Abstract
Background
Emerging evidence suggests that myeloid cells play a critical role in glioblastoma (GBM) immunosuppression. Disappointing results of recent checkpoint inhibitor trials suggest that combination immunotherapy with alternative agents could be fruitful in overcoming immunosuppression. Overexpression of chemokine receptor CXCR4 is associated with poor prognosis in GBM. We investigate the treatment effects of combination immunotherapy with anti-PD-1 and anti-CXCR4 in a murine glioma model.
Methods
C57BL/6 mice were implanted with GL261-Luc+ glioma cells and randomized into 4 arms: (1) control (2) anti-PD-1 (3) anti-CXCR4, and (4) anti-PD-1 and anti-CXCR4 therapy. Overall survival and median survival were assessed. Cell populations were assessed by flow cytometry.
Results
Combination therapy conferred a significant survival benefit compared to control and monotherapy arms. Mice that received combination therapy demonstrated immune memory and decreased populations of immunosuppressive tumor-infiltrating leukocytes, such as monocytic myeloid-derived suppressor cells and microglia within the brain. Furthermore, combination therapy improved CD4+/CD8+ ratios in the brain as well as contributed to increased levels of pro-inflammatory cytokines.
Conclusions
Anti-CXCR4 and anti-PD-1 combination immunotherapy modulates tumor-infiltrating populations of the glioma microenvironment. Targeting myeloid cells with anti-CXCR4 facilitates anti-PD-1 to promote an antitumor immune response and improved survival rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM) is the most common primary brain tumor in adults, accounting for 45.6% of all primary brain malignancies [1]. Current treatment for GBM consists of maximum surgical resection, adjuvant radiotherapy, and chemotherapeutics [1, 2]. GBM is still a devastating diagnosis, with survival at 5 years of < 10% and median survival of 14 months [1].
While immunotherapy has been effective in many solid tumors, results in GBM have been disappointing [3, 4]. Anti-programmed death 1 (anti-PD-1) is a well-known checkpoint inhibitor, and multiple groups have found that anti-PD-1 synergizes with other immune checkpoint inhibitors in preclinical glioma models [5, 6]. Unfortunately, human trials with anti-PD-1 have been negative to-date. The failure of GBM to respond to anti-PD-1 may be due to the immunologic milieu, which is consistent with other “cold” tumors, and is characterized by a paucity of tumor infiltrating lymphocytes (TILs) and a predominance of immunosuppressive myeloid cells [7]. Evidence suggests that myeloid cells are key mediators of immunosuppression in GBM and overcoming this immunosuppression may allow an effective antitumor immune response [6, 8]. A recent study showed that tumor-associated macrophages (TAMs) significantly contribute to resistance against anti-PD-1 therapy by diverting the therapeutic antibodies from PD-1+ tumor-infiltrating CD8+ T cells [9].
The CXCR4/CXCL12 chemokine signaling axis affects immune cell homing and migration and regulates hematopoietic cell development [10]. CXCR4 is normally expressed on hematopoietic cells, such as T and B lymphocytes, macrophages, monocytes, and progenitor cells, as well as microglia and vascular endothelial cells. CXCR4 is overexpressed in over 23 types of cancers, including GBM, contributing to tumor treatment resistance by promoting tumor growth, survival, and metastasis as well as recruiting immunosuppressive myeloid cells and promoting aberrant tumor angiogenesis [10, 11].
In this study, we hypothesized that disrupting tumor immunosuppression, such as through the myeloid cell compartment, with blockade of CXCR4 can augment an anti-PD-1 mediated cytolytic T-cell response.
Materials and methods
Mice and cell lines
Six- to eight-week-old C57BL/6J wild-type female mice (Jackson, ME; in-house breeding) were maintained at the Johns Hopkins University Animal Facility. All animal experiments were performed in accordance with protocols approved by the Johns Hopkins Institutional Animal Care and Use Committee (IACUC).
