Abstract
In patients with pituitary adenomas (PA) who are unable to undergo complete surgical resection, radiation therapy (RT), specifically stereotactic radiosurgery (SRS), results in excellent local control. However, the utility of radiosurgery may be limited by the proximity of the lesion to the optic chiasm (OC). We evaluate the efficacy of debulking surgery in increasing the PA-OC separation to convert patients into SRS candidates. From 2007 to 2015, 31 patients with PA < 2 mm from the OC underwent debulking surgery followed by RT within 2 years of resection. Coronal and sagittal T1-pre- and post-contrast sequences were used to determine PA-OC separation. Time interval between postoperative and pre-radiotherapy MRI scans and type of radiation therapy were analyzed. Functional tumor status, tumor characteristics [cavernous sinus (CS) or suprasellar (SS) involvement, chiasm/nerve encasement (NE)], and presence of ≥ 2 of these characteristics (multiple factors, MF) was also noted. Surgery converted 9 of 31 patients (29%) to SRS candidates. Median time from surgery to pre-RT planning MRI was 8 months (range 2–20). Of the 31 patients initially ineligible for SRS, 6 became eligible immediately after surgery, and another 3 were deemed eligible on follow-up. Mean PA-OC separation was 0.3 mm preoperative, 1.4 mm postoperative, and 2.1 mm at time of SRS (p = 0.002). Preoperative SS, NE, and MF involvement predicted pre-RT separation < 2 mm. Debulking surgery of unresectable pituitary tumors is a successful strategy for converting select radiosurgery-ineligible patients to radiosurgery candidates. Absence of preoperative SS, NE, and MF predicts for successful conversion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pituitary adenomas (PA) are benign neoplasms representing approximately 10% of primary intracranial neoplasms [1]. They are classified according to their functional status (non-secretory versus secretory) and can cause suppression of normal pituitary function or hypersecretion of pituitary hormones leading to specific syndromes (i.e. acromegaly, Cushing’s disease, hyperprolactinemia, and hyperthyroidism) [2]. While all tumors may cause some degree of hypopituitarism due to mass effect on surrounding structures [3], non-secreting tumors are less likely to present with hormone specific syndromes and more likely to present with neurologic deficits due to optic chiasm (OC) compression and cavernous sinus invasion, prompting early medical evaluation. Goals of treatment include reversal of hypersecretion (if present), restoration of pituitary function, removal of tumor mass, and/or restoration of normal neurologic function. Pharmacotherapy is frequently used for suppression of prolactinomas and growth hormone-secreting adenomas, but surgical resection followed by observation or RT is the current standard of care for refractory hypersecreting tumors, progressive non-secretory tumors, or tumors causing neurologic symptoms [4, 5]. Patients who are not surgical candidates are treated with fractionated stereotactic radiotherapy (fSRT) or stereotactic radiosurgery (SRS) as the sole modality [6]. SRS is an effective and convenient definitive or adjuvant treatment [7, 8]. However, due to anatomical limitations, the optic chiasm is often a primary dose-limiting structure for SRS with a maximum tolerated single fraction dose of approximately 10 Gy. This often necessitates the use of fSRT to minimize the risk of developing radiation-induced optic neuropathy [9]. Therefore, PA-OC separation strongly dictates the feasibility of SRS over fSRT. The purpose of this retrospective study is to evaluate a strategy of debulking surgery to increase PA-OC separation in order to facilitate SRS in patients initially deemed unsuitable for this procedure.
Methods
We conducted an institutional review board–approved retrospective review of all patients with the diagnosis of PA treated at our institution from 2007 to 2015. Eligible patients had a diagnosis of PA treated with LINAC-based frameless SRS or fSRT, had undergone debulking surgery within 2 years prior to radiotherapy, and had PA < 2 mm from the OC prior to surgery. All patients were 18 years or older and had preoperative, postoperative, and pre-RT imaging. Coronal and sagittal T1-pre and post-contrast sequences were used to measure the closest separation between PA and OC/optic structure using coronal and sagittal MRI images. Data regarding functional tumor status, time intervals between postoperative and pre-RT MRI scans and type of radiation therapy (SRS versus fSRT) was collected. Additionally, tumor characteristics of cavernous sinus (CS) involvement, suprasellar (SS) involvement, and chiasm/nerve encasement (NE) were recorded. All patients were discussed at a multi-disciplinary neuro-oncology tumor board. Surgical debulking was performed by the same group of experienced neurosurgeons, and all received routine follow-up evaluation by both a radiation oncologist and neurosurgeon. MRI of the brain with and without contrast was performed every 3–6 months in the postoperative setting. T-test analyses were used to compare PA-OC separation averages at different points in time. Statistical analysis using Fisher’s exact tests were used to predict how PA characteristics impacted PA/OC separation (SAS version 9.4, Cary, NC).
