Abstract
Surgical resection is not the standard of care for primary central nervous system lymphoma (PCNSL), as historical studies have demonstrated unfavorable complication rates and limited benefits. Some recent studies suggest that resection may provide a therapeutic benefit, yet the safety of these procedures has not been systematically investigated in the setting of modern neurosurgery. We examined the safety of surgical resection for PCNSL. We retrospectively analyzed all patients with PCNSL treated at Columbia University Medical Center between 2000 and 2015 to assess complications rates following biopsy or resection using the Glioma Outcomes Project system. We identified predictors of complications and selection for resection. Well-validated scales were used to quantify patients’ baseline clinical characteristics, including functional status, comorbid disease burden, and cardiac risk. The overall complication rate was 17.2% after resection, and 28.2% after biopsy. Cardiac risk (p = 0.047, OR 1.72 [1.01, 2.95]), and comorbid diagnoses (p = 0.004, OR 3.05 [1.42, 6.57]) predicted complications on multivariable regression. Patients who underwent resection had better KPS scores (median 70 v. 80, p = 0.0068, ∆ 10 [0.0, 10.00]), and were less likely to have multiple (46.5% v. 27.6%, p = 0.030, OR 1.42 [1.05, 1.92]) or deep lesions (70.4% v. 39.7%, p = 0.001, OR 1.83 [1.26, 2.65]). Age (p = 0.048, OR 0.75 per 10-year increase [0.56, 1.00]) and deep lesions (p = 0.003, OR 0.29 [0.13, 0.65]) influenced selection for resection on multivariable regression. Surgical resection of PCNSL is safe for select patients, with complication rates comparable to rates for other intracranial neoplasms. Whether there is a clinical benefit to resection cannot be concluded.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare disease with poor prognosis: in recent studies, the median survival for patients with PCNSL ranges between 12 and 32 months [1, 2]. While some investigations have proposed specific treatment algorithms [1], there is considerable debate concerning the standard of care for this disease [2–4].
Surgical resection is not part of the standard of care for PCNSL. Historical studies have demonstrated unfavorable complication rates and marginal benefits [5, 6]. Investigations encompassing study periods as late as 1995 have shown no benefit to resection, or have identified resection as a predictor of poor outcome for patients with PCNSL [1, 2, 6–13]. Consensus has therefore been that surgical resection should not to be recommended for PCNSL, and stereotactic needle brain biopsy has been the standard diagnostic procedure [14].
Recent studies suggest that resection is associated with a survival benefit for select patients with PCNSL [2, 15]. Historically, complication rates following resection for PCNSL have been high, making it hard to justify exploring the potential therapeutic benefit of resection. However, complication rates associated with a variety of surgical procedures have decreased over time [16], including neurosurgical procedures [17, 18], and complications rates from general anesthesia have also decreased [19]. Moreover, technological innovations have proliferated since the early literature on PCNSL resective surgery, including increased use of MRI [20], frameless stereotaxy [21], tumor visualization technologies [22], and improved perioperative care. These technologies and practices have contributed to the safety of modern intracranial surgery [19]. In light of the new evidence suggesting a role for surgery in PCNSL treatment, some investigators have called for a reexamination of surgical resection for PCNSL [2–4], and some professional societies have recommended resection in certain circumstances [23]. Nevertheless, data on the complication rates following resection of PCNSL in the modern era are limited.
As the use of surgical resection for PCNSL will depend on the safety of the procedure, we examined complication rates following either biopsy or resection for PCNSL. In order to facilitate comparisons to other intracranial neoplasms for which surgical resection is routine, we employed a system of quantifying complications that is widely used in neurosurgical oncology. Furthermore, we examined factors affecting selection for resection, as well as factors predictive of complications.
