Abstract
We hypothesized that sorafenib (BAY 43-9006), an oral multi-kinase inhibitor, used in combination with SRS will improve overall intracranial control. This Phase I study assesses the safety, tolerability, and maximal tolerated dose of sorafenib administered with SRS to treat 1–4 brain metastases. This was an open label phase I dose escalation study with an expansion cohort. Eligible adults had 1–4 brain metastases from solid malignancies. Sorafenib was begun 5–7 days prior to SRS and continued for 14 days thereafter. Dose escalation of sorafenib was conducted via a “3 + 3” dose escalation design. Dose limiting toxicities (DLT) were determined 1 month after SRS and defined as ≥grade 3 neurologic toxicities. Twenty-three patients were enrolled. There were no DLTs at dose level 1 (400 mg per day) or dose level 2 (400 mg twice per day). An expansion cohort of 17 patients was treated at dose level 2. There were six grade 3 toxicities: hypertension (n = 2), rash (n = 1), lymphopenia (n = 1), hypokalemia (n = 1), fatigue (n = 1) and hand-foot syndrome (n = 1). All of these were attributable to sorafenib and not to the combination with SRS. The median time to CNS progression was 10 months, 1 year CNS progression-free survival was 46%, the median overall survival was 11.6 months and the 1 year overall survival was 46%. The use of sorafenib concurrent with SRS for the treatment of 1–4 brain metastases is safe and well tolerated at 400 mg twice a day. Our recommended phase II dose of concurrent sorafenib with SRS would be 400 mg twice daily.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases are a major source of cancer morbidity and mortality for patients. Failure to control brain metastases including both radiographically visible disease as well as micrometastatic lesions significantly impact cognitive function, quality of life, performance status and overall survival. Approximately 10–30% of cancer patients develop brain metastases during the course of their disease [1]. Treatment options for clinically apparent brain metastases include surgery, stereotactic radiosurgery (SRS), whole brain radiation therapy (WBRT), or a combination of these treatments.
WBRT treating both the grossly visible disease as well as the micrometastatic sites of disease has been the mainstay of treatment for decades. It provides a 4–6 months survival benefit, but is associated with a high risk of neurocognitive toxicity and is less effective at controlling gross disease as compared to surgery or SRS. Clinical trials have validated that for appropriately selected patients, SRS alone results in equivalent overall survival (OS) [2, 3] and improved neurocognition as compared to WBRT plus SRS [4]. Despite SRS’s ability to achieve excellent local control of the target lesion, patients remain at high risk for intracranial progression [2, 3]. In a recent subset analysis of good prognosis patients, there was a benefit in overall survival to the addition of WBRT to SRS [5]. Thus a regimen that treats both gross as well as micrometastatic disease while minimizing treatment related side effects and neurotoxicity is highly desirable.
Historically, chemotherapy has not been used to treat brain metastases due to the lack of penetration of drugs due to the blood–brain barrier. Although several chemotherapeutic agents including topotecan [6], carboplatin [7] and temozolomide [8,9,10] have been used in combination with WBRT with the hopes of improving CNS disease control, thus far they have failed to demonstrate a benefit in overall survival. A Phase III trial (RTOG 0320) of WBRT and SRS alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1–3 brain metastases found no benefit to the addition of chemotherapy to WBRT/SRS in these patients with 1–3 brain metastases. The radiation alone arm had less deterioration in performance status at 6 months [11].
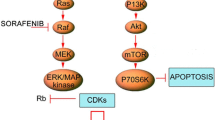
Preclinical studies have shown sorafenib (BAY 43-9006), an oral multi-kinase inhibitor, to induce complete tumor stasis and inhibition of tumor angiogenesis in a variety of tumor types [12]. Preclinical data has also suggested that combining angiogenic blockade with radiation may result in increased DNA damage [13]. We hypothesized that sorafenib would reduce intracranial disease progression by exerting its broad anti-tumor coverage on microscopic disease in the brain while SRS would be used to treat the macroscopic disease. This Phase I clinical trial is designed to assess the safety, tolerability, and maximal tolerated dose of sorafenib when administered in combination with SRS in the treatment of 1–4 brain metastases.
