Abstract
Cetuximab conjugated iron-oxide nanoparticles (cetuximab-IONPs) have shown both in-vitro and in-vivo anti-tumor efficacy against gliomas. The purpose of this pilot study was to evaluate the safety and potential efficacy of cetuximab-IONPs for treatment of spontaneously occurring intracranial gliomas in canines after convection-enhanced delivery (CED). The use of CED allowed for direct infusion of the cetuximab-IONPs both intratumorally and peritumorally avoiding the blood brain barrier (BBB) and limiting systemic effects. A total of eight dogs participated in the study and only two developed mild post-operative complications, which resolved with medical therapy. All canines underwent a single CED treatment of the cetuximab-IONPs over 3 days and did not receive any further adjuvant treatments. Volumetric analysis showed a median reduction in tumor size of 54.9% by MRI at 1-month (4–6 weeks) follow-up. Five dogs were euthanized due to recurrence of neurological signs other than seizures, two due to recurrent seizures, and one dog died in his sleep. Median survival time after surgery was 248 days (mean 367 days).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomas are the second most common type of brain tumor in canines, reported to account for up to 36% of central nervous system (CNS) tumors [1, 2]. These tumors have a similar prevalence and poor prognosis in humans as in canines [3, 4]. Although accurate data regarding prognosis following treatment in canines with gliomas is limited, median survival rates are reported to be < 1 year even when considered low-grade [5]. Canine spontaneous glioma tumors share many similarities including clinical, pathologic, molecular, and genetic properties with human gliomas, making dogs an attractive naturally-occurring, large animal model for validation of novel therapies [6,7,8,9].
Recently, there have been advances in understanding the molecular biology of gliomas, which has led to targeted therapy of these tumors [10, 11]. Identification of molecular signaling pathways implicated in gliomagenesis in canines can lead to development and translation of novel targeted therapies for canine gliomas [12, 13]. Potential targets that are over-expressed in spontaneous canine gliomas include proteins such as the epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor alpha (PDGFRα), insulin-like growth factor binding protein-2 (IGFBP-2), interleukin 13 receptor alpha 2 (IL-13RA2), and activating transcription factor 5 (ATF5) [14,15,16]. EGFR is an attractive molecular therapeutic target and a known oncogenic factor in both human and canine gliomas [9, 17]. EGFR is amplified in 40–50% of glioblastomas (GBMs) in humans and has been associated with a poor prognosis [18,19,20,21]. Additionally, EGFR was shown to be the most common glioma mutation with The Cancer Genome Atlas (TCGA) [22, 23]. In a canine glioma study, EGFR expression was found to be increased in all canine tumor types and grades, being more consistently increased than other cell surface receptors [17]. Small molecule tyrosine kinase inhibitors (TKIs) and monoclonal antibodies have been developed to target EGFR [24, 25]. Cetuximab (Erbitux; ImClone LLC), a human-mouse chimeric monoclonal antibody, binds specifically to EGFR preventing dimerization and activation of the tyrosine kinase and thereby inhibiting downstream signal transduction [24, 25]. Canine and human tumor-associated antigens, ErbB-1 and -2, share over 90% amino acid homology [26]. Cetuximab is an anti-EGFR (anti-ErbB-1) antibody and was found to bind to canine mammary carcinoma cells with EGFR expression resulting in inhibition of canine tumor cell proliferation [26]. Cetuximab has been shown to reduce cell proliferation, inhibit tumor angiogenesis, and enhance radiosensitivity by promoting radiation-induced apoptosis with inhibition of radiation-induced damage repair [27]. It has shown efficacy as a single agent in advanced non-small cell lung cancer (NSCLC), ovarian cancer, head and neck cancer, and colorectal cancer [27]. Systemic administration of cetuximab has been evaluated in Phase II clinical trials in human patients with recurrent GBM and demonstrated minimal toxicity but limited efficacy and survival benefit [18, 28, 29].
Magnetic iron oxide nanoparticles (IONPs) have recently been shown to be effective for glioma targeting and can be modified by the addition of a peptide or antibody specific for tumor cells on their surface [30]. Due to their ferromagnetic properties, IONPs also provide magnetic resonance imaging (MRI) contrast enhancement and serve as a useful tool for imaging brain tumors [31]. Theranostic IONPs conjugated to an EGFR antibody have been shown to provide selective MRI contrast enhancement of human GBM tumor cells and significant antitumor efficacy with orthotropic human GBM xenografts [30]. Treatment with cetuximab conjugated IONPs (cetuximab-IONPs) resulted in a significant anti-tumor effect that was greater than with cetuximab alone due to more efficient cellular targeting and uptake, EGFR signaling alterations, EGFR internalization, and apoptosis induction in EGFR-expressing glioma stem-like cells (GSCs), as well as tumor non-stem cells [32].