Orthotopic gliomas were established using GL261-Luciferase-tagged (GL261-Luc+) cells grown in Dulbecco’s Modified Eagle Medium (DMEM, Gibco) + 10% fetal bovine serum (FBS, Sigma-Aldrich) + 1% penicillin–streptomycin (Sigma-Aldrich) with the addition of 100 μg/mL G418 (Corning) selection media.
Intracranial murine glioma model
130,000 GL261-Luc+ cells were stereotactically injected into the left cortical hemisphere as previously described in Kim et al [5].
Mice were randomly assigned to treatment arms, and tumor burden was monitored by bioluminescent IVIS® imaging (Perkin Elmer) on post-tumor implantation day 7, 21, and every week thereafter. Survival experiments were repeated in duplicate and animals accordingly euthanized as previously described [5]. Long-term survival was defined as 90 days post-tumor implantation. For the survival experiments performed in duplicate, at least 7–8 mice were included in the control along with monotherapy and combination therapy arms involving 9–20 mice in each group.
Therapeutic antibodies
Anti-murine CXCR4 and anti-murine PD-1 antibodies were generously provided by Bristol-Myers Squibb (BMS) and stored at − 80 °C in 2 mg/mL aliquots. Final treatment dose for each antibody was 200 μg per animal. Antibody treatments were administered intra-peritoneally on days 10, 12, and 14 following tumor implantation.
Tumor rechallenge
Long-term survivors were re-challenged with 260,000 GL261-Luc+ cells injected into the contralateral hemisphere, 2 mm anterior and 2 mm lateral to lambda. Tumor presence was assessed on day 7 post-implantation. Animals would be euthanized when they demonstrated morbidity signs or after 100 days post-rechallenge for remaining mice. The number of mice within each experiment arm depended on the number of long-term survivors, ranging from 1–2 mice for anti-CXCR4 monotherapy, 4–5 mice for anti-PD-1 monotherapy, and 6–8 mice for combination therapy. A group of 10 mice were included for each of the rechallenge experiments for the control arm.
Immune cell isolation
To isolate peripheral cells, lymph nodes (deep cervical), and spleens were harvested from mice in all groups sacrificed on post-implantation day 20. Solid organs were mechanically homogenized in harvest media (Roswell Park Memorial Institute (RPMI) medium + 10% FBS + 1% penicillin–streptomycin) and filtered through 100-μm mesh cell strainers (BD Falcon). Lymph nodes were centrifuged at 1300 rpm for 10 min. Spleen samples were lysed (ACK lysing buffer, Quality Bio) and washed with PBS.
To isolate brain cells, brains were harvested on post-implantation day 20. Brains were mechanically homogenized, filtered, resuspended in 5 mL 72% Percoll in 1X HBSS without phenol red, layered below 7 mL of 36% Percoll in 1X HBSS and centrifuged at 2000 rpm for 20 min at room temperature. Cell layer at the 36%/72% interface was collected and washed with PBS.
Flow cytometry and immunophenotyping
Isolated immune cells were plated for staining in 200 μL PBS.
For myeloid cell panel, cells were pre-treated, washed, and stained with Live-Dead Aqua AmCyan (Life Technologies), F4/80 PeCy7 (clone BM8, BioLegend), CD45 APC/Cy7 (clone 30-F11, BioLegend), CD11b AF700 (clone M1/70, BioLegend), CD11c FITC (clone N418, BioLegend), IA/IE PerCP/Cy5.5 (clone M5/114.15.2, BioLegend), Ly6C BV605 (clone HK1.6, BioLegend), Ly6G BV421 (clone 1A8, BioLegend), and CXCR4 PE-eFluor610 (clone 2B11, Invitrogen).