Materials and methods
Patient eligibility
The study was conducted in accordance with the principles of the Declaration of Helsinki and in accordance with the International Conference on Harmonization Guideline for Good Clinical Practice (ClinicalTrials.gov Identifier: NCT01276210). Informed consent was obtained from the patient prior to study enrollment. Eligible patients were ≥18 years old with histologically confirmed solid tumors (except for lymphomas and small cell lung cancer) who had 1–4 brain metastases less than 4 cm in size for which the treating radiation oncologist recommended treatment with SRS. Patients who had prior radiation or surgery for CNS metastatic disease were eligible as long as 1 month had elapsed prior to enrollment. Patients were required to have an Eastern Cooperative Oncology Group performance status of ≤1 with adequate metabolic function. Prior treatment with sorafenib or other tyrosine kinase inhibitors was allowed as long it had been discontinued either more than 28 days or five half-lives prior to enrollment.
Exclusion criteria included concurrent chemotherapy, grade 3 hemorrhage within 4 weeks of enrollment, concurrent medications that could potentially affect hepatic metabolism, uncontrolled hypertension, heart failure > New York Heart Association class II, angina, ventricular arrhythmias or thromboembolic event within the previous 6 months.
Stereotactic radiosurgery
SRS could be single fraction or fractionated (up to five fractions per lesion was allowed). Target volume and isocenter determination were based on MRI (T1 post-Gadolinium enhancing sequences, 1 mm thick slices) fused with CT images obtained with the patient’s head in a stereotactic frame and custom thermoplast mask (Brainlab, Chicago, IL). The target volume included the enhancing portion of the metastatic lesion or the surgical resection cavity with a 1–2 mm margin. The prescription dose was delivered to the 50–90% (maximum = 100%) isodose surface, and was defined as the minimum dose to the target volume. Dose and fractionation were determined by location, prior treatment, size of target lesion and left to the discretion of the treating radiation oncologist. SRS was delivered by a linear accelerator based treatment system (Novalis TX, Varian, Palo Alto, CA and Brainlab).
Dosage and drug administration
This was a single institution phase I trial. Sorafenib was started 5–7 days prior to SRS and continued for 14 days after SRS. Dose escalation of sorafenib was conducted using a “3 + 3” dose escalation design. Dose level 1 began with sorafenib 400 mg once per day with dose level 2 being 400 mg twice a day. No further dose escalations were planned. If the 400 mg dose twice a day was well tolerated, that would be considered the recommended phase II dose. Intra-patient dose escalation was not allowed. Patients were followed weekly for 1 month after SRS to assess for DLTs.
The Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 was utilized. Study drug was held or dose reduced based on development of grade 3 hematologic or non-hematologic toxicities if this occurred during the 3 weeks the patient was receiving drug. For hand and foot syndrome, dose reductions were for grade 3 toxicity or persistent (>7 days) of grade 2 toxicity. For hypertension, dose reductions occurred for grade 3 or symptomatic grade 2 toxicities that persisted despite anti-hypertensives. A dose limiting toxicity (DLT) was defined as ≥grade 3 neurologic toxicities which were attributable to the combination of sorafenib and SRS. Grade 3 toxicity that was a result of known toxicities of sorafenib alone were not considered dose limiting.
Patients had brain MRIs at 2, 6, and 12 months after SRS to assess the target lesion(s) as well as intracranial tumor control. Further brain imaging was left to the discretion of the treating radiation oncologist.
This study was designed as a phase I study of the safety and tolerability of combining sorafenib with SRS. The primary endpoint was to establish the maximum tolerated dose (MTD) and determine the toxicity profile of combining sorafenib with SRS. Secondary endpoints included overall survival (OS) using the Kaplan–Meier method. Overall survival was measured from time of study enrollment until death due to any cause. At the discretion of the treating physician, sorafenib could be continued after completion of the study.