Overcoming the blood brain barrier (BBB) and maximizing therapeutic efficacy directly impacts the success of a targeted therapeutic for GBM [10]. The large size of cetuximab precludes its ability to cross an intact BBB [28]. Convection-enhanced delivery (CED) of agents into the brain is designed to overcome this barrier and limit the systemic effects of pharmacologic agents by direct pressure dependent infusion into the brain [7, 33,34,35]. Although CED has been used in dogs with intracranial gliomas, it has only been evaluated with the animals under continuous general anesthesia using real-time MRI [36]. Our group has successfully performed intracranial CED of cetuximab-IONPs in a healthy canine model previously [37]. Robust delivery of nanoparticles into the brain was achieved by CED and effective quantitative monitoring of distribution and dispersion of nanoparticles after CED was provided by MRI. No safety, intracranial or systemic toxicity issues were found after CED of cetuximab-IONPs [37]. The aim of this pilot study was to evaluate the safety and potential efficacy of cetuximab-IONPs CED for treatment of spontaneously occurring gliomas in canines.
Materials and methods
Patient recruitment
Dogs with a pre-operative MRI documenting a suspected glioma amenable to surgery were recruited for participation in the study conducted at the University of Georgia, Veterinary Teaching Hospital (UGA VTH). Inclusion criteria were ultimately based on the following MRI characteristics consistent with spontaneously-occurring intracranial gliomas [38]: intra-axial mass, iso- to hyperintense on T2-weighted imaging, iso- to hypo-intense on pre-contrast T1-weighted imaging, with variable peri-lesional edema and contrast enhancement. All procedures were approved by the UGA-VTH clinical research committee conforming to Institutional Animal Care and Use Committee (IACUC) regulations.
MRI and anesthesia
All dogs had a complete blood count, biochemical analysis, and urinalysis prior to anesthesia for imaging. The brain MRI scans were performed at UGA either with a 3.0 T MRI unitFootnote 1, Footnote 2 with a single-channel quadrature knee coil or a 1.5TFootnote 3 unit with a 15-channel transmit-receive quadrature knee coil. MRI scans performed at other centers were performed using the units available at the practice. Dogs received butorphanolFootnote 4 (0.2 mg/kg IV) and diazepamFootnote 5 (0.2 mg/kg IV) as a premedication; anticholinergic drugs (atropineFootnote 6 or glycopyrrolateFootnote 7) were administered based on heart rate and blood pressure. Anesthesia was induced with propofolFootnote 8 (4 mg/kg IV or to effect) and was maintained with isofluraneFootnote 9 or propofol CRI.
Dogs were positioned in sternal recumbency. MR images of the brain were obtained in the sagittal, transverse (slice thickness 3 mm), and dorsal planes using the following pulse sequences: T1-weighted fluid attenuated inversion recovery (FLAIR), T2-weighted, and T2W FLAIR, Gradient Echo, and additional pulse sequences based on clinician preference. Following the intravenous administration of 0.1 mmol/kg of gadopentetate dimeglumine,Footnote 10 further T1W FLAIR sequences were obtained in sagittal, dorsal, and transverse planes and fast spoiled gradient echo (FSPGR) T1W images.
Surgery and CED catheter placement
A peripheral intravenous (IV) catheter was placed in all dogs prior to surgical procedures. Dogs were premedicated with IV fentanyl (5 µg/kg) and diazepam (0.25 mg/kg) separately, and anesthesia was then induced with IV propofol (4 mg/kg titrated to effect). Once intubated, dogs were maintained on oxygen, and a low percentage of isoflurane to minimize increases in intracranial pressure. During the surgical procedure, a continuous rate of infusion (CRI) of fentanyl (5–10 µg/kg/h) was administered, in addition to a propofol CRI (6–20 mg/kg/h titrated to effect). All dogs were mechanically ventilated to maintain a low normal end tidal carbon dioxide (CO2). Homeostasis was maintained using an IV administration of lactated Ringer’s solution (5 ml/kg/h). Administration of IV buprenorphine (0.02 mg/kg) provided post-operative analgesia.
A craniotomy was performed in all cases for either open biopsy or partial tumor resection, followed by CED of the cetuximab-IONPs. The tumor extent of resection, ranged from 0 to 67.2%, with a mean extent of tumor resection of 12.5%. Intraoperative samples of the tumor were submitted for frozen histopathologic confirmation of glioma in 3/8 patients. In the remaining patients, histopathologic diagnosis was confirmed post-operatively. After partial tumor resection or open biopsy, two FDA-approved catheters (MedtronicFootnote 11 intrathecal catheters Indura 8709SC and Ascenda 8780) for intrathecal use, were implanted within the residual tumor. Intrathecal catheters were used for cetuximab-IONP CED based upon our prior experience with healthy canines [37]. An attempt was made to separate the catheter tips by at least 1 cm if possible. The two catheters were then tunneled through the subcutaneous tissue under the skin and connected each to an external programmable reservoir infusion pump [MedtronicFootnote 12 SynchroMed II Programmable Pump 8637] (Fig. 1). The pumps were secured on the head or body of each patient with sutures and bandaging material, after which the dogs were recovered from anesthesia. (Fig. 1).
Cetuximab-IONPs infusion
Each pump was pre-filled with cetuximab-IONPs, which were prepared in the Brain Tumor Nanotechnology Laboratory at Emory University using a technique previously described [32]. Infusions were performed after programming each external reservoir pump by utilizing a handheld device (MedtronicFootnote 13 N’Vision Clinician Programmer 8840). Each canine patient underwent a 72 h infusion of cetuximab-IONPs at a concentration of 0.5 mg/ml and at a CED rate of 0.5 µl/min based upon our prior CED experience with healthy canines [37]. A total of 4.32 ml (2.16 ml per pump/catheter) of infusate (2.16 mg of cetuximab-IONPs) was administered by CED to each canine patient. The CED infusions were performed while the dogs were awake in the hospital. After the completion of the infusion, the catheters were removed without the need for sedation or anesthesia.