Methods
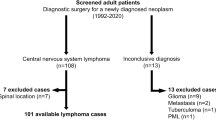
We used our pathology database to identify patients with CNS lymphoma diagnosed at Columbia University Medical Center between 2000 and 2015. We then distinguished cases of cranial PCNSL by excluding all patients with a prior diagnosis of lymphoma elsewhere, patients who had disseminated disease found on further workup, or patients whose lymphoma was located in the spine. We identified a total of 129 patients with cranial PCNSL. We retrospectively reviewed patient medical records for clinical information, including age at diagnosis, past medical history, functional status at diagnosis, tumor location, operations performed, postoperative complications, and survival data.
Functional status was quantified using the Karnofsky Performance Status (KPS) [24]. Postoperative complications were classified using a widely-used method for intra-axial tumors, the Glioma Outcomes Project classification system (GOP system) [25]. Cardiovascular risk was quantified using The Simple Index for Prediction of Cardiac Risk, an index retrospectively derived using data from 2893 patients undergoing major non-cardiac surgery, then prospectively shown to be superior to other common preoperative decision aids in predicting postoperative complications in a cohort of 1422 patients [26]. Immunocompromised patients were defined as patients diagnosed with HIV, or patients with a history of solid organ transplantation. Comorbid diagnoses were quantified using the Charlson Comorbidity Index (CCI) [27].
Tumor location category variables included left hemispheric involvement, infratentorial location, deep location, and multiple lesions. These categories were included as binary variables, and a given patient’s tumor could belong to multiple or none of the different categories.
Complication rates following resection of PCNSL were compared to a previously published institutional series for patients with lobar glioblastoma (GBM) [28]. Both series use the same method of classifying complications, and the same group of surgeons performed the surgeries. As the GBM series only studied patients age 65 and older, only PCNSL patients age 65 and over were included for the comparison. Unlike the current series, the GBM series was restricted to patients with unifocal, lobar disease. The baseline age, KPS, and cardiovascular risk of each group were also compared.
Statistical analysis was performed using SAS 9.1 (SAS Institute, Cary, NC) and Prism 6.0b (GraphPad Software, Inc., 1994–2012). Baseline clinical data between groups were compared using T tests, Mann–Whitney tests, and Fisher’s exact tests, as appropriate. Multiple-variable logistic regression was performed to identify predictors of selection for craniotomy and predictors of complications. Explanatory variables included age, KPS, cardiac risk, comorbid diagnoses, multiple lesions, immunocompromised status, infratentorial location, deep location, and left hemispheric involvement. Age was treated as a continuous variable in our analysis, and KPS, cardiac risk, and comorbid diagnoses were treated as ordinal. When calculating the rate of complications among all patients regardless of procedure type, procedure type (resection or biopsy) was also included as a variable. All variables with p ≤ 0.20 on single-variable logistic regression were included in the multiple variable models. p values of <0.05 were considered statistically significant.
Results
Baseline patient characteristics
A total of 129 patients were identified. Their median age was 65 (range 21–88), median KPS was 70 (range 20 to 100), and the median cardiac risk factor was 0 (range 0–4) (Table 1). Sixteen patients (12.4%) were immunocompromised, 71 patients (55.0%) underwent biopsy, and 58 patients (45.0%) underwent surgical resection. 63 patients (48.8%) had tumors with left-hemispheric involvement, 29 patients (22.5%) had infratentorial tumors, 73 (56.6%) had tumors with a deep location, and 49 patients (38.0%) had multiple tumors.
Selection for resection
Patients who underwent biopsy and patients who underwent resection were comparable with respect to age (64.7 years v. 60.5 years, p = 0.105, ∆ −4.22 [−9.3, 0.9]), cardiac risk (p = 0.743, ∆ 0.0 [0.0, 0.0]), comorbid diagnoses (p = 0.190, ∆ 0.00 [0.0, 0.0]), and the proportion of patients with immunocompromised status (p = 1.000, OR 1.03 [0.64, 1.63]). There was no difference favoring biopsy versus resection for tumors involving the left hemisphere (p = 0.475, OR 1.16 [0.84, 1.60]), or with tumors involving infratentorial location (p = 0.525, OR 0.85 [0.56, 1.28]). On single-variable logistic regression, KPS, not having multiple lesions, and not having deep lesions were predictors of selection for resection (KPS p = 0.022, OR 1.31 [1.04, 1.64]); multiple lesions p = 0.014, OR 0.39 [0.19, 0.83]; deep lesions p = 0.000, OR 0.26 [0.13, 0.55]) (Table 2). Age and absence of deep lesion were predictors of resection on multivariable regression (age p = 0.048, OR 0.75 per 10-year increase [0.56, 1.00]; deep location p = 0.003, OR 0.29 [0.13, 0.65]).