Response criteria
RECIST criteria were used to measure response [14]. MRI scans were used to determine response to therapy. Complete response (CR) was defined as complete disappearance of all tumor. Partial response (PR) was defined as greater than 50% reduction in tumor size on bidimensional measurements. CNS progression was defined as ≥25% increase in size of the tumor or the emergence of new brain metastases or leptomeningeal spread of disease (PD). Stable disease (SD) was defined as measurements not meeting the criteria for CR, PR or PD.
Statistical methods
As this was a phase I study, descriptive statistics were used to summarize the demographics and adverse events. Kaplan–Meier curve was used to estimate survival and CNS-progression free survival.
Results
From July 2011 to July 2015, 29 patients consented and 23 eligible patients were enrolled. The treatment schema is summarized in Fig. 1. Reasons for exclusion included abnormal lab values (n = 3), active bleeding (n = 1) and a decline in performance status (n = 2). Twenty-three patients are eligible for toxicity analysis. Six patients enrolled in the “3 + 3” dose escalation design. There were no DLTs at dose level 1 (400 mg per day) or dose level 2 (400 mg twice a day). The remaining 17 patients were enrolled in the expansion cohort at dose level 2. None of these patients suffered a DLT.
Table 1 summarizes the patient characteristics of the group as a whole. The median age was 63 with 70% being females. The most common primaries were non-small cell lung cancer (57%) and breast (22%). Forty-three percent of patients had a single brain lesion.
Table 2 summarizes the patient and treatment characteristics of each patient on study. The details of radiation treatment was left to the discretion of the treating radiation oncologist and varied based on histology, size, location and number of lesions. One patient was enrolled on study and developed severe back pain which revealed leptomeningeal disease. This patient received whole brain radiation instead of SRS. As he had had initiated sorafenib, he was evaluable for toxicity and therefore is included in the analysis. He had a dramatic response to sorafenib and continued on this regimen.
Table 3 summarizes grade 2 and grade 3 treatment related toxicities. All of these toxicities are well established side effects of sorafenib alone [15]. These included rash, hand foot syndrome, hypertension, mucositis, fatigue, lymphopenia and hypokalemia. None of these were intracranial and therefore not a result of concurrent sorafenib and SRS and were therefore not deemed to be DLTs. There were no Grade 4 or 5 treatment related toxicities. Two patients in the expansion cohort at dose level 2 had their sorafenib dose reduced because of known sorafenib induced side effects. One patient developed hand-foot syndrome the other severe maculopapular skin rash with associated skin pain. In both instances, their skin reactions were assessed as Grade 2 toxicities which persisted >7 days. Dose reductions were not required for any of the Grade 3 toxicities that our patients experienced as they occurred after completion of the course of study drug.
Of the 23 patients, only one had a complete response. Of the patients (n = 20) with measurable disease, the overall response rate (stable disease + partial response) was 70%. There were three patients who did not have measurable disease by RECIST criteria. At 1 year, 80% of the SRS treated index lesions remained stable but 46% of patients experienced intracranial progression due to the development of new intracranial metastases.
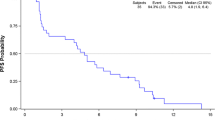
Median follow up for all 23 enrolled patients is 23 months with a minimum follow up of at least 3 months after SRS. The median time to CNS progression was 10 months. Of the 11 patients who have not developed CNS progression, 4 (36%) remain alive at 3.5 2.3, 2 and 0.8 years. The 1 year CNS progression-free survival was 46% (Fig. 2). The median overall survival was 11.6 months. The 1 year overall survival was 46% (Fig. 3). Sixteen patients have died of disease and 7 patients remain alive with disease.
Figure 4 is a representative case of a 54 year old woman who underwent SRS for a single lesion. Her 8 week scan confirms partial response of the lesion. She remains alive 48 months following completion of SRS.