Follow-up
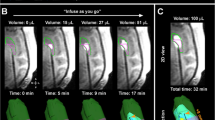
Follow-up MRI scans were performed in canine patients at the following time points: 24 h or immediately post-operatively (8/8), 3/8 at 7 days, 8/8 at 4–6 weeks (1 month visit), 5/8 at 3 months, 2/8 at 6 months, and at 1 year, 18 and 24 months in one dog. Postoperative SPACE (Sampling Perfection with Application optimized Contrasts using different flip angle Evolution) MRI sequences were obtained in addition to True FISP (Fast Imaging with Steady Precession) to further define catheter placement and IONP visualization. True FISP suppresses the signal from hemorrhage while maintaining the susceptibility associated with the IONPs. These produce distinct magnetic dipole areas of signal loss for direct imaging of the IONPs (Fig. 2).
Postoperative MRI of a canine glioma patient following surgical placement of two catheters and cetuximab-IONP CED. a Transverse 0.5 mm thick proton-density SPACE (Sampling Perfection with Application optimized Contrasts using different flip angle Evolution) MRI sequence reveals two linear hypointense catheters (white arrows) that are intra-tumoral. b Transverse 2.5 mm thick T2-weighted and c dorsal/coronal 1.2 mm thick True FISP (Fast Imaging with Steady Precession) MRI. It is more difficult to evaluate the catheter and IONPs on standard T2-weighted MRI (b) due to the blooming artifact of the IONPs and post-surgical hemorrhagic products. However, IONPs can be visualized by T2-weighted imaging at the tip of the more medially extending catheter (b, arrow), as well as intratumorally by the other catheter. True FISP suppresses the signal from hemorrhage while maintaining the susceptibility associated with the IONPs. These produce distinct magnetic dipole areas of signal loss and permit direct visualization of the IONPs after CED (c, arrows)
Following euthanasia, brains from two dogs were fixed in 10% buffered formalin and examined microscopically for residual tumor.
Volumetric evaluation
OsirixFootnote 14 imaging software was used to evaluate pre-operative and post-operative MRIs of all patients. OsirixFootnote 15 was also used to determine volumetric data. Tumors were measured in the immediate post-operative scan and the 1 month post-operative scan using the following technique: on each T2-weighted FLAIR transverse image of a complete series, the tumor mass was manually outlined for each patient in OsirixFootnote 16, and the program used this data to calculate the volume of the lesion in cm3.
Tumor identification, grading, and EGFR status
Formalin fixed biopsy and tumor tissue was processed routinely and embedded in paraffin. Four micron sections were stained with hematoxylin and eosin (H&E), oligodendrocyte transcription factor 2 (Olig2), and glial fibrillary acidic protein (GFAP) by immunohistochemistry (IHC) to confirm tumor type. Grading of tumors was performed by a pathologist and followed the World Health Organization (WHO) classification of human brain tumors [39]. When adequate tissue was available, the tumors were also stained for EGFR by IHC and staining was subjectively scored 0–3 (0 = none; 1 = mild; 2 = moderate; 3 = marked) as compared to a positive control (canine squamous cell carcinoma that had marked immunoreactivity).
For immunohistochemistry, sections were stained with rabbit anti-Olig2Footnote 17 (citrate buffer and heat retrieval, 1:400), mouse anti-GFAPFootnote 18 (citrate buffer and heat retrieval, 1:4000), and mouse anti-EGFRFootnote 19 (31G7 + GFR1195; citrate buffer and heat retrieval, 1:8000) followed by biotinylated secondary antibodies,Footnote 20 a streptavidin-HRP conjugated labelFootnote 21, and 3,3 diaminobenzidine (DAB)Footnote 22 as the chromogen. Appropriate positive controls were used and in the negative control, isotype rabbit or mouse control serumFootnote 23 replaced the primary antibody.
Results
The eight dogs included three Boxers, two Boston terriers, one Pit bull, one French bulldog, and one mixed breed dog (Table 1). Clinical signs at presentation included seizures (7/8), mentation changes (5/8), and circling (3/8). All tumors were rostrotentorial and oligodendrogliomas based on WHO criteria. Seven of eight were WHO grade II tumors; one had insufficient tissue for grading. There was sufficient tissue for EGFR immunohistochemistry in 5/8 tumors. One tumor did not have EGFR expression (score = 0), two tumors had mild staining (score = 1), one tumor had moderate staining (score = 2), and one tumor had marked staining (score = 3) (Table 2). Both dogs that had brains examined post mortem had tumor regrowth with tumors having similar histopathology to the original biopsy submission.
In our study, one dog had an EGFR negative tumor and three canine tumors had unknown EGFR status. The patient with an EGFR negative tumor had a 42.6% reduction in residual tumor size at 1-month postoperatively based on volumetric MRI measurements. Only one dog did not show a noticeable reduction in tumor size at the 1 month post-op MRI, but the EGFR status could not be determined for this patient due to insufficient tissue availability for histologic analysis. EGFR status was also not available for two additional patients, but these patients did have a reduction in tumor size (100% and 33.4%) (Fig. 3).