For cytokines panel, samples were stimulated in 200μL RPMI containing ionomycin (1:2000, Sigma-Aldrich), phorbol 12-myristate 13-acetate (PMA, 1:2000, Sigma-Aldrich), and Golgi Stop (1:500, BD Biosciences) for 4 h at 37 °C and was washed in PBS following incubation. Checkpoint panel was stained separately. Extracellular markers included Live-Dead Aqua AmCyan, CD45 APC/Cy7, CD3 BV421 (clone 17A2, BioLegend), CD4 FITC (clone RM4-4, eBioscience), CD8 BV605 (clone 53–6.7, BioLegend), PD1 PeCy7 (clone J43, eBioscience), CXCR4 PE-eFluor610, CD62L PerCP-Cy5.5 (clone MEL-14, eBioscience), CD44 AF700 (clone IM7, Biolegend). Samples were fixed in 1:3 fixation/permeabilization buffer overnight. Cells were subsequently stained for IFNγ PeCy7 (clone XMG1.2, eBioscience), TNFα (clone MP6-XT22, eBioscience), FoxP3 AF700/PE (FJK-16s, eBioscience) in intracellular permeabilization buffer.
Samples were processed using LSR II flow cytometer (BD Biosciences). Data was analyzed using FlowJo v10.2 (FlowJo, LLC). Table 1 indicates cell population markers.
Statistics
Survival was analyzed by Kaplan–Meier survival curves and compared by log-rank Mantel Cox test. One-way ANOVA was used to analyze for significance among all groups. Unpaired t-test was used to compare two groups. Comparisons within groups were presented as mean ± standard error of the mean (SEM). Data were analyzed using GraphPad Prism 7 and values of p < 0.05 were considered significant.
Results
Anti-CXCR4 in combination therapy modulates immunosuppressive myeloid cell populations
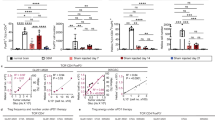
In human glioma samples, the level of CXCR4 expression correlates with tumor malignancy and grade as well as poor prognosis [12]. To elucidate the potential mechanism by which combination therapy confers survival benefit and glioma regression, we investigated immunosuppressive and tumor-promoting myeloid cell populations within all experiment arms.
Resident microglia can contribute to glioma progression [11]. Gliomas also direct glioma-associated microglia or macrophages (GAMs) to convert to the pro-tumor, pro-angiogenesis, anti-inflammatory polarity. While we did not demonstrate effects on activated antigen-presenting cells, which would have been otherwise denoted as MHCII+ F4/80+ macrophages or MHCII+ CD11c+ dendritic cells, in our study, all treatment groups including anti-CXCR4 had significantly reduced populations of tumor-promoting CD11b+ microglia when compared to control (anti-CXCR4: p = 0.0036; combination therapy: p = 0.0065) (Fig. 1a). When we were assessing the proportion of microglia bearing CXCR4, we discovered that the population of CXCR4+ CD11b+ microglia was significantly diminished in the group receiving combination therapy when compared to control (p = 0.0490) (Fig. 1b).
In our study, there was a significant decrease in the proportion of immunosuppressive monocytic myeloid-derived suppressor cells (M-MDSCs) for the combination group when compared to control (p = 0.0119) and both monotherapy arms (anti-CXCR4: p = 0.0465; anti-PD-1: p = 0.0118) (Fig. 1c).
Tumor-infiltrating dendritic cells (DCs) contribute to tumor promotion in several cancer models [13, 14]. For proportions of CD11b+ CD11c+ CD45+ DCs, there was a significant decrease with combination therapy relative to control (p = 0.0022) (Fig. 1d). There was a significant decrease in DC proportions in the combination therapy group when compared to anti-PD-1 monotherapy as well (p = 0.0073).
Combination anti-PD-1 and anti-CXCR4 therapy confers survival benefit and long-term protective immunity
We hypothesized CXCR4 and anti-PD-1 blockade administered in combination will result in a greater treatment effect and survival benefit than monotherapy alone. We utilized the following experimental arms: non-treated control, anti-PD-1 alone, anti-CXCR4 alone, and anti-PD-1 plus anti-CXCR4 (Fig. 2a).
