Abstract
Introduction
Arterial hypertension and proteinuria are common side effects of antiangiogenic treatment and might represent a biomarker of response in patients with glioblastoma. The aim of this study was to assess the impact of these side effects in predicting therapeutic response to second line chemotherapy with bevacizumab.
Methods
We evaluated clinical and survival data of glioblastoma patients who underwent treatment with bevacizumab after progression under temozolomide, at CHUSJ between 2010 and 2017. We analysed treatment-related arterial hypertension, proteinuria grade, thrombotic and haemorrhagic events during treatment. Overall survival (OS) and progression free survival (PFS) under bevacizumab were calculated according to the Kaplan–Meier method. Multivariate analysis was performed using Cox proportional hazards method.
Results
We evaluated 140 patients. Arterial hypertension and proteinuria occurred in 23 (16.3%) and 17 (12.1%) patients, respectively. PFS during treatment with bevacizumab was 12 months (95% CI 7.9–16.1) in the hypertensive group and 4 months (95% CI 3.2–4.8) in the normotensive group (p = 0.005). Patients with proteinuria had a PFS of 10 months (95% CI 4.9–15.0) versus 4 months (95% CI 3.4–4.8) in patients without proteinuria (p = 0.002). Multivariate analysis revealed hypertension and proteinuria as independent prognostic factors of PFS and OS.
Conclusion
Our data suggest that hypertension and proteinuria can be effective predictors of response to antiangiogenic therapy in recurrent glioblastoma and are associated with longer disease control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM), the most common malignant primary brain tumour, maintains a poor prognosis despite all efforts of therapeutic research with a 2-year survival rate of 26.5% in the temozolomide era [1, 2]. GBM is one of the most vascularized tumours in humans, consistently showing high expression of human vascular endothelial growth factor (VEGF). Bevacizumab, a recombinant human monoclonal immunoglobulin G1 antibody that selectively binds to and neutralizes VEGF, has been approved in 2009 by the US Food and Drug Administration for the treatment of recurrent GBM [3,4,5,6]. Despite overall survival has not increased with bevacizumab in randomized clinical trials, it has shown significant impact on progression-free survival. However, efficacy and toxicity vary from patient to patient, and it is crucial to distinguish patients who benefit from its administration from patients in which this therapy may be futile or even harmful. There is already evidence that the development of side effects of bevacizumab as hypertension (HTN) is associated with higher progression-free survival (PFS) and overall survival (OS) in metastatic colorectal cancer (mCRC) and that developing proteinuria might lead to poorer survival [7,8,9]. Few studies have looked at these variables as possible clinical biomarkers of response or resistance to bevacizumab in patients with glioblastoma [8, 10].
The aim of this study was to assess whether the development bevacizumab induced hypertension and proteinuria predicts higher PFS and OS comparing to patients that do not present such side effects.
Materials and methods
Study design and patient selection
This is a retrospective cohort study of all patients with GBM treated at Centro Hospitalar Universitário São João, Oporto, between January 2010 and December 2017, with radiochemotherapy according to Stupp protocol, followed, upon recurrence, by treatment with bevacizumab-based therapy.
The inclusion criteria were patients with histologically proven GBM, age ≥ 18 years, that were submitted to second-line treatment with bevacizumab-based regimen after neuro-oncology multidisciplinary team meeting (MDT) decision.
All patients were treated according to standard first-line chemotherapy with temozolomide, administered 75 mg/m2 concurrent with daily external-beam radiation therapy (RT) (2 Gy/fraction, for a total of 60 Gy in 30 fractions) and followed by adjuvant TMZ at 150–200 mg/m2 for 5 days every 28 days until progression. All patients were treated with second-line bevacizumab-based therapy: bevacizumab monotherapy (10 mg/kg); bevacizumab (10 mg/kg) + irinotecan (340 or 125 mg/m2, with or without concomitant enzyme inducing antiepileptic drugs, respectively) every 2 weeks or bevacizumab (10 mg/kg) every 2 weeks with lomustine (90 mg/m2) every 6 weeks.