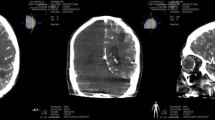
Antitumor effect of cetuximab-IONP CED in a canine glioma patient. a Preoperative T2-weighted sagittal MRI scan revealing a large glioma tumor causing mass effect on the brain (white arrow). b Postoperative T2-weighted sagittal MRI at 1 month showing residual tumor (white arrow) with cetuximab-IONP artifact present within the tumor (red arrows). c Postoperative MRI at 18 months showing complete resolution of tumor after cetuximab-IONP CED treatment
Complications
Six out of the eight dogs did not have any post-operative complications. One dog developed an elevated body temperature that resolved with antibiotics and one dog became hypertensive, which was controlled with an antihypertensive (amlodipine).
MRI volumetric evaluation
The immediate post-operative MRI confirmed correct catheter placement of both catheters into the residual tumor in six dogs (Fig. 2). In two dogs, only one catheter resided in the residual tumor cavity. In these two dogs, one of the catheters was within the lateral ventricle and the adjacent brain parenchyma, respectively. One of these canines did not have a measurable reduction in tumor size by volumetric MRI analysis.
MRI-assisted volumetric evaluation of the neoplastic lesions was compatible with a decreased tumor size at 1 month (4–6 weeks) when compared to the post-surgical lesion size (Table 3, Graph 1) (Fig. 3). Median residual tumor volume immediately following surgery was 5.11 cm3 (mean 6.48 cm3). At 1 month, median tumor volume was 2.81 cm3 (mean 3.29 cm3), indicating a 54.9% reduction in tumor size (mean 50.7%). One patient (patient 8) did not have a measurable reduction in tumor size.
Outcome
Five dogs were euthanized due to suspected tumor recurrence and progressive worsening of neurological signs (circling, mentation changes) and two were euthanized due to refractory seizures (one developed status epilepticus). One dog died in his sleep. Median survival time (MST) after surgery was 248 days (mean 367 days) (Table 1). None of the canines treated underwent any further adjuvant therapy after their single cetuximab-IONP CED treatment.
Discussion
Cetuximab-IONPs CED was originally evaluated by our group in a study of healthy laboratory canines and found to be a safe and effective delivery method in a larger animal model [37]. The dogs were infused with either free IONPs or cetuximab-conjugated IONPs at different CED infusion parameters (rates and volumes) and monitored with serial MRI scans. Volume of distribution was found to be linearly proportional to infusion volume and dispersion of the cetuximab-IONPs continued to occur 5 days following CED [37]. Slower infusion rates provided a more uniform distribution of IONPs and reduced infusate leak-back along the catheter track [37]. Infusion leakage along cannula tracks has also been shown in a separate study to occur at higher infusion rates (> 5 µl/min) with other therapeutics [7]. By securing the CED pumps and catheters to the patient, we were able to apply these concepts into a clinical canine model of naturally-occurring intracranial gliomas and demonstrate that infusion can be delivered in awake dogs safely and effectively over 3 days.
Cetuximab has been used to treat human GBM patients and has been shown to have an inhibitory effect on GBM cells in vitro and in vivo with pre-clinical models [40,41,42,43]. However, cetuximab has shown questionable efficacy in human GBM patients after systemic administration. Recently, cetuximab has been shown to potentially target recurrent GBM tumors by direct intra-arterial administration [44]. Multiple studies have confirmed that cetuximab can bind to the extracellular domain of human EGFR, targeting both the wild type EGFR (wtEGFR) and the EGFR variant III (EGFRvIII) deletion mutant [29, 40, 45]. Interestingly, cetuximab has also shown efficacy in EGFR negative tumors [46, 47]. We included canine patients without knowing their tumor EGFR status and found similar results. Possible reported explanations of response in EGFR negative tumors include lack of a consistent methodology and interpretation of EGFR IHC expression in tumor samples, variability in EGFR immunoreactivity depending on fixation method, a dramatic decline in staining intensity over time, and possibly lower levels of EGFR expression in tumors (false-negative IHC) [47]. Another explanation involves variability in the EGFR especially when compared to the commercially available anti-EGFR antibody for IHC [47].
Magnetic nanoparticles, most commonly composed of ferromagnetic iron-oxide, are small theranostic particles measuring under 100 nm [48, 49], which are biocompatible and non-toxic [50]. Although uptake of magnetic nanoparticles by malignant tumor cells has been demonstrated in culture and in vivo, the ability to modify their surface with molecules specific for tumors allows for more specific targeting [50]. Bioconjugated cetuximab-IONPs can be used for therapeutic targeting of GBM and simultaneous MR imaging both in vitro and in vivo after intracranial CED [32]. No toxicity of normal human astrocytes was observed with cetuximab-IONPs treatment [32]. Furthermore, CED of cetuximab-IONPs has shown significant efficacy in multiple rodent glioma models with prolonged animal survival [32]. The use of cetuximab-IONPs has been found to be more efficacious than cetuximab alone by greater cellular targeting and uptake, EGFR signaling alterations, EGFR internalization, and apoptosis induction in EGFR-expressing GBM tumor cells [32]. Furthermore, cetuximab-IONP CED in a rodent glioma model has shown radiosensitivity enhancement of tumors by decreased DNA damage repair and increased reactive oxygen species formation [51]. In our current study in canines with spontaneous gliomas, volumetric evaluation showed a reduction of tumor size by an average of over 50% from the immediate post-operative MR imaging to the 1 month (4–6 weeks) time-point. We believe our single cetuximab-IONP treatment was efficacious in our canine glioma patients even in patients with EGFR negative tumors (Fig. 3).