Survival study experimental schema. a Experimental treatment schedule. Intracranial tumor implantation was performed on Day 0, with intraperitoneal injections of anti-PD-1 and anti-CXCR4 administered on Days 10, 12, and 14. b Representative Kaplan–Meier survival curve. *p < 0.05 for the combination treatment arm (n = 20) when compared to control (n = 7) and all monotherapy arms (n = 9 for anti-CXCR4, n = 10 for anti-PD-1) by log-rank Mantel-Cox test. Survival studies were repeated in duplicate. c Animals from initial survival studies that have tumor regression and long-term survival were implanted with 260,000 GL261-Luc+ cells in contralateral brain hemisphere and followed over time for clinical decline. *p < 0.05 for all treatment groups compared with control group by log-rank Mantel-Cox test. Re-challenge experiments were repeated in duplicate
In comparison to control and to both monotherapy arms, combination therapy improved overall survival (Fig. 2b). Median survival was 24 days and overall long-term survival rate of 0% for the control arm while anti-CXCR4 monotherapy had a median survival of 25 days and overall survival rate of 11.1% (p = 0.8670). Anti-PD-1 resulted in median survival of 30 days and overall long-term survival rate of 30.0%, which was statistically significant when compared to control (p = 0.0265). However, combining anti-CXCR4 with anti-PD-1 further improved the overall survival to 60.0%, which was a significant improvement when compared to control (p < 0.0001) and both monotherapies (anti-CXCR4: p = 0.0006, anti-PD-1: p = 0.0404).
To assess the development of immunologic memory, we performed tumor re-challenge survival studies. All mice that had received antibody inoculation, with either monotherapy or combination therapy had overall survival rates of 100% (Fig. 2c). The control group had a median survival of 24 days with 0% survival rate by day 35 post-implantation. In comparison, the treated groups all retained 100% survival by day 100 (p < 0.0001). The re-challenge experiment demonstrates the development of long-term protective immune memory against GL261-Luc+ glioma cells.
Combination therapy improves TIL CD4/CD8 ratio and brain CD8+ subpopulation profile
In all treatment groups when compared to control, there was a statistically significant decrease in the CD4+/CD8+ ratio (p < 0.0001). Combination therapy also resulted in a significantly decreased CD4+/CD8+ TC ratio compared to anti-PD-1 alone (p = 0.0180) (Fig. 3a).
CD4+/CD8+ ratios and regulatory T cell proportions in brain. a CD4+/CD8+ ratio in brain control and treatment groups. b Proportion of FoxP3+ CD4+ regulatory T cells in brain, lymph nodes, and spleen control mice. c Regulatory T cell/CD8+ ratio in brain. d Proportion of FoxP3+ of CD4+ T cells in brain
The pro-tumor effects may be mediated by CD4+ FoxP3+ regulatory T cells (Tregs), which suppress activation of effector cells and provide immune escape for gliomas [15]. The proportion of Tregs was found to be significantly higher within the brain than in lymph nodes (p < 0.0001) and spleen (p < 0.0001) in the control group. (Fig. 3b). Combination therapy significantly improved the Treg to CD8+ T cell ratio within the brain among all arms (p < 0.0001) (Fig. 3c).
Immunotherapy increases levels of circulating inflammatory anti-tumor cytokines
Anti-tumor CD8+ effector and Th1 CD4+ TCs release pro-inflammatory cytokines, IFNγ and TNFα, following stimulation. All treatment groups demonstrated significantly elevated levels of IFNγ production by CD8+ cells compared to control (anti-CXCR4: p = 0.0039; anti-PD-1: p = 0.0435; combination therapy: p = 0.0039) (Fig. 4a). Likewise, all treatment arms had significantly elevated levels of TNFα production by CD8+TCs with no significant difference between the combination therapy and the monotherapy groups. Similarly for CD4+ populations, treatment groups exhibited significantly elevated levels of both IFNγ (anti-CXCR4: p = 0.0278; anti-PD-1: p = 0.0012; combination therapy: p = 0.0009) and TNFα production (anti-PD-1: p = 0.0012; combination therapy: p = 0.0054) compared to control (Fig. 4c, d).