Data were collected from digital and written clinical records as well as nursing registries and analytical studies performed at Oncology Day Unit setting. We analysed demographic, clinical, therapeutic, adverse events and survival data of all patients.
The study was approved by local ethics committee.
Diagnostic criteria
Overall survival (OS) was considered as the time frame between surgery and the date of death or to the last day of follow-up if alive. Progression-free survival (PFS) was calculated from the beginning of bevacizumab until the date of disease progression that determined the switch to a third-line chemotherapy agent or to best supportive care.
We evaluated the most frequently reported bevacizumab related side effects, namely: arterial hypertension, proteinuria, arterial and venous thrombosis, haemorrhages, infections, wound healing problems and gastrointestinal perforation.
Treatment related arterial hypertension (HTN) was defined as the initiation of new antihypertensive medication during antiangiogenic therapy or newly documented measurements of systolic blood pressure > 140 mm Hg or diastolic blood pressure > 90 mmHg. Previous hypertensive patients with no modification in dose or number of antihypertensive drugs were not considered in treatment-related HTN group. Neither were patients with corticosteroid associated hypertension. Proteinuria was evaluated based on 24-h urine collection, protein/creatinine ratio and dipstick, and its evolution during bevacizumab-based therapy. HTN and proteinuria were evaluated according to National Cancer Institute – Common Toxicity Criteria scale version 5.0 (CTCAE v5.0) [11].
Data and statistical analysis
The primary endpoint of this study was the effect of anti-angiogenic side effects (HTN and proteinuria) on PFS. Additionally, as secondary endpoints, we evaluated the impact on overall survival and the frequency of other adverse effects. Differences between groups were analysed using Chi square, Fisher’s exact test, Student’s T test or Mann–Whitney U test as appropriate, after assessing distribution and equality of variances. Survival data was evaluated using Kaplan–Meier product-limit analysis and 95% confidence intervals were calculated. We used Log-rank test to detect statistically significant differences in survival distribution. Data of patients who have not progressed or died were right-censored in our analysis. Multivariate analysis for PFS and OS was conducted for variables with p values < 0.2 on univariate analysis and performed using forward stepwise Cox proportional hazard method to determine independent prognostic factors. Differences were considered statistically significant at p < 0.05. The software used for statistical analysis was IBM SPSS Statistics 25.
Results
Patients demographics and characteristics
We identified 153 patients, from which 140 patients were included. Ten patients were excluded due to rapid clinical deterioration before initiating therapy, 2 patients were excluded since they had participated in the active arm of the AVAglio study and 1 patient had an allergic reaction and immediately discontinued therapy.
The median age at diagnosis was 57 years (26–77 years). The majority of patients were male (60%, n = 84) and 89% (n = 125) had an ECOG performance status of 0 or 1. Most patients did not have history of cardiovascular disease (55.0%, n = 77), however 32.1% (n = 45) had been previously diagnosed with HTN.
Clinical and demographic characteristics of the cohort are detailed in Table 1.
Bevacizumab-related adverse events
Among 140 patients, 23 (16.4%) initiated new anti-hypertensive treatment, 12 patients developed grade 3 HTN, 8 patients developed grade 2 HTN, one patient developed grade 4 HTN and one patient developed grade 1 HTN. Nine patients (39.1%) already had a past medical history of HTN controlled with medical treatment. Eleven patients needed one drug, seven patients needed 2 drugs, and five patients required 3 drugs to control arterial blood pressure. None of these patients increased corticosteroid dose during anti-angiogenic therapy. In fact, 14 patients (61%) were corticosteroid free; 6 patients (26%) reduced corticosteroid dose, and 3 patients (13%) maintained a stable low dose (5-20 mg prednisolone).