Discussion
This Phase I trial of sorafenib concurrent with SRS for the treatment of 1–4 brain metastases demonstrates that this multidisciplinary approach is safe and well tolerated at doses as high as 400 mg twice a day. The grade 3 treatment-related toxicities seen were known systemic side effects of sorafenib alone and unrelated to its concurrent use with SRS. No DLTs were encountered in the phase I component of this study (n = 6) or in the expansion cohort (n = 17). Therefore, sorafenib 400 mg twice a day with SRS is the recommended phase II dosing. This Phase I trial incorporates aggressive local therapy with SRS to control gross disease with a systemic agent, sorafenib, that can help to reduce intracranial failures with minimal neurotoxicity as compared to WBRT. This has the potential to delay or avoid the use of WBRT and its associated neurotoxicity.
There is a well-established dose-volume relationship when assessing acute and long term organ toxicity from radiation. The addition of chemotherapy to radiation often further narrows this therapeutic window. Many different chemotherapeutic and biologic agents have been combined with whole brain radiation therapy in the hopes of improving the outcomes of patients with brain metastases with disappointing results [6, 7, 16]. The RTOG recently published their results of a Phase III trial (RTOG 0320) of WBRT and SRS with temozolomide (TMZ) or erlotinib (ETN) for non-small cell lung cancer and 1–3 brain metastases [11]. The authors concluded that the addition of these agents to WBRT/SRS did not improve overall survival and may have even decreased median survival times. It is presumed that the increased side effect profile of concurrent chemotherapy and WBRT helped contribute to the decreased median survival times. Our study, on the other hand, finds that the additional toxicity introduced by concurrent systemic agents is minimal when a much smaller volume of brain is treated with radiation using SRS.
This dose-volume relationship was also evident in the Phase I trial of sorafenib with stereotactic body radiosurgery (SBRT) for patients with non-resectable hepatocellular carcinoma. The unacceptably high toxicity profiles with concurrent sorafenib with SBRT was found when larger volumes of liver were irradiated [17]. This is in contrast to the much smaller CNS treatment volumes needed to treat focal brain metastases and can explain why no DLTs were seen in our study.
The idea of using chemotherapy alone to attempt to control brain metastases has been explored. In the recently reported Phase II LANDSCAPE trial, patients with previously untreated brain metastases from HER2-positive metastatic breast cancer were treated with lapatinib plus capecitabine. There was a significant proportion (49%) of grade 3 or grade 4 treatment-related adverse events [18]. The authors concluded that this combination chemotherapy is active in HER2-positive brain metastases and a Phase III trial is warranted. These results are intriguing and worthy of further investigation though it is unlikely that systemic therapy alone can control radiologically evident CNS disease in the long term.
In a retrospective analysis of patients with metastatic renal cell cancer patients who were randomly assigned to receive sorafenib or placebo, the patients who received sorafenib were less likely to develop brain metastases as compared to the control group. This benefit persisted 1 and 2 years after treatment [19]. Though not currently approved for this indication, there are reports of sorafenib being clinically effective in patients with metastatic disease to the brain and in patients with primary brain tumors [20, 21]. A distinct mechanism of tumor control and non-overlapping toxicity profiles from radiation makes sorafenib an ideal agent to combine with focal radiation to maximize control of both gross (SRS) as well as microscopic (sorafenib) intracranial metastasis.
Our study confirms those of others that SRS achieves high local control rates of 80% in the SRS-treated lesions. Although we had hypothesized that the addition of a systemic agent that penetrates the blood–brain barrier would result in better tumor control by killing microscopic intracranial disease, the 1 year CNS progression free survival of 46% and the median survival of 11.6 months seen in this study is not significantly different compared to patients treated with SRS alone [3, 4].
There are several limitations to this study. The small number of patients, the variety of histologies, the wide range of total dose and fractionation schemas used for SRS as well as patients with a range of disease burden from CNS only presentations to a significant burden of systemic disease. Studies of patients with brain metastases have always been difficult given the wide variation in tumor-specific factors such as number, size, location and histologies of the lesions. Patient-specific factors such as medical comorbidities as well as performance status all of which can substantially effect survival. Neurologic control can also be affected by additional treatments including the newer more effective systemic treatments that can penetrate the blood brain barrier as well as salvage SRS or whole brain radiation.