Discussion
Glioblastoma (GBM) have poor prognoses despite standard of care treatment that involves maximal surgical resection with adjuvant temozolomide chemotherapy or radiation [2]. Numerous studies have described the altered immunosuppressive GBM tumor microenvironment that contributes to treatment difficulties. Our study demonstrated that anti-PD-1 plus anti-CXCR4 combination blockade conferred a significant survival benefit in a GL261 murine glioma model through modulation of the myeloid and T cell tumor microenvironment and potentially tumor bed vasculature. Furthermore, administration of immunotherapeutic antibody, whether as monotherapy or synergistic therapy, resulted in long-term immunity against GL-261-Luc glioma cells in surviving animals, a phenomenon also seen in other immunotherapy regimens, such as dosage with IL-15 superagonist and with anti-PD-1 and anti-TIM3 combination therapy [5, 16]. In these cases, CD8+ T cells can become primed as memory cells for long-term maintenance of the effector CD8+ phenotype.
CXCR4 is a chemokine receptor normally involved in immune cell homing and cell chemotaxis among other functions along with its ligand CXCL12/SDF-1. It is ubiquitously expressed on most types of immune cells, including T lymphocytes, macrophages, monocytes, dendritic cells, and progenitor cells, as well as vascular endothelial cells and microglia. In addition, CXCR4 is overexpressed in over 23 different cancers, leading to tumor proliferation, aberrant angiogenesis, metastasis, and treatment resistance [10].
First of all, it is known that gliomas may direct glioma-associated microglia or macrophages (GAMs) to convert to the pro-tumor, pro-angiogenesis, anti-inflammatory M2 polarity instead of the pro-inflammatory M1 phenotype. Appropriate immunotherapeutics may be successful in modulating the pro-tumor microenvironment by affecting these immunosuppressive cell populations. For instance, CXCR4 itself is upregulated on endothelial cells within tumor microenvironments that are also characterized by M2 GAM polarization [17] When we were assessing CXCR4+ populations of these myeloid cells in particular, we discovered that the population of CXCR4+ CD11b+ microglia was significantly diminished in the group receiving combination therapy when compared to control. Another group also found that CD11b+ GAMs were significantly decreased in GBM-bearing animals receiving another CXCR4 antagonist, peptide R, as well [18]. In fact, CXCR4, its ligand CXCL12, and other chemokine receptors and ligands (CXCL16, CXCR7) were found to be highly transcribed and expressed on GAMs, further emphasizing the important role of GAMs in tumor promotion [19, 20]. In addition to CXCR4, CXCL12 also binds CXCR7, which is implicated in poor prognosis in glioma as well as involved in aberrant glioma cell proliferation and invasion [21, 22].
The glioma microenvironment may also induce recruitment and accumulation of immunosuppressive populations, such as myeloid-derived suppressor cells (MDSCs). For example, GAMs produce the chemokine CCL2, which attracts monocytic MDSCs (M-MDSC; CD45brightCD11b+ Ly6C+) to the tumor region [7]. Furthermore, blood and brain tissue samples from GBM patients showed a tumor grade-associated increase in MDSCs [23]. In our study, the control group had significantly increased populations than the combination treatment group. In an ovarian cancer murine model, a similar combination of anti-PD-1 with the anti-CXCR4 agent, plerixafor, decreased the intratumoral population of MDSCs [24]. Tumor-infiltrating dendritic cells (DCs), characterized by the surface markers CD11b+ and MHCII+, also contribute to tumor promotion in melanoma, ovarian cancer, and lung cancer models [13, 14, 25]. Our study identified significant decreases in the tumor-infiltrating DCs population in all treatment groups. Unfortunately, not much is known about tumor-infiltrating DCs specific to glioma and GBM models, other than their modulation by checkpoint receptor inhibition and their activation and anti-tumor activity following IL-4 tumor cell vaccine administration [26]. However, recently it was found that survival benefit could be mediated by increased activation of tumor-infiltrating dendritic cells following combination treatment of anti-PD-1 with toll-like receptor (TLR3) agonist [8]. Upregulation of MHCII+ antigen-presenting cells and microglia aids the adaptive immune system by enhancing T cell population stimulation. Thus, given the modulation of various myeloid cell types within the tumor microenvironment, it would be fruitful to pursue potential treatments focusing on the myeloid compartment.