Proteinuria was detected during bevacizumab therapy in 17 (12.1%) patients, 13 (9.3%) were grade 1, 3 (2.1%) were grade 2 and 1 (0.7%) patient developed nephrotic syndrome. 4 (23.5%) of these patients had a past medical history of controlled HTN with medical treatment.
Median timing of onset of hypertension and proteinuria was 15 and 18 weeks after initiation of anti-angiogenic treatment, respectively.
There were no arterial thrombosis events detected. Deep vein thrombosis occurred in 5 (3.6%) patients, and one of them developed pulmonary embolism. One patient had a minor asymptomatic intracerebral haemorrhage, 4 patients had wound healing problems, one patient had gastrointestinal perforation and one patient developed heart failure.
Bevacizumab-related side effects are detailed in Table 2.
Survival analysis
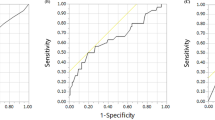
The median PFS while on bevacizumab for all patients was 5 months. The PFS was significantly different between normotensive and hypertensive groups with a median PFS of 4 months (95% confidence interval [95% CI] 3.23–4.77 months) versus 12 months (95% CI 7.90–16.1) (p = 0.005), respectively. The PFS was also significantly different between patients that did not develop proteinuria with a PFS of 4 months (95% CI 3.24–4.76) versus those who developed proteinuria with a PFS of 10 months (95% CI 4.96–15.04) (p = 0.002) (Fig. 1).
Median OS was 19 months for the entire cohort. OS was also significantly higher in the hypertensive group (27 months, [95% CI 18.94–35.06], versus normotensive group (18 months, [95% CI 16.29–19.71] (p = 0.035). Although there was a trend toward better survival in patients with proteinuria (25 months vs 18 months), that difference was not statistically significant (p = 0.054) in univariate analysis.
A comparative analysis of the baseline characteristics between normotensive and hypertensive groups showed no significant differences regarding age (p = 0.704, Mann–Whitney U test), gender (p = 0.901, Chi square test), ECOG performance status (p = 0.465, Fisher’s exact test), type of surgery (p = 1.00, Fisher’s exact test), number of temozolomide cycles (p = 0.112, Mann–Whitney U test) or multifocality (p = 0.327, Fisher’s exact test). The same was true for proteinuria and non-proteinuria groups.
Two patients (2/5 = 40%) on bevacizumab monotherapy (BEV), 10 patients (10/87 = 11.5%) on bevacizumab associated to irinotecan (BEV + ITC) and 11 patients (11/48 = 23%) on bevacizumab with lomustin (BEV + LOM) developed treatment-related HTN. There were no statistically significant differences in survival data between different bevacizumab-based regimens.
Multivariate analysis revealed treatment-related HTN (p = 0.033, HR = 0.58) and proteinuria (p = 0.014, HR = 0.48) as independent predictors of better PFS on bevacizumab-based therapy. Uni and multivariate PFS analysis are detailed in Table 3.
Regarding OS, multivariate analysis identified age ≥ 60 years (p = 0.023, HR = 1.54), number of TMZ cycles > 6 (p < 0.001, HR = 0.48), treatment-related HTN (p = 0.009, HR = 0.483), treatment-related proteinuria (p = 0.018, HR 0.465) and beginning of a third-line chemotherapy (p = 0.016, HR = 0.64) as independent prognostic factors significantly associated with increased overall survival. Gender, ECOG, resection type, localisation and focality had no statistical impact on OS in this group of patients. Uni and multivariate OS analysis are depicted in Table 4.
Discussion
Although survival benefit from bevacizumab containing regimens is modest, some patients respond better than others and it is critical to establish predictive and therapy monitoring markers for selection of patients for this specific therapy [12]. Several authors have reported bevacizumab adverse events as predictors of response in advanced or metastatic non-small cell lung cancer, colorectal cancer, breast cancer and ovarian cancer [13,14,15]. Khoja et al. found that HTN predicts significantly higher response rates in metastatic colorectal cancer, while the development of proteinuria during treatment with antiangiogenics portends poorer survival [7].