One of the major obstacles impeding effective treatment of brain tumors is the BBB. CED avoids the BBB allowing for direct delivery of an infusate into a tumor or tumor cavity, providing high intra-tumoral drug concentrations and limiting systemic toxicity [13]. In two out of eight canine patients, only one catheter was correctly placed in the tumor bed. Although incorrect catheter placement can impact the infusate delivery, one of these patients also had the longest survival. Despite this observation, the importance of optimal CED catheter positioning has been described in human patients with high-grade gliomas [52].
Spontaneous intracranial gliomas in dogs represent an attractive model to study the delivery and efficacy of potential therapeutic agents that could be translated to human GBM patients. Although GBM tumors are the most common high-grade glioma in humans, they tend to be rare in spontaneous canine gliomas, which explains why no canine patient in this study was found to have a GBM diagnosis [53]. Interestingly, the median survival of dogs with low-grade gliomas is reported to be about 1 year, which is similar to that of humans with GBM [54]. A decrease in the size of the residual tumor (median reduction in tumor size of 54.9%) was found in almost all of our patients by MRI volumetric analysis after a single cetuximab-IONP CED treatment. An effect was demonstrated with both EGFR-expressing and non-EGFR expressing canine glioma tumors. In the single patient that did not have an antitumor effect, only one of the catheters was properly placed within the tumor, which may have had an impact on response. Furthermore, our animals did not undergo any further adjuvant therapy and we believe the addition of fractionated external beam radiotherapy may have resulted in further enhanced treatment effect based on our preclinical studies. A future study investigating combination therapy of cetuximab-IONP CED with fractionated external beam radiotherapy after partial surgical resection in canines with spontaneous gliomas is required.
The authors understand that there are several limitations to this pilot study. It is difficult to draw conclusions about efficacy based on the low number of cases and lack of a control population. Additionally, the initial tumor size and amount of tumor removed likely affected long-term survival and varied between canine patients. Another limitation to veterinary studies is that survival is determined by euthanasia and time of euthanasia can vary between patients depending on the owner’s wishes. Unfortunately, two out of the eight patients (25%) were euthanized due to recurrent seizures; however, no necropsy information or imaging was available from these patients to evaluate tumor regrowth. Finally, necropsies were only performed in two patients, so we were unable to evaluate recurrence histopathologically or toxicity associated with the infusion. Regardless, none of the canine patients showed clinical signs of either CNS or systemic toxicities after cetuximab-IONP CED treatment.
In this study, we have demonstrated for the first time that cetuximab-IONP CED is a safe and effective adjuvant therapy for spontaneous canine glioma patients at the time of their initial tumor surgery. The IONPs can be directly imaged by MRI to confirm tumor targeting (Fig. 2). Furthermore, CED infusions can be delivered in awake patients without general anesthesia over 3 days (Fig. 3), a duration which has been shown experimentally to improve the volume of distribution within the tumor. CED of cetuximab-IONPs after surgical resection in canines with spontaneous gliomas may represent a unique and translational large animal model for targeting infiltrative cancer cells away from the tumor bulk, which are responsible for tumor recurrence in human patients. A large placebo controlled blinded clinical trial is necessary to further understand the role of this treatment for canine intracranial gliomas.
Notes
GE 3.0T Signa HDx; GE Healthcare, Milwaukee, WI.
3.0T Siemens Skyra.
Siemens Symphony, TIM technology.
Butorphanol tartrate; Torbugesic®, Fort Dodge, Fort Dodge, IA.
Diazepam, Valium®; Hospira, Lakeforest, IL.
Atropine sulfate; Med Pharmex, Pomona, CA.
Glycopyrrolate; Baxter Healthcare Corp., Deerfield, IL.
PropoFlo; Abbott Laboratories, North Chicago, IL.
Isoflurane, MDI, Boise, ID.
Gadopentetate dimeglumine, Magnevist®; Bayer HealthCare Pharmaceuticals, Wayne, NJ.
Medtronic, Inc., Minneapolis, MN.
See footnote 11.
See footnote 11.
OsiriX 3.6, Pixmeo, Bernex, Switzerland.
See footnote 14
See footnote 14
Rabbit anti-olig2; GeneTex, Irvine, CA.
Mouse anti-GFAP, Biogenex, San Ramon, CA.
Mouse anti-EGFR, Lifespan Biosciences.
Biotinylated secondary antibodies, Vector Laboratories, Burlingame, CA.
A streptavidin-HRP conjugated label, Biocare Medical, LLC, Concord, CA.
DAB, DAKO, Carpinteria, CA.
Isotype rabbit or mouse control serum, Biocare Medical, LLC, Concord, CA.