CD4+ and CD8+ tumor-infiltrating lymphocytes (TILs) have been shown to have important antitumor effects in several studies [27,28,29]. However, a high CD4+/CD8+ TIL ratio itself was correlated with poor prognosis as well as with tumor grade and malignancy in human GBM [30]. In addition, CXCR4 not only promotes migration of immunosuppressive cell populations but also negatively affects cytotoxic CD8+ T cell function [31]. Our study identified significant decrease in the CD4+/CD8+ ratio in the combination therapy group, indicating the concurrent proliferation of cytotoxic CD8+ TIL following dual antibody treatment. The pro-tumor effects may be mediated by CD4+ FoxP3+ regulatory T cells (Tregs), which suppress activation of effector cells and provide immune escape for gliomas [9, 15, 28]. The level of Tregs was found to be significantly higher within the brain than in peripheral compartments in our study. In addition, antibody treatment improves pro-inflammatory cytokine production, namely IFNγ and TNFα. Our data suggest that TNFα production is driven primarily by anti-PD-1 while both anti-CXCR4 and anti-PD-1 play roles in influencing IFNγ production. Likewise, CXCR4 blockade in a murine model of allergic lung inflammation resulted in an increase in IFNγ production [4].
While we have shown significant survival benefit in a murine glioma model with anti-CXCR4 and anti-PD-1 immunotherapy, it would be critical to characterize anti-CXCR4′s effect on the immunosuppressive tumor microenvironment further and to develop future experiments with patient-derived xenografts and potentially patient clinical trials as well.
Conclusion
In this study, we co-administered a novel CXCR4 chemokine receptor antagonist with anti-PD-1 to disrupt immunosuppression and induced a significant survival benefit as well as long-term immunity. We assessed the immune cell composition of the tumor microenvironment, which showed decreased density of immunosuppressive myeloid cell populations, more favorable T cell ratios and increased production of pro-inflammatory cytokines following combination treatment. As a result, CXCR4 may be a useful target to disrupt immunosuppression in GBM to facilitate an antitumor immune response.
References
Theeler BJ, Gilbert MR (2015) Advances in the treatment of newly diagnosed glioblastoma. BMC Med 13(1):293. https://doi.org/10.1186/s12916-015-0536-8
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996. https://doi.org/10.1056/NEJMoa043330
Weller M, Butowski N, Tran DD et al (2017) Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol 18(10):1373–1385. https://doi.org/10.1016/S1470-2045(17)30517-X
Reardon DA, Omuro A, Brandes AA et al (2017) Randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. Neuro Oncol 19(Suppl 3):iii21
Kim JE, Patel MA, Mangraviti A et al (2017) Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin Cancer Res 23(1):124–136. https://doi.org/10.1158/1078-0432.CCR-15-1535
Wainwright DA, Chang AL, Dey M et al (2014) Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res 20(20):5290–5301. https://doi.org/10.1158/1078-0432.CCR-14-0514
Chang AL, Miska J, Wainwright DA et al (2016) CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory t cells and myeloid-derived suppressor cells. Cancer Res 76(19):5671–5682. https://doi.org/10.1158/0008-5472.CAN-16-0144
Garzon-Muvdi T, Theodros D, Luksik AS et al (2018) Dendritic cell activation enhances anti-PD-1 mediated immunotherapy against glioblastoma. Oncotarget 9(29):20681–20697. https://doi.org/10.18632/oncotarget.25061
Arlauckas SP, Garris CS, Kohler RH et al (2017) In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med 9(389):eaal3604. https://doi.org/10.1126/scitranslmed.aal3604
Chatterjee S, Behnam Azad B, Nimmagadda S (2014) The intricate role of CXCR10 in cancer. Adv Cancer Res 124:31–82. https://doi.org/10.1016/B978-0-12-411638-2.00002-1
Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM (2010) Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest 120(3):694–705. https://doi.org/10.1172/JCI40283
Bian X, Yang S, Chen J et al (2007) Preferential expression of chemokine receptor CXCR12 by highly malignant human gliomas and its association with poor patient survival. Neurosurgery 61(3):570–579. https://doi.org/10.1227/01.NEU.0000290905.53685.A2
Pyfferoen L, Brabants E, Everaert C et al (2017) The transcriptome of lung tumor-infiltrating dendritic cells reveals a tumor-supporting phenotype and a microRNA signature with negative impact on clinical outcome. Oncoimmunology 6(1):e1253655. https://doi.org/10.1080/2162402X.2016.1253655
Karyampudi L, Lamichhane P, Krempski J et al (2016) PD-1 blunts the function of ovarian tumor–infiltrating dendritic cells by inactivating NF-κB. Cancer Res 76(2):239–250. https://doi.org/10.1158/0008-5472.CAN-15-0748
Sayour EJ, McLendon P, McLendon R et al (2015) Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol Immunother 64(4):419–427. https://doi.org/10.1007/s00262-014-1651-7
Mathios D, Park C-K, Marcus WD et al (2016) Therapeutic administration of IL-15 superagonist complex ALT-803 leads to long-term survival and durable antitumor immune response in a murine glioblastoma model. Int J cancer 138(1):187–194. https://doi.org/10.1002/ijc.29686
Choi S-H, Kim A-R, Nam J-K et al (2018) Tumour-vasculature development via endothelial-to-mesenchymal transition after radiotherapy controls CD44v6+ cancer cell and macrophage polarization. Nat Commun 9(1):5108. https://doi.org/10.1038/s41467-018-07470-w
Mercurio L, Ajmone-Cat MA, Cecchetti S et al (2016) Targeting CXCR19 by a selective peptide antagonist modulates tumor microenvironment and microglia reactivity in a human glioblastoma model. J Exp Clin Cancer Res 35(1):55. https://doi.org/10.1186/s13046-016-0326-y
Hattermann K, Sebens S, Helm O et al (2014) Chemokine expression profile of freshly isolated human glioblastoma-associated macrophages/microglia. Oncol Rep 32(1):270–276. https://doi.org/10.3892/or.2014.3214
Hattermann K, Held-Feindt J, Lucius R et al (2010) The Chemokine Receptor CXCR21 Is highly expressed in human glioma cells and mediates antiapoptotic effects. Cancer Res 70(8):3299–3308. https://doi.org/10.1158/0008-5472.CAN-09-3642
Birner P, Tchorbanov A, Natchev S, Tuettenberg J, Guentchev M (2015) The chemokine receptor CXCR22 influences prognosis in human glioma in an IDH1-dependent manner. J Clin Pathol 68(10):830–834. https://doi.org/10.1136/jclinpath-2015-202886
Liu Y, Carson-Walter E, Walter KA (2015) Targeting chemokine receptor CXCR2 inhibits glioma cell proliferation and mobility. Anticancer Res 35(1):53–64
Gielen PR, Schulte BM, Kers-Rebel ED et al (2016) Elevated levels of polymorphonuclear myeloid-derived suppressor cells in patients with glioblastoma highly express S100A8/9 and arginase and suppress T cell function. Neuro Oncol 18(9):1253–1264. https://doi.org/10.1093/neuonc/now034
Zeng Y, Li B, Liang Y et al (2019) Dual blockade of CXCL12-CXCR1 and PD-1-PD-L1 pathways prolongs survival of ovarian tumor-bearing mice by prevention of immunosuppression in the tumor microenvironment. FASEB J 23:12. https://doi.org/10.1096/fj.201802067RR
Nakahara T, Oba J, Shimomura C, Kido-Nakahara M, Furue M (2016) Early tumor-infiltrating dendritic cells change their characteristics drastically in association with murine melanoma progression. J Invest Dermatol 136(1):146–153. https://doi.org/10.1038/JID.2015.359
Eguchi J, Kuwashima N, Hatano M et al (2005) IL-4-transfected tumor cell vaccines activate tumor-infiltrating dendritic cells and promote type-1 immunity. J Immunol 174(11):7194–7201