Results
Thirty-one patients met inclusion criteria for this study (15 male and 16 female), with median age of 63 years at the time of radiotherapy. Thirty patients had surgery as the initial treatment of their adenoma and one patient had surgery at the time of recurrence after an initial resection. Twenty-seven patients underwent transsphenoidal tumor resection and four underwent craniotomy based on neurosurgeon preference. Twenty patients had non-functional tumors and 11 had functional tumors; one ACTH-secreting, five prolactin-secreting, and five growth hormone-secreting. Cavernous sinus involvement was present in 16 patients, suprasellar extension in 22 patients, nerve encasement in seven patients, and multiple factors in 17 patients (Table 1).
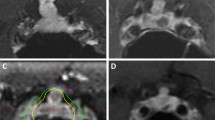
Following resection, nine patients were able to undergo SRS (one of which was treated with 5-fraction SRS). All remaining patients were treated with fSRT. All patients who were converted to SRS candidates had a transsphenoidal surgical approach. The number of patients with PA within 2 mm of OC at different times in their treatment course was analyzed using MRI (Table 2). Mean PA-OC separations were determined preoperatively, immediately postoperatively, and pre-RT (Table 3). All 31 patients had initial PA less than 2 mm from the OC. Preoperative mean PA-OC separation was 0.3 mm (range 0–2). Postoperative mean PA-OC separation was 1.4 mm (range 0-6.5). Six patients had a postoperative MRI showing PA greater than 2 mm from the OC (Fig. 1). Three additional patients had subsequent MRI scans showing PA greater than 2 mm from the OC. The mean time from surgery to RT was 9 months (median 8 months, range 2.6 to 15.6 months). Pre-RT mean PA-OC separation was 2.1 mm (range 0-7.9). Postoperative PA-OC mean separation was significantly more than preoperative PA-OC mean separation (p = 0.002). SRS doses ranged from 15 Gy to 24 Gy (Fig. 2) with one patient receiving 30 Gy in five fractions. All radiosurgery patients were able to receive the prescription dose to the entire tumor while keeping OC dose < 10 Gy. fSRT doses in the radiosurgery ineligible patients were 50.4–54 Gy. No patients in either group developed RION with follow up ranging from 0.5 to 8 years.
Mean changes in PA-OC separation between preoperative versus immediate postoperative MRI, and preoperative versus pre-RT MRI were evaluated. Compared to the preoperative MRI, the mean change in the postoperative minus PA-OC separation was 0.9 mm (range 0–4) and mean change in pre-RT PA-OC separation was 1.6 mm (range 0–5.9, p = 0.0001).
The presence of preoperative characteristics of suprasellar extension, nerve encasement, and multiple factors predicted for pre-RT separation of PA-OC of less than 2 mm (p = 0.001, p = 0.02, and p = 0.004, respectively). Cavernous sinus involvement did not predict for pre-RT separation of PA-OC of less than 2 mm (p = 0.2) (Table 4). Five of 20 non-functional and four of 11 functional adenoma patients were converted to radiosurgery candidates. One of four functional adenomas subsequently recurred, with no recurrences of the 5 non-functional adenomas (mean follow-up 2.6 years, range 0.5 to 8 years). One of the nine radiosurgery candidates had re-resection of PA prior to SRS while four of 22 fSRT patients had re-resection prior to fSRT.
Discussion
While SRS and fSRT are both effective treatments for pituitary adenoma, SRS offers multiple advantages over fSRT. “Separation surgery” has been described for the management of metastatic spine tumors but, to our knowledge, its use has not been reported for treatment of pituitary adenoma [10,11,12]. Here we report the success of converting 9/31 (29%) patients with pituitary adenoma who were initially ineligible for SRS into potential candidates using debulking surgery.
SRS may be more effective than fSRT in the management of functioning pituitary adenomas. Functioning pituitary adenomas pose a significant management challenge because successful treatment requires both tumor control and normalization of endocrine levels. These tumors often require a higher dose of radiation therapy to achieve these goals, with lower tumor control probability than nonfunctioning adenomas [13, 14]. The value of SRS in this setting was demonstrated in a review of SRS for PA outcomes with significantly improved hormone normalization in functional adenomas compared with fSRT [7, 15].