In glioblastoma, AVAglio and RTOG 0825 tried to identify a subset of patients who might benefit from upfront treatment with bevacizumab, but no biomarker proved consistently effective in predicting either response or resistance to bevacizumab [5, 6]. In the recurrent setting, Wick et al. conducted a randomized phase 3 trial of bevacizumab plus lomustine at first progression of glioblastoma after standard chemoradiotherapy and showed significant improvement in PFS, but no overall survival advantage [16].
Despite some dismay in antiangiogenic clinical trial results with respect to overall survival, bevacizumab remains a cornerstone therapy in the treatment of recurrent GBM, providing symptom alleviation, increased PFS and probably, in a subgroup of patients, a real survival benefit. In this perspective, identification of biomarkers of tumour response and stratification of patients according to antiangiogenic treatment efficacy remain an important evolving area of research.
In our group of patients 16.4% (n = 23) developed antiangiogenic-related HTN, with the majority (87%) being grade 2 or 3. Our data show a rate of HTN comparable to data reported in the literature: which varies between 3 and 20% [4, 17,18,19,20]. 12.1% (n = 17) of our patients developed proteinuria, with the majority of these (76.5%, n = 13) having grade 1. Nangia et al. at the ASCO meeting in 2011 reported higher rates, with 39% of bevacizumab-treated patients developing proteinuria and 75% developing HTN [21].
The results of our study demonstrate that development of HTN and proteinuria in patients receiving bevacizumab is predictive of better disease control, with longer progression-free survival and overall survival. Our study also showed that differences in tumour control and survival were not attributable to differences in clinical and therapeutic variables in the two groups.
The mechanisms underlying bevacizumab-related arterial hypertension and proteinuria and their association with patient outcome are not yet elucidated. One hypothesis is that anti-VEGF therapy can result in vessel normalization in tumors but endothelial dysfunction and capillary rarefaction in normal tissues, which may thus be responsible for the improved outcome and subsequent toxicity [7, 22].
Other investigators have reported differences in clinical outcomes in glioblastoma depending on whether patients developed arterial HTN as an antiangiogenic side effect. Nangia et al. observed a median PFS of 7 versus 2.9 months and OS 11 versus 5.7 months in hypertensive versus normotensive patients, respectively [21]. Lombardi et al. studied 53 patients with glioblastoma treated with antiangiogenic drugs upon recurrence (30 with sorafenib and 23 with bevacizumab) and showed significantly associations between HTN and disease control rate, and HTN and progression-free survival at 6 months. Additionally, multivariate analysis showed that HTN onset was independently associated with a longer survival [8]. Zhong et al. studied 83 patients submitted to bevacizumab for recurrent GBM and reported significantly different PFS with 2.5 months for the normotensive patients and 6.7 months for the hypertensive patients [10]. Thus, our robust data and large number of patients confirm these results, showing a PFS of 12 months in hypertensive patients versus 4 months in normotensive patients (p = 0.033, hazard ratio = 0.584).
As a secondary endpoint, OS was significantly longer in patients who developed treatment-related HTN, 27 months, versus those who did not, 18 months (p = 0.035).
Proteinuria, to our current knowledge, has not been consistently reported as a potential biomarker. On the contrary some authors report a negative impact on its prognosis in other malignancies [7]. Nangia et al. showed a statistically significant increase in PFS (7.1 vs 6.0 months) and OS (13.2 vs 9.3 months) associated with the development of proteinuria [21]. Our data demonstrated that treatment-related proteinuria was associated with increase in PFS (10 vs 4 months) (p = 0. 002).
Interestingly, co-existence of treatment-related HTN and proteinuria occurred in five patients with median PFS of 16 months (95% CI 11.71–20.29) while on bevacizumab and median OS of 32 months (95% CI 18.92–45.08).