References
Snyder JM, Shofer FS, Van Winkle TJ, Massicotte C (2006) Canine intracranial primary neoplasia: 173 cases (1986–2003). J Vet Intern Med 20:669–675
Song RB, Vite CH, Bradley CW, Cross JR (2013) Postmortem evaluation of 435 cases of intracranial neoplasia in dogs and relationship of neoplasm with breed, age, and body weight. J Vet Intern Med 27:1143–1152. https://doi.org/10.1111/jvim.12136
Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol 14(Suppl 5): v1–v49. https://doi.org/10.1093/neuonc/nos218
Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, Burkhard C, Schuler D, Probst-Hensch NM, Maiorka PC, Baeza N, Pisani P, Yonekawa Y, Yasargil MG, Lutolf UM, Kleihues P (2004) Genetic pathways to glioblastoma: a population-based study. Cancer Res 64:6892–6899. https://doi.org/10.1158/0008-5472.CAN-04-1337
Hu H, Barker A, Harcourt-Brown T, Jeffery N (2015) Systematic review of brain tumor treatment in dogs. J Vet Intern Med 29:1456–1463. https://doi.org/10.1111/jvim.13617
Rossmeisl JH, Duncan RB, Huckle WR, Troy GC (2007) Expression of vascular endothelial growth factor in tumors and plasma from dogs with primary intracranial neoplasms. Am J Vet Res 68:1239–1245. https://doi.org/10.2460/ajvr.68.11.1239
Dickinson PJ, LeCouteur RA, Higgins RJ, Bringas JR, Larson RF, Yamashita Y, Krauze MT, Forsayeth J, Noble CO, Drummond DC, Kirpotin DB, Park JW, Berger MS, Bankiewicz KS (2010) Canine spontaneous glioma: a translational model system for convection-enhanced delivery. Neuro Oncol 12:928–940. https://doi.org/10.1093/neuonc/noq046
Stoica G, Kim HT, Hall DG, Coates JR (2004) Morphology, immunohistochemistry, and genetic alterations in dog astrocytomas. Vet Pathol 41:10–19. https://doi.org/10.1354/vp.41-1-10
Chakravarti A, Dicker A, Mehta M (2004) The contribution of epidermal growth factor receptor (EGFR) signaling pathway to radioresistance in human gliomas: a review of preclinical and correlative clinical data. Int J Radiat Oncol Biol Phys 58:927–931. https://doi.org/10.1016/j.ijrobp.2003.09.092
Sathornsumetee S, Rich JN (2008) Designer therapies for glioblastoma multiforme. Ann N Y Acad Sci 1142:108–132. https://doi.org/10.1196/annals.1444.009
Zhang X, Zhang W, Cao WD, Cheng G, Zhang YQ (2012) Glioblastoma multiforme: Molecular characterization and current treatment strategy (review). Exp Ther Med 3:9–14. https://doi.org/10.3892/etm.2011.367
Boudreau CE, York D, Higgins RJ, LeCouteur RA, Dickinson PJ (2017) Molecular signalling pathways in canine gliomas. Vet Comp Oncol 15:133–150. https://doi.org/10.1111/vco.12147
Dickinson PJ (2014) Advances in diagnostic and treatment modalities for intracranial tumors. J Vet Intern Med 28:1165–1185. https://doi.org/10.1111/jvim.12370
Higgins RJ, Dickinson PJ, LeCouteur RA, Bollen AW, Wang H, Wang H, Corely LJ, Moore LM, Zang W, Fuller GN (2010) Spontaneous canine gliomas: overexpression of EGFR, PDGFRalpha and IGFBP2 demonstrated by tissue microarray immunophenotyping. J Neuro-oncol 98:49–55. https://doi.org/10.1007/s11060-009-0072-5
Debinski W, Dickinson P, Rossmeisl JH, Robertson J, Gibo DM (2013) New agents for targeting of IL-13RA2 expressed in primary human and canine brain tumors. PLoS ONE 8:e77719. https://doi.org/10.1371/journal.pone.0077719
York D, Sproul CD, Chikere N, Dickinson PJ, Angelastro JM (2017) Expression and targeting of transcription factor ATF5 in dog gliomas. Vet Comp Oncol. https://doi.org/10.1111/vco.12317
Dickinson PJ, Roberts BN, Higgins RJ, Leutenegger CM, Bollen AW, Kass PH, LeCouteur RA (2006) Expression of receptor tyrosine kinases VEGFR-1 (FLT-1), VEGFR-2 (KDR), EGFR-1, PDGFRalpha and c-Met in canine primary brain tumours. Vet Comp Oncol 4:132–140. https://doi.org/10.1111/j.1476-5829.2006.00101.x
Neyns B, Sadones J, Joosens E, Bouttens F, Verbeke L, Baurain JF, D’Hondt L, Strauven T, Chaskis C, In’t Veld P, Michotte A, De Greve J (2009) Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol 20: 1596–1603. https://doi.org/10.1093/annonc/mdp032
Dunn IF, Heese O, Black PM (2000) Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. J Neuro-oncol 50:121–137
Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B (1987) Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci USA 84:6899–6903
Smith JS, Tachibana I, Passe SM, Huntley BK, Borell TJ, Iturria N, O’Fallon JR, Schaefer PL, Scheithauer BW, James CD, Buckner JC, Jenkins RB (2001) PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst 93:1246–1256
Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455: 1061–1068. https://doi.org/10.1038/nature07385
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN, Cancer Genome Atlas Research Network (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110. https://doi.org/10.1016/j.ccr.2009.12.020
Jutten B, Dubois L, Li Y, Aerts H, Wouters BG, Lambin P, Theys J, Lammering G (2009) Binding of cetuximab to the EGFRvIII deletion mutant and its biological consequences in malignant glioma cells. Radiother Oncol 92:393–398. https://doi.org/10.1016/j.radonc.2009.06.021
Combs SE, Heeger S, Haselmann R, Edler L, Debus J, Schulz-Ertner D (2006) Treatment of primary glioblastoma multiforme with cetuximab, radiotherapy and temozolomide (GERT)—phase I/II trial: study protocol. BMC Cancer 6:133. https://doi.org/10.1186/1471-2407-6-133
Singer J, Weichselbaumer M, Stockner T, Mechtcheriakova D, Sobanov Y, Bajna E, Wrba F, Horvat R, Thalhammer JG, Willmann M, Jensen-Jarolim E (2012) Comparative oncology: ErbB-1 and ErbB-2 homologues in canine cancer are susceptible to cetuximab and trastuzumab targeting. Mol Immunol 50:200–209. https://doi.org/10.1016/j.molimm.2012.01.002
Baumann M, Krause M (2004) Targeting the epidermal growth factor receptor in radiotherapy: radiobiological mechanisms, preclinical and clinical results. Radiother Oncol 72:257–266. https://doi.org/10.1016/j.radonc.2004.07.007
Hasselbalch B, Lassen U, Hansen S, Holmberg M, Sorensen M, Kosteljanetz M, Broholm H, Stockhausen MT, Poulsen HS (2010) Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a phase II trial. Neuro Oncol 12:508–516. https://doi.org/10.1093/neuonc/nop063
Belda-Iniesta C, Carpeno Jde C, Saenz EC, Gutierrez M, Perona R, Baron MG (2006) Long term responses with cetuximab therapy in glioblastoma multiforme. Cancer Biol Ther 5:912–914
Hadjipanayis CG, Machaidze R, Kaluzova M, Wang L, Schuette AJ, Chen H, Wu X, Mao H (2010) EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res 70:6303–6312. https://doi.org/10.1158/0008-5472.CAN-10-1022
Liu HL, Hua MY, Yang HW, Huang CY, Chu PC, Wu JS, Tseng IC, Wang JJ, Yen TC, Chen PY, Wei KC (2010) Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc Natl Acad Sci USA 107:15205–15210. https://doi.org/10.1073/pnas.1003388107
Kaluzova M, Bouras A, Machaidze R, Hadjipanayis CG (2015) Targeted therapy of glioblastoma stem-like cells and tumor non-stem cells using cetuximab-conjugated iron-oxide nanoparticles. Oncotarget 6:8788–8806. https://doi.org/10.18632/oncotarget.3554
Barua NU, Gill SS, Love S (2013) Convection-enhanced drug delivery to the brain: therapeutic potential and neuropathological considerations. Brain Pathology. https://doi.org/10.1111/bpa.12082
Yun J, Rothrock RJ, Canoll P, Bruce JN (2013) Convection-enhanced delivery for targeted delivery of antiglioma agents: the translational experience. J Drug Deliv 2013:107573. https://doi.org/10.1155/2013/107573
Saito R, Tominaga T (2012) Convection-enhanced delivery: from mechanisms to clinical drug delivery for diseases of the central nervous system. Neurol Med Chir 52:531–538
Dickinson PJ, LeCouteur RA, Higgins RJ, Bringas JR, Roberts B, Larson RF, Yamashita Y, Krauze M, Noble CO, Drummond D, Kirpotin DB, Park JW, Berger MS, Bankiewicz KS (2008) Canine model of convection-enhanced delivery of liposomes containing CPT-11 monitored with real-time magnetic resonance imaging: laboratory investigation. J Neurosurg 108:989–998. https://doi.org/10.3171/JNS/2008/108/5/0989
Platt S, Nduom E, Kent M, Freeman C, Machaidze R, Kaluzova M, Wang L, Mao H, Hadjipanayis CG (2012) Canine model of convection-enhanced delivery of cetuximab-conjugated iron-oxide nanoparticles monitored with magnetic resonance imaging. Clin Neurosurg 59:107–113. https://doi.org/10.1227/NEU.0b013e31826989ef
Young BD, Levine JM, Porter BF, Chen-Allen AV, Rossmeisl JH, Platt SR, Kent M, Fosgate GT, Schatzberg SJ (2011) Magnetic resonance imaging features of intracranial astrocytomas and oligodendrogliomas in dogs. Vet Radiol Ultrasound 52:132–141. https://doi.org/10.1111/j.1740-8261.2010.01758.x
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. https://doi.org/10.1007/s00401-007-0243-4
Martens T, Laabs Y, Gunther HS, Kemming D, Zhu Z, Witte L, Hagel C, Westphal M, Lamszus K (2008) Inhibition of glioblastoma growth in a highly invasive nude mouse model can be achieved by targeting epidermal growth factor receptor but not vascular endothelial growth factor receptor-2. Clin Cancer Res 14:5447–5458. https://doi.org/10.1158/1078-0432.CCR-08-0147