Additionally, SRS is completed in a single treatment session compared to 25–30 treatments for fractionated stereotactic radiotherapy resulting in a significant improvement in convenience for the patient. Long-term recurrence rates of nonfunctioning pituitary adenomas following surgery alone can be as high as 50% [7, 16, 17], prompting the addition of adjuvant fractionated radiation therapy in 25–30 treatments to improve 10 year progression-free survival rates to greater than 90% [2]. However, multiple large SRS studies of nonfunctioning pituitary adenomas have resulted in comparable or improved control rates > 90% at five years and > 80% at 10 years with acceptable treatment-related toxicities [7, 18].
Finally, SRS can prescribe a higher biologically effective dose to the tumor while limiting dose to surrounding normal structures. This higher BED damages adenoma cells both directly and indirectly by killing tumor cells through DNA damage as well as damage to the tumor’s vascular supply. This may be the explanation for the higher rates of tumor control with SRS previously reported.
An important consideration for SRS treatment involves limiting the dose of radiation to parasellar cranial nerves, specifically the anterior visual pathway (AVP). Improvements in thin-slice MRI imaging, fusion software, and on-board imaging have improved the confidence of dose delivery to tumor and normal structures. Radiation induced optic neuritis (RION) rates are low if the maximum dose to the AVP is less than 12 Gy. For example, in a retrospective review of 222 patients treated using Gamma Knife® radiosurgery (GKRS), rates of RION were 10% with dose to AVP greater than 12 Gy and 0% otherwise [9]. To deliver prescription dose to the tumor while keeping the dose to the optic chiasm and nerves below tolerance, adequate separation between the tumor and AVP is required. The necessary separation has generally been 2–3 mm, and therefore a treatment approach that could increase this separation and therefore decrease dose to the AVP could potentially decrease the risk of RION.
In our analysis of 31 patients with PA within 2 mm of the optic chiasm or optic structures, nine total patients were converted to SRS candidates with PA greater than 2 mm from the optic chiasm. Six patients were converted in the immediate postoperative setting and three additional patients were converted with some delay (3–15 months) between surgery and initiation of radiation therapy for an overall conversion rate of 29%. These patients were then able to undergo SRS rather than fSRT. Predictably, preoperative suprasellar involvement, nerve encasement, and the presence of multiple adverse factors predicted for a final separation of < 2 mm and inability to undergo radiosurgery. Of interest, all patients who were converted to SRS candidates had undergone transsphenoidal surgery, though it not possible to determine from our data if this approach was more effective for conversion than other surgical techniques.
Others have recognized the potential benefit of neurosurgical resection prior to SRS in PA patients. Fu, et al. describe their use of pterional craniotomy and microneurosurgery on 59 PA patients, treating with GKRS to residual tumor 3 months following surgery. However, optic chiasm dose was limited to 9.5 Gy in all cases, resulting in compromised tumor coverage for some patients. Our study, in contrast, did not compromise tumor coverage to spare dose to optic chiasm or nerves, but rather evaluated the separation between tumor and chiasm to select for SRS feasibility. In the study by Fu et al. seven of 59 patients (11.9%) experienced complications of visual deterioration, but only one of those seven had decreased vision after completion of GKRS as opposed to six with visual deterioration before GKRS [19].
Timing of radiosurgery after neurosurgical debulking was also a consideration for the current study. Pomeraniec and colleagues evaluated clinical outcomes of patients treated with transsphenoidal resection (TSR) for non-functioning PA followed by early (< 6 months) versus late (> 6 months) GKRS. Longer time intervals allowed their patients time to recover from TSR, improved post-surgical changes, and allowed clearer imaging for GKRS purposes. However, they noted a greater risk of tumor progression after delayed GKRS, with significantly more patients with residual tumor at last follow-up [20]. We hypothesized that a longer time interval between surgery and initiation of radiotherapy would allow the tumor more time to separate from the optic structures. We completed imaging every 3 to 6 months in the postoperative setting to evaluate this separation.
The current study has limitations, including its retrospective nature. MRI images were not all taken using the same slice thickness on coronal, sagittal and axial collections, and pre-RT and post-RT imaging typically utilized thinner slices with higher resolution for RT planning and fusion purposes. All MRI images were not taken at the same institution nor on the same scanner. Patient head position variability was also a confounding factor in making these measurements. In addition, neuro-endocrine follow-up was not available for most patients.
Despite these limitations, which are representative of the heterogeneity in imaging in clinical practice, our study succeeds in demonstrating the feasibility of a novel approach to facilitating SRS for pituitary adenomas in close proximity to optic structures. Of the 31 patients who initially were non-SRS candidates, debulking surgery converted 9 of them into SRS candidates within 15 months of surgery. Therefore, this study supports debulking as a practical approach to managing patients with PA close to AVP.