Our data are in agreement with other studies namely regarding HTN development impact on disease control and survival in patients with glioblastoma submitted to treatment with bevacizumab. Additionally, we demonstrate that proteinuria, a commonly negative predictor of outcome in malignancies, might also be a predictor of response in this group of patients. We hypothesize that both adverse effects might synergistically predict better disease control and survival. Further studies are necessary to confirm this.
Our study has some limitations. Although oncology day unit protocols and comprehensive registries are well established in our hospital for several years, this was a retrospective study and, thus, susceptible to potential bias. Molecular markers, namely IDH1/2 mutations and MGMT methylation, which have been suggested as being responsible for differential response to bevacizumab therapy was not performed routinely and therefore could not be assessed [23]. Nonetheless, we present a large number of patients with well described clinical and therapeutic data, with relevant statistically significant results.
Conclusion
Our study shows that hypertension and proteinuria developed during bevacizumab therapy are correlated to prolonged progression-free survival and may constitute an indicator of active anti-tumour effect. The absence of these side effects may be an alert for neurooncologists anticipating resistance to antiangiogenic treatment. Additionally, newly-developed hypertension was associated with prolonged survival. In an era of ever-increasing demand of personalized medicine, knowing which patients might benefit from a treatment and avoiding unnecessary or toxic therapies is of the utmost importance. Additional studies should be conducted to prospectively validate these clinical biomarkers.
References
Darefsky AS, King JT Jr, Dubrow R (2012) Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer 118(8):2163–2172. https://doi.org/10.1002/cncr.26494
Stupp RJNEJM (2005) European Organisation for Research and Treatment of Cancer brain tumor and radiotherapy groups; National Cancer Institute of Canada clinical trials group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma 352:987–996
Cohen MH, Shen YL, Keegan P, Pazdur R (2009) FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist 14(11):1131–1138. https://doi.org/10.1634/theoncologist.2009-0121
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740. https://doi.org/10.1200/JCO.2008.19.8721
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370(8):709–722. https://doi.org/10.1056/NEJMoa1308345
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ Jr, Mehta MP (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370(8):699–708. https://doi.org/10.1056/NEJMoa1308573
Khoja Leila, Kumaran G, Zee YK, Murukesh Nishanth, Swindell Ric, Saunders MP, Clamp AR, Valle JW, Jayson GWGC, Hasan J (2014) Evaluation of hypertension and proteinuria as markers of efficacy in antiangiogenic therapy for metastatic colorectal cancer. J Clin Gastroenterol 48:4
Lombardi G, Zustovich F, Farina P, Fiduccia P, Della Puppa A, Polo V, Bertorelle R, Gardiman MP, Banzato A, Ciccarino P, Denaro L, Zagonel V (2013) Hypertension as a biomarker in patients with recurrent glioblastoma treated with antiangiogenic drugs: a single-center experience and a critical review of the literature. Anticancer Drugs 24(1):90–97. https://doi.org/10.1097/CAD.0b013e32835aa5fd
Osterlund P, Soveri LM, Isoniemi H, Poussa T, Alanko T, Bono P (2011) Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy. Br J Cancer 104(4):599–604. https://doi.org/10.1038/bjc.2011.2
Zhong J, Ali AN, Voloschin AD, Liu Y, Curran WJ Jr, Crocker IR, Shu HK (2015) Bevacizumab-induced hypertension is a predictive marker for improved outcomes in patients with recurrent glioblastoma treated with bevacizumab. Cancer 121(9):1456–1462. https://doi.org/10.1002/cncr.29234