Postoperative complications
Patients undergoing biopsy and those undergoing resection had comparable rates of complications for all complication types. Overall, 10 resection patients (17.2%) and 20 biopsy patients (28.2%) experienced at least one complication (Table 3). Five resection patients (8.6%) and 13 biopsy patients (18.3%) experienced a systemic complication, 2 resection patients (3.4%) and 5 biopsy patients (7.0%) experienced a regional complication, and 3 resection patients (5.2%) and 4 biopsy patients (5.6%) experienced a neurologic complication.
Predictors of complications among all patients
Among all patients, KPS, comorbid diagnoses, and immunocompromised status were predictive of overall complications on single-variable regression (n = 129; KPS p = 0.008, OR 0.72 [0.57, 0.92]; comorbid diagnoses p = 0.001, OR 3.52 [1.66, 7.49]; immunocompromised status p = 0.045, OR 3.04 [1.03, 9.05]). Comorbid diagnoses predicted overall complications on multivariable regression (p = 0.004, OR 3.05 [1.42, 6.57]). For systemic complications, KPS, cardiac risk, comorbid diagnoses, and the presence of multiple lesions were predictive on single-variable regression (KPS p = 0.008, OR 0.69 [0.52, 0.91]; cardiac risk p = 0.011, OR 1.72 [1.13, 2.61]; comorbid diagnoses p = 0.015, OR 2.19 [1.16, 4.12]; presence of multiple lesions p = 0.048, 2.99 [1.01, 8.86]). Cardiac risk and comorbid diagnoses were predictive on multivariable regression (cardiac risk p = 0.047, 1.72 [1.01, 2.95]; comorbid diagnoses p = 0.044, OR 1.97 [1.02, 3.82]). For regional complications, comorbid diagnoses were predictive on single-variable regression (p = 0.004, OR 3.12 [1.45, 6.69]), and no other variables met criteria for inclusion as candidate variables in a multiple variable regression model. No variables were significant on single- or multiple variable regressions as predictors of neurologic complications among all patients.
Predictors of complications among patients undergoing resection
Among patients undergoing resection (n = 58), KPS and comorbid diagnoses predicted overall complications (KPS p = 0.021, OR 0.62 [0.42, 0.93]; comorbid diagnoses p = 0.022, OR 5.40 [1.28, 22.75]) (Table 4). Both variables showed trends toward significance as predictors of overall complications on multivariable regression. Regarding systemic complications, KPS, comorbid diagnoses, and multiple lesions were predictive on single-variable regression (KPS p = 0.018, OR 0.53 [0.31, 0.90]; comorbid diagnoses p = 0.005, OR 15.45 [2.34, 101.93]; multiple lesions p = 0.025, OR 13.67 [1.39, 134.12]). KPS showed a trend as a predictor of systemic complications on multiple-variable regression. No variables were significant on single- or multiple-variable regressions as predictors of regional or neurologic complications among resection patients.