In the current study, gross disease was well controlled by SRS concurrent with sorafenib but patients continued to develop intracranial failures. Our study used sorafenib for approximately 1 week prior to SRS and 2 weeks after SRS and therefore this short duration of exposure may not be long enough to assess long term intracranial control, but is an acceptable duration to assess potential toxicities of concurrent therapy which was primary objective of this Phase I trial. Although a few patients continued sorafenib as the systemic disease was also responding to sorafenib, the large majority of patients only received a total of 3 weeks of treatment. In a phase II study of adjuvant sunitinib to control distant intracranial failure in combination with SRS also showed no increase in acute or long term side effects. Despite a 4 week course of sunitinib, the 6 month disease control of 43% was no different from historical controls [22]. Additional study is needed to optimize the timing and duration of systemic therapy in conjunction with SRS.
This proposed treatment schema of SRS with systemic therapy is novel in that SRS can provide excellent local control of known disease and it aims to utilize systemic therapy to help control subclinical micrometastatic disease in the brain. With better intracranial control of disease, it is hoped that WBRT and its neurocognitive side effects can be avoided or delayed and reserved for salvage therapy only when needed. The first step is to assess if systemic therapies can be safely given concurrently with SRS in order to design rigorous clinical trials to assess intracranial disease control to this multimodality approach to the treatment of brain metastases.
In summary, this Phase I trial shows that the concurrent use of sorafenib with SRS for the treatment of 1–4 brain metastases was safe and well tolerated at doses as high as 400 mg twice a day. This lays the foundation for future Phase II clinical trials to assess treatment efficacy for sorafenib with SRS as well as providing the framework for future investigations into multimodality treatment approaches to brain metastases. Our recommended phase II dose for this combination would be sorafenib 400 mg twice daily concurrently with SRS.
References
Stewart BW (2014) Wild CPe: World Cancer Report 2014. International Agency for Research on Cancer, Lyon
Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC (1999) Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 45:427–434
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G (2006) Stereotactic radiosurgery plus whole-brain radiation therapy versus stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483–2491. doi:10.1001/jama.295.21.2483
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037–1044. doi:10.1016/s1470-2045(09)70263-3
Aoyama H, Tago M, Shirato H, Investigators JROSG-J- (2015) Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99–1 Randomized Clinical Trial. JAMA Oncol 1:457–464. doi:10.1001/jamaoncol.2015.1145
Neuhaus T, Ko Y, Muller RP, Grabenbauer GG, Hedde JP, Schueller H, Kocher M, Stier S, Fietkau R (2009) A phase III trial of topotecan and whole brain radiation therapy for patients with CNS-metastases due to lung cancer. Br J Cancer 100:291–297. doi:10.1038/sj.bjc.6604835
Guerrieri M, Wong K, Ryan G, Millward M, Quong G, Ball DL (2004) A randomised phase III study of palliative radiation with concomitant carboplatin for brain metastases from non-small cell carcinoma of the lung. Lung Cancer 46:107–111. doi:10.1016/j.lungcan.2004.02.019
Antonadou D, Paraskevaidis M, Sarris G, Coliarakis N, Economou I, Karageorgis P, Throuvalas N (2002) Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. J Clin Oncol 20:3644–3650
Agarwala SS, Kirkwood JM, Gore M, Dreno B, Thatcher N, Czarnetski B, Atkins M, Buzaid A, Skarlos D, Rankin EM (2004) Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol 22:2101–2107. doi:10.1200/JCO.2004.11.044
Verger E, Gil M, Yaya R, Viñolas N, Villà S, Pujol T, Quintó L, Graus F (2005) Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int J Radiat Oncol Biol Phys 61:185–191. doi:10.1016/j.ijrobp.2004.04.061