Diaz Miqueli A, Rolff J, Lemm M, Fichtner I, Perez R, Montero E (2009) Radiosensitisation of U87MG brain tumours by anti-epidermal growth factor receptor monoclonal antibodies. Br J Cancer 100:950–958. https://doi.org/10.1038/sj.bjc.6604943
Eller JL, Longo SL, Hicklin DJ, Canute GW (2002) Activity of anti-epidermal growth factor receptor monoclonal antibody C225 against glioblastoma multiforme. Neurosurgery 51:1005–1013 (discussion 1013-1004)
Eller JL, Longo SL, Kyle MM, Bassano D, Hicklin DJ, Canute GW (2005) Anti-epidermal growth factor receptor monoclonal antibody cetuximab augments radiation effects in glioblastoma multiforme in vitro and in vivo. Neurosurgery 56:155–162 (discussion 162)
Chakraborty S, Filippi CG, Wong T, Ray A, Fralin S, Tsiouris AJ, Praminick B, Demopoulos A, McCrea HJ, Bodhinayake I, Ortiz R, Langer DJ, Boockvar JA (2016) Superselective intraarterial cerebral infusion of cetuximab after osmotic blood/brain barrier disruption for recurrent malignant glioma: phase I study. J Neuro-oncol 128:405–415. https://doi.org/10.1007/s11060-016-2099-8
Garrett CR, Eng C (2011) Cetuximab in the treatment of patients with colorectal cancer. Expert Opin Biol Ther 11:937–949. https://doi.org/10.1517/14712598.2011.582464
Hebbar M, Wacrenier A, Desauw C, Romano O, Cattan S, Triboulet JP, Pruvot FR (2006) Lack of usefulness of epidermal growth factor receptor expression determination for cetuximab therapy in patients with colorectal cancer. Anticancer Drugs 17:855–857. https://doi.org/10.1097/01.cad.0000217425.44584.9f
Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L, Klimstra DS, Fridman D, Kelsen DP, Saltz LB (2005) Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol 23:1803–1810. https://doi.org/10.1200/JCO.2005.08.037
Sandhiya S, Dkhar SA, Surendiran A (2009) Emerging trends of nanomedicine–an overview. Fundam Clin Pharmacol 23:263–269. https://doi.org/10.1111/j.1472-8206.2009.00692.x
Mahmoudi K, Hadjipanayis CG (2014) The application of magnetic nanoparticles for the treatment of brain tumors. Front Chem 2:109. https://doi.org/10.3389/fchem.2014.00109
Wankhede M, Bouras A, Kaluzova M, Hadjipanayis CG (2012) Magnetic nanoparticles: an emerging technology for malignant brain tumor imaging and therapy. Expert Rev Clin Pharmacol 5:173–186. https://doi.org/10.1586/ecp.12.1
Bouras A, Kaluzova M, Hadjipanayis CG (2015) Radiosensitivity enhancement of radioresistant glioblastoma by epidermal growth factor receptor antibody-conjugated iron-oxide nanoparticles. J Neuro-oncol 124:13–22. https://doi.org/10.1007/s11060-015-1807-0
Sampson JH, Archer G, Pedain C, Wembacher-Schroder E, Westphal M, Kunwar S, Vogelbaum MA, Coan A, Herndon JE, Raghavan R, Brady ML, Reardon DA, Friedman AH, Friedman HS, Rodriguez-Ponce MI, Chang SM, Mittermeyer S, Croteau D, Puri RK, Investigators PT (2010) Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg 113:301–309. https://doi.org/10.3171/2009.11.JNS091052
Lipsitz D, Higgins RJ, Kortz GD, Dickinson PJ, Bollen AW, Naydan DK, LeCouteur RA (2003) Glioblastoma multiforme: clinical findings, magnetic resonance imaging, and pathology in five dogs. Vet Pathol 40:659–669. https://doi.org/10.1354/vp.40-6-659
Dolera M, Malfassi L, Bianchi C, Carrara N, Finesso S, Marcarini S, Mazza G, Pavesi S, Sala M, Urso G (2017) Frameless stereotactic radiotherapy alone and combined with temozolomide for presumed canine gliomas. Vet Comp Oncol. https://doi.org/10.1111/vco.12316
Acknowledgements
The authors thank Ken Johnson with the Boo Radley Foundation, Wilder Grummon with Medtronics, the UGA Bioimaging Research Center, Lisa Reno, Tim Jarrett, Kim Mason, referral neurologists including Gillian Irving and Jason King.
Funding
Funding provided in part by American Kennel Club Canine Health Foundation, the NIH (NS053454; P50CA128301-01A10003), the Georgia Cancer Coalition, Distinguished Cancer Clinicians and Scientists Program, and the Dana Foundation, the Boo Radley Foundation, and UGA clinical research grants.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Freeman, A.C., Platt, S.R., Holmes, S. et al. Convection-enhanced delivery of cetuximab conjugated iron-oxide nanoparticles for treatment of spontaneous canine intracranial gliomas. J Neurooncol 137, 653–663 (2018). https://doi.org/10.1007/s11060-018-2764-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2764-1