Belcaid Z, Phallen JA, Zeng J et al (2014) Focal radiation therapy combined with 4–1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. Chen M, ed. PLoS ONE 9(7):e101764. https://doi.org/10.1371/journal.pone.0101764
Grauer OM, Nierkens S, Bennink E et al (2007) CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responsesin vivo. Int J Cancer 121(1):95–105. https://doi.org/10.1002/ijc.22607
Driessens G, Gordower L, Nuttin L et al (2008) Therapeutic efficacy of antitumor dendritic cell vaccinations correlates with persistent Th1 responses, high intratumor CD8+ T cell recruitment and low relative regulatory T cell infiltration. Cancer Immunol Immunother 57(12):1745–1756. https://doi.org/10.1007/s00262-008-0500-y
Han S, Zhang C, Li Q et al (2014) Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer 110(10):2560–2568. https://doi.org/10.1038/bjc.2014.162
Zhuang Y, Peng L-S, Zhao Y-L et al (2012) CD8(+) T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology 143(4):951–962.e8. https://doi.org/10.1053/j.gastro.2012.06.010
Ooi YC, Tran P, Ung N et al (2014) The role of regulatory T-cells in glioma immunology. Clin Neurol Neurosurg 119:125–132. https://doi.org/10.1016/j.clineuro.2013.12.004
Lukacs NW, Berlin A, Schols D, Skerlj RT, Bridger GJ (2002) AMD3100, a CxCR34 antagonist, attenuates allergic lung inflammation and airway hyperreactivity. Am J Pathol 160(4):1353–1360. https://doi.org/10.1016/S0002-9440(10)62562-X
Author information
Authors and Affiliations
Contributions
Project design: AW, ML, PC, ZB, HB, DP. Conduction of experiments: AW, RM, YX, MO, ZB, EK, AH, AL, DT, JC. Manuscript Draft: AW. Editing and reviewing of manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
This research was partly supported by Bristol-Myers Squibb, who generously provided anti-CXCR4 and anti-PD-1 antibodies.
Michael Lim, MD Research support: Arbor, Aegenus, Altor, BMS, Accuray, DNAtrix. Consultant: Tocagen, SQZ Technologies, VBI. Non-research: Consultant - Stryker, Baxter.
Henry Brem, MD Research support: Arbor Pharmaceuticals, Bristol-Myers Squibb, and Accurexa. Consultant: AsclepiX Therapeutics, Celsion-EGEN, Perosphere Inc., StemGen, Accelerating Combination Therapies, Camden Partners, LikeMinds, Inc, Acuity Bio Corp.
Drew Pardoll, MD, PhD Grants and Research support: Bristol-Myers Squibb, Melanoma Research Alliance. Consultant: Five Prime Therapeutics, Aduro, Compugen, GlaxoSmithKline, Medimmune/AstraZeneca, Merck, Potenza Therapeutics, Sanofi, Tizona, DNatrix, Amgen, Rock Springs Capital, Immunomic Therapeutics, Janssen, Astellas, WindMill Therapeutics, Bayer. Patents: patent for biomarkers useful for determining response to PD-1 blockade pending, patent for cancer therapy via combination of epigenetic modulation and immune modulation pending, patent for method of preventing organ transplant rejections using agonists to PD-1 checkpoint pathway pending, patent for cancer immunotherapy pending, patent for compositions and methods for targeting activin signaling to treat cancer pending, patent for combinatorial therapy of cancer and infectious diseases with anti-B7-H1 antibodies pending, patent for combination of immunotherapy with local chemotherapy for the treatment of malignancies pending, patent for inhibition of YAP for breaking tumor immune tolerance pending, patent for compositions and methods for cancer immunotherapy licensed to Aduro Biotech, and a patent for T cell regulation licensed to Bristol- Myers-Squibb.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, A., Maxwell, R., Xia, Y. et al. Combination anti-CXCR4 and anti-PD-1 immunotherapy provides survival benefit in glioblastoma through immune cell modulation of tumor microenvironment. J Neurooncol 143, 241–249 (2019). https://doi.org/10.1007/s11060-019-03172-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03172-5