Predictors of complications among patients undergoing biopsy
Among patients undergoing biopsy (n = 71), cardiac risk, comorbid diagnoses, and immunocompromised status were predictors of overall complications on single-variable regression (cardiac risk p = 0.150, OR 1.40 [0.89, 2.20]; comorbid diagnoses (p = 0.017, 2.81 [1.20, 6.59]; immunocompromised status p = 0.003, OR 13.19 [2.44, 71.21]), and all three variables remained significant on multiple-variable regression (cardiac risk p = 0.143, OR 1.50 [0.87, 2.59]; comorbid diagnoses: p = 0.015, OR 2.60 [1.20, 5.63]; immunocompromised p = 0.003, OR 14.59 [2.42, 87.82]). Cardiac risk predicted systemic complications on single-variable regression (p = 0.028, OR 1.75 [1.06, 2.89]), and no other variables met criteria for inclusion as candidate variables. Comorbid diagnoses were a significant predictor of regional complications on both single-variable (p = 0.006, OR 3.76 [1.47, 9.65]) and multiple-variable regression (p = 0.006, OR 4.46 [1.55, 12.79]). Immunocompromised status predicted neurologic complications on single-variable regression (p = 0.046, OR 8.57 [1.04, 70.74]), but no variables were significant predictors of neurologic complications after biopsy on multiple-variable regression.
Complication rates for resection of PCNSL compared to GBM
There were 68 PCNSL patients age 65 and older who underwent surgery, who were compared to 243 GBM patients age 65 and older from a previously published institutional series for elderly patients with GBM [28]. Both series use the same method of classifying complications, and the same group of surgeons performed the surgeries in each series, but the GBM series only included patients with lobar disease. There was no difference in age between the two groups (PCNSL 74.1 years v. GBM 73.1 years, p = 0.246, ∆ −0.98 [−2.64, 0.68]). The PCNSL patients had a lower KPS and higher cardiac risk than the GBM patients (KPS: PCNSL 68.6 v. GBM 76.3, p = 0.001, ∆ −7.67 [−12.03, −3.30]; Cardiac risk: PCNSL 0.94 v. GBM 0.44, p = 0.001, ∆ 0.50 [0.20, 0.79]). The overall rate of complications, as well was the rate of regional complications and neurological complications, were equivalent between PCNSL patients and GBM patients (overall complications for PCNSL 25.0% v. GBM 21.7%, p = 0.514, OR 1.22 [0.65, 2.30]; regional complications for PCNSL 5.9% v. GBM 8.2%, p = 0.617, OR 0.70 [0.23, 2.11]; neurologic complications for PCNSL 4.4% v. GBM 7.8%, p = 0.430, OR 0.54 [0.16, 1.90]). PCNSL patients had a higher rate of systemic complications than GBM patients (systemic complications for PCNSL 17.6% v. GBM 7.0%, p = 0.158, OR 2.85 [1.29, 6.30]).
Discussion
Surgical resection is not the standard of care for PCNSL because of historical studies demonstrating unfavorable complication rates and minimal benefits [1, 5, 6]. A few recent studies have suggested that resection is associated with a survival benefit in select patients [2, 15]. However, these studies include a post-hoc analysis of selected patients enrolled in a clinical trial, which may underestimate the morbidity associated with resection surgery. The safety of resection of PCNSL has not been systematically investigated in the setting of standard modern neurosurgical techniques and perioperative care. Existing surgical literature suggests that surgical care has become safer [16], including literature from within the field of neurosurgery [17, 18, 29–31], so a re-examination of the safety of resection for PCNSL is warranted. Furthermore, patient selection affects resection outcomes for CNS tumors [25, 32], but remains under-examined for patients with PCNSL. Our results show that refined patient selection might allow for reasonably safe surgical resection for PCNSL, as has been the case for other “high-risk” populations that have been traditionally, a priori, deemed to be too risky to undergo brain tumor resections [28].
