35–420 days). Treatment suspension occurred in one patient after 168 days on selumetinib due to the lack of clear clinical benefit and occurrence of left ventricular ejection fraction drop that resolved utterly within a month after treatment suspension. The remaining 18 patients showed clinical improvement, mainly in the first 60–90 days: nerve function improvement translated in chronic pain resolution (100%, 4/4); motor function improvement in the limb located PN subgroup (100%, 3/3, either by functional assessment n = 2, or segmental muscle strength increase n = 1); urinary incontinence resolution (75%, 3/4, all in the pelvic located PN subgroup), disfigurement improvement (50%, 4/8). All patients had at least one MRI re-evaluation, and there was a PN size reduction in 47.3% (9/19).
Clinical or radiological progression was not observed in any patient. Table 2 shows that all patients reported adverse events, mainly CTCAE grade 1. Grade 2 complications were acneiform rash (n = 7), asymptomatic left ventricular ejection fraction reduction (n = 4), paronychia (n = 3), nausea and vomiting (n = 1), erythematous rash (n = 1) and neutrophil count decrease (n = 1). Grade 3 adverse effects occurred in two patients (asymptomatic CPK increase).
Discussion
We present the first observational study describing the experience of selumetinib treatment for PN in patients with inoperable NF1. Selumetinib is a mitogen-activated protein kinase enzyme (MEK1/2) inhibitor and works by targeting the MAPK pathway [6]. Inoperable PN causes severe clinical and social burden, and up until now, no consistent pharmacological treatment options exist. We have recently published a case of clinical improvement of a massive plexiform neurofibroma with selumetinib [8].The study showed that the majority of patients improved clinically, had their tumor volume reduced. These findings are consistent with the results from a previous trial with selumetinib, that showed clinical and radiological improvement or stability in patients treated with this drug [3, 6]. Our study has important limitations to be discussed. Firstly, the sample was small and heterogeneous compromising strong conclusions when analyzing specific characteristics such as symptom distribution and location of PN. For instance, urinary incontinence which was a major symptom in a small subgroup of four patients, all with pelvic PN, improved in three quarter of them. Consequently, with this diminutive number it is hard to value this finding. Secondly, because of its unavailability, imaging response was not assessed using the recommended volumetric analysis for PN [9] used in the clinical trials [3, 6]. The RECIST criteria was used to assess tumor reduction [7] instead which of course limits comparison with the results from the pivotal trials of selumetinib for inoperable PN. Despite these limitations, the study results consistently point that the selumetinib improves the functional prognosis of patients with inoperable. It is also worthwhile to highlight that selumetinib treatment occurred without significant side effects. An optimal treatment initiation window is not well defined yet. A progressing PN may cause irreversible nerve damage and disfigurement, which argues in favor of universal screening for PN detection, close monitoring, and early medical treatment. This should of course be balanced against the implications of more prolonged treatment exposure to a drug whose long-term safety profile is unknown.
Conclusion
In the absence of valid treatments for most patients with PN [2, 3], selumetinib emerges as an option to improve the quality of life of NF1 patients.
In our sample, selumetinib allowed disease control, namely in clinical symptoms but also tumor volume size reduction, at the cost of a few grade 3 and 4 side effects.
If further studies confirm the safety profile and favorable response we hereby report, it would be of interest to discuss the role of early medical treatment in preventing morbidity related to either PN growth or surgical interventions.
References
Huson SM, Harper PS, Compston DA (1988) Von Recklinghausen neurofibromatosis. A clinical and population study in south-east Wales. Brain 111(Pt 6):1355–1381
Hirbe AC, Gutmann DH (2014) Neurofi bromatosis type 1: a multidisciplinary approach to care. Lancet Neurol 13:835–843
Andrea Gross BCW, Wolters P, Baldwin A, Dombi E, Fisher MJ, Weiss B, Kim AR, Blakeley J, Whitcomb P, Holmblad M, Maritin S, Roderick MC, Paul SM, Therrien J, Heisey K, Doyle A, Malc (2018) Neuro-Oncology. Neuro-Oncol 1:i143–i144
Evans DGR, Baser ME, McGaughran J, Sharif S, Howard E, Moran A (2002) Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet 39(5):311–314
Dombi E et al (2007) NF1 plexiform neurofibroma growth rate by volumetric MRI Relationship to age and body weight. Neurology 68:643–648
Dombi E et al (2016) Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med 375(26):2550–2560
Eisenhauer EA (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxford, England : 1990) 45(2):228–247
Passos J, Nzwalo H, Azevedo M, Tavares M, Nunes S (2019) Dramatic improvement of a massive plexiform neurofibroma after administration of selumetinib. Pediatr Neurol 19(11):30928–30932
Dombi E et al (2013) Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology 81(21 Suppl 1):S33–40
Acknowledgements
The authors would like to thank all the patients and their relatives for their collaboration and AstraZeneca for providing selumetinib under the "early access drug program."
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was approved by the institutional ethics committee and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Espírito Santo, V., Passos, J., Nzwalo, H. et al. Selumetinib for plexiform neurofibromas in neurofibromatosis type 1: a single-institution experience. J Neurooncol 147, 459–463 (2020). https://doi.org/10.1007/s11060-020-03443-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03443-6