Conclusions
For patients with pituitary adenomas, SRS is both convenient and effective. However, patients with PA adjacent to AVP are suboptimal SRS candidates given their risk of radiation-related toxicity. In nearly 30% of these patients, debulking surgery to increase the separation between the PA and AVP, followed by radiosurgery, may represent a feasible treatment paradigm.
References
Pamir MN, Kiliç T, Belirgen M, Abacioglu U, Karabekiroglu N (2007) Pituitary adenomas treated with gamma knife radiosurgery: Volumetric analysis of 100 cases with minimum 3 year follow-up. Neurosurgery 61:270 – 80
Brada M, Rajan B, Traish D et al (1993) The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clinical Endocrinol 38:571–578
Alameda C, Lucas T, Pineda E et al (2005) Experience in management of 51 non-functioning pituitary adenomas: indications for post-operative radiotherapy. J Endocrinol Investig 28:18–22
Park P, Chandler WF, Barkan AL et al (2004) The role of radiation therapy after surgical resection of nonfunctional pituitary macroadenomas. Neurosurgery 55:100–107
van den Bergh AC, Van den Berg G, Schoorl MA et al (2007) Immediate postoperative radiotherapy in residual nonfunctioning pituitary adenoma: beneficial effect on local control without additional negative impact on pituitary function and life expectancy. Int J Radiat Oncol Biol Phys 67:863–869
Castinetti F, Nagai M, Morange I et al (2009) Long-term results of stereotactic radiosurgery in secretory pituitary adenomas. J Clin Endocrinol Metab 94:3400–3407
Sheehan JP, Niranjan A, Sheehan JM et al (2005) Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J Neurosurg 102:678–691
Minniti G, Gilbert D, Brada M (2009) Modern techniques for pituitary radiotherapy. Rev Endocr Metab Disord 10:135–144
Leavitt JA, Stafford SL, Link MJ, Pollock BE (2013) Long-term evaluation of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 87:524–527
Fisher C, Batke J (2013) Editorial: separation surgery. J Neurosurg Spine 18:205–206; discussion p 6
Laufer I, Iorgulescu JB, Chapman T et al (2013) Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine 18:207–214
Moulding HD, Elder JB, Lis E et al (2010) Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases. J Neurosurg Spine 13:87–93
Zierhut D, Flentje M, Adolph J, Erdmann J, Raue F, Wannenmacher M (1995) External radiotherapy of pituitary adenomas. Int J Radiat Oncol Biol Phys 33:307–314
McCord MW, Buatti JM, Fennell EM et al (1997) Radiotherapy for pituitary adenoma: long-term outcome and sequelae. Int J Radiat Oncol Biol Phys 39:437–444
Kim JW, Kim DG (2014) Stereotactic radiosurgery for functioning pituitary adenomas. World Neurosurg 82:58–59
Laws ER, Sheehan JP, Sheehan JM, Jagnathan J, Jane JA Jr, Oskouian R (2004) Stereotactic radiosurgery for pituitary adenomas: a review of the literature. J Neuro 69:257–272
Greenman Y, Ouaknine G, Veshchev I, Reider-Groswasser I, Segev Y, Stern N (2003) Postoperative surveillance of clinically nonfunctioning pituitary macroadenomas: markers of tumour quiescence and regrowth. Clin Endocrinol 58:763–769
Mitsumori M, Shrieve DC, Alexander E, 3rd, et al (1998) Initial clinical results of LINAC-based stereotactic radiosurgery and stereotactic radiotherapy for pituitary adenomas. Int J Radiat Oncol Biol Phys 42:573–580
Fu P, He Y-s, Cen Y-c et al (2016) Microneurosurgery and subsequent gamma knife radiosurgery for functioning pituitary macroadenomas or giant adenomas: one institution’s experience. Clin Neurol Neurosurg 145:8–13
Pomeraniec IJ, Dallapiazza RF, Xu Z, Jane JA Jr, Sheehan JP. Early versus late Gamma Knife radiosurgery following transsphenoidal resection for nonfunctioning pituitary macroadenomas: a matched cohort study. J Neurosurg 2015:1–11
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Rights and permissions
About this article
Cite this article
Forster, N., Warnick, R., Takiar, V. et al. Debulking surgery of pituitary adenoma as a strategy to facilitate definitive stereotactic radiosurgery. J Neurooncol 138, 335–340 (2018). https://doi.org/10.1007/s11060-018-2801-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2801-0