Institute NC (2017) Common terminology criteria for adverse events, version 5.0. 28/03/2019 edn. National Cancer Institute, National Institutes of Health, Department of Health and Human Services
Jain RK, Duda DG, Willett CG, Sahani DV, Zhu AX, Loeffler JS, Batchelor TT, Sorensen AG (2009) Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol 6(6):327
Mir O, Coriat R, Cabanes L, Ropert S, Billemont B, Alexandre J, Durand JP, Treluyer JM, Knebelmann B, Goldwasser F (2011) An observational study of bevacizumab-induced hypertension as a clinical biomarker of antitumor activity. Oncologist 16(9):1325–1332. https://doi.org/10.1634/theoncologist.2010-0002
Gampenrieder SP, Romeder F, Muss C, Pircher M, Ressler S, Rinnerthaler G, Bartsch R, Sattlberger C, Mlineritsch B, Greil R (2014) Hypertension as a predictive marker for bevacizumab in metastatic breast cancer: results from a retrospective matched-pair analysis. Anticancer Res 34(1):227–233
Scartozzi M, Galizia E, Chiorrini S, Giampieri R, Berardi R, Pierantoni C, Cascinu S (2009) Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol 20(2):227–230. https://doi.org/10.1093/annonc/mdn637
Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, Brandes AA, Taal W, Domont J, Idbaih A, Campone M, Clement PM, Stupp R, Fabbro M, Le Rhun E, Dubois F, Weller M, von Deimling A, Golfinopoulos V, Bromberg JC, Platten M, Klein M, van den Bent MJ (2017) Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med 377(20):1954–1963. https://doi.org/10.1056/NEJMoa1707358
Batchelor TT, Duda DG, di Tomaso E, Ancukiewicz M, Plotkin SR, Gerstner E, Eichler AF, Drappatz J, Hochberg FH, Benner T, Louis DN, Cohen KS, Chea H, Exarhopoulos A, Loeffler JS, Moses MA, Ivy P, Sorensen AG, Wen PY, Jain RK (2010) Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol 28(17):2817–2823. https://doi.org/10.1200/JCO.2009.26.3988
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27(5):740–745. https://doi.org/10.1200/JCO.2008.16.3055
Reardon DA, Vredenburgh JJ, Desjardins A, Peters K, Gururangan S, Sampson JH, Marcello J, Herndon JE 2nd, McLendon RE, Janney D, Friedman AH, Bigner DD, Friedman HS (2011) Effect of CYP3A-inducing anti-epileptics on sorafenib exposure: results of a phase II study of sorafenib plus daily temozolomide in adults with recurrent glioblastoma. J Neurooncol 101(1):57–66. https://doi.org/10.1007/s11060-010-0217-6
Wick A, Schafer N, Dorner N, Schemmer D, Platten M, Bendszus M, Wick W (2010) Arterial hypertension and bevacizumab treatment in glioblastoma: no correlation with clinical outcome. J Neurooncol 97(1):157–158. https://doi.org/10.1007/s11060-009-0003-5
Nangia CS, Wang D, Scarpace L, Schultz L, Khanshour A, Mikkelsen T (2011) The role of the development of hypertension or proteinuria in predicting outcome with the use of bevacizumab for patients with glioblastoma multiforme. J Clin Oncol. https://doi.org/10.1200/jco.2011.29.15_suppl.2021
Mourad JJ, des Guetz G, Debbabi H, Levy BI (2008) Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol 19(5):927–934. https://doi.org/10.1093/annonc/mdm550
Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D, de Vos FY, Dinjens WN, Enting RH, Taphoorn MJ, van den Berkmortel FW, Jansen RL, Brandsma D, Bromberg JE, van Heuvel I, Vernhout RM, van der Holt B, van den Bent MJ (2014) Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15(9):943–953. https://doi.org/10.1016/S1470-2045(14)70314-6
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Carvalho, B., Lopes, R.G., Linhares, P. et al. Hypertension and proteinuria as clinical biomarkers of response to bevacizumab in glioblastoma patients. J Neurooncol 147, 109–116 (2020). https://doi.org/10.1007/s11060-020-03404-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03404-z