The complication rates we observed after resection of PCNSL are lower than historical complication rates. Notably, our rate of neurological complications following resection (5.2%) was significantly lower than the complication rates seen in historical series, such as the 40% rate observed by DeAngelis et al. [6]. Multiple factors may account for this finding, including national trends toward centralization of care at specialty centers [31], patient selection, and newer technologies that have improved surgical safety. Regarding technology, many investigations showing that resection is not safe for PCNSL were conducted without tools that are now common: the series from De Angelis et al. predates the widespread use of MRI [6], and the series from Henry et al. predates the very invention of MRI [5]. An influential series from Bataille et al. only included patients as late as 1995 [1], when MRI use was not the standard of care. Indeed, from 1996 to 2010, MRI use increased by 3.8 fold [20], and one early neuronavigation system, VectorVision from BrainLab, only received FDA approval in 1997, after which literature supported its widespread use [21]. Moreover, our finding may be part of a larger trend: complication rates have decreased for a variety of surgical procedures over time [16], including neurosurgical procedures [17, 18], and the complication rate associated with general anesthesia has also decreased [19].
Our series demonstrates that complication rates following resection of PCNSL are reasonable. The complication rates we observed after resection of PCNSL are comparable to complication rates from series on other CNS tumors that used the same method of assessing complications (Fig. 1). Chang et al. reported a series of 408 patients undergoing resection of WHO grade III or IV gliomas in whom the overall rate of complications was 24.2% [25]. Sawaya et al. (n = 400) and Brell et al. (n = 200) report overall complication rates of 32 and 27.5%, respectively, when investigating patients undergoing resection of WHO grade II-IV gliomas or metastases [33, 34]. Studies on glioma patients from this decade that use the same reporting method show rates of complications that are comparable to ours (17.2%), including studies from Hoover et al. (12.8%), Moiyadi et al. (18%), and Talacchi et al. (23%) [35–37]. Indeed, Malone et al. examined data on craniotomy for multiple tumor types (glioma, metastases, meningioma, acoustic neuroma) from the Nationwide Inpatient sample, and found overall complication rates to be between 14.3 and 15.7% [38]. Moreover, our complication rate in this institutional series is comparable to the complication rate in our recently-published institutional series on elderly patients with lobar GBM [28]. Of note, the Glioma Outcome morbidity metric used for our study and others overstates neurological complications, as it does not distinguish transient and expected perioperative neurological deficits from permanent neurological injury. It appears, therefore, that the complication rate following resection of PCNSL observed in our series is no higher than rates following resection of tumors for which surgical resection is the standard of care, and that the rate of complications is acceptable.
Complication rates from craniotomy for resection in series with complete complications data classified according to the Glioma Outcomes Project system.[25] Superscripts denote what types of tumors were treated in each series, as follows: a glioblastoma, b malignant glioma (WHO grade II–IV), and c three or more intra-axial tumor types, including both gliomas and metastases
We found that comorbid diagnoses predict complications, which can help refine patient selection for improving the safety of PCNSL resection surgery. This finding runs contrary to the results of Benz et al., who also used the CCI to quantify complications, and found no relationship between comorbid conditions and complications following spine surgery [39]. However, surgical literature from outside the sphere of neurosurgery has repeatedly found that the CCI predicts postoperative complications [40, 41], and similar comorbidity indices have been demonstrated to predict complications following craniotomy [30], as well as following other neurosurgical procedures [29]. We believe that the CCI is a useful metric for identifying PCNSL patients who are likely to experience perioperative complications.
In this study, we also identified patient characteristics that are associated with resection versus biopsy as the procedure of choice. Our finding that age, multiple lesions, and deep lesion location were factors associated with selection for biopsy is consistent with prior series on intracranial neoplasms. Weller et al. also found in their PCNSL series that patients with multiple lesions were less likely to undergo resection [2]. Tomlinson et al. found that age predicts a poor prognosis for PCNSL [9], and age has also been shown to predict complications following resection of intra-axial tumors [34]. Tanaka et al. found that deep lesion location was a negative predictor of selection for resection for other intra-axial tumors [42], and multiple series have demonstrated that deep lesion location is a poor prognostic indicator for both PCNSL [7, 43–45], and other CNS malignancies [42, 46]. Indeed, some investigators suggest resection should be avoided for deep PCNSL [6, 8], and current guidelines only recommend resection when there