Sperduto PW, Wang M, Robins HI, Schell MC, Werner-Wasik M, Komaki R, Souhami L, Buyyounouski MK, Khuntia D, Demas W, Shah SA, Nedzi LA, Perry G, Suh JH, Mehta MP (2013) A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys 85:1312–1318. doi:10.1016/j.ijrobp.2012.11.042
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA (2004) BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64:7099–7109. doi:10.1158/0008-5472.CAN-04-1443
Mazeron R, Anderson B, Supiot S, Paris F, Deutsch E (2011) Current state of knowledge regarding the use of antiangiogenic agents with radiation therapy. Cancer Treat Rev 37:476–486. doi:10.1016/j.ctrv.2011.03.004
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. doi:10.1016/j.ejca.2008.10.026
Bayer HealthCare Pharmaceuticals Inc. (2013) Nexavar® (sorafenib tosylate) tablets prescribing information. Wayne, NJ
Krown SE, Niedzwiecki D, Hwu WJ, Hodgson L, Houghton AN, Haluska FG, CaLG B (2006) Phase II study of temozolomide and thalidomide in patients with metastatic melanoma in the brain: high rate of thromboembolic events (CALGB 500102). Cancer 107:1883–1890. doi:10.1002/cncr.22239
Brade AM, Ng S, Brierley J, Kim J, Dinniwell R, Ringash J, Wong RR, Cho C, Knox J, Dawson LA (2016) Phase 1 Trial of sorafenib and stereotactic body radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 94:580–587. doi:10.1016/j.ijrobp.2015.11.048
Bachelot T, Romieu G, Campone M, Diéras V, Cropet C, Dalenc F, Jimenez M, Le Rhun E, Pierga JY, Gonçalves A, Leheurteur M, Domont J, Gutierrez M, Curé H, Ferrero JM, Labbe-Devilliers C (2013) Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 14:64–71. doi:10.1016/S1470-2045(12)70432-1
Massard C, Zonierek J, Gross-Goupil M, Fizazi K, Szczylik C, Escudier B (2010) Incidence of brain metastases in renal cell carcinoma treated with sorafenib. Ann Oncol 21:1027–1031. doi:10.1093/annonc/mdp411
Ranze O, Hofmann E, Distelrath A, Hoeffkes HG (2007) Renal cell cancer presented with leptomeningeal carcinomatosis effectively treated with sorafenib. Onkologie 30:450–451. doi:10.1159/0000105131
Valcamonico F, Ferrari V, Amoroso V, Rangoni G, Simoncini E, Marpicati P, Vassalli L, Grisanti S, Marini G (2009) Long-lasting successful cerebral response with sorafenib in advanced renal cell carcinoma. J Neurooncol 91:47–50. doi:10.1007/s11060-008-9676-4
Ahluwalia MS, Chao ST, Parsons MW, Suh JH, Wang D, Mikkelsen T, Brewer CJ, Smolenski KN, Schilero C, Rump M, Elson P, Angelov L, Barnett GH, Vogelbaum MA, Weil RJ, Peereboom DM (2015) Phase II trial of sunitinib as adjuvant therapy after stereotactic radiosurgery in patients with 1–3 newly diagnosed brain metastases. J Neurooncol 124:485–491. doi:10.1007/s11060-015-1862-6
Acknowledgements
Supported by the National Center for Research Resources, Grant UL1 RR024975-01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06 (BC), K12 CA090625 (JM), NCI P30 CA068485, Clinical Research Grant from Bayer-Onyx (BC) The research in this publication was supported, in part, through funding from the NIH-NCI Cancer Center Support Grant P30 CA0068485.
Funding
The funding sources had no role in the study design, collection, analysis, and interpretation of data, writing of the report, or the decision to submit for publication. The corresponding author had full access to all of the data and the final responsibility to submit for publication. The authors are solely responsible for the study design as well as the collection, analysis and interpretation of data; writing of the manuscript; and in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kyle Arneson, Joshua Mondschein, Mark Stavas, Anthony J. Cmelak, Albert Attia, Leora Horn, Kenneth Niermann, Igor Puzanov, Fen Xia have no conflicts of interest. A. Bapsi Chakravarthy: clinical research grant Bayer/Onyx.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Arneson, K., Mondschein, J., Stavas, M. et al. A phase I trial of concurrent sorafenib and stereotactic radiosurgery for patients with brain metastases. J Neurooncol 133, 435–442 (2017). https://doi.org/10.1007/s11060-017-2455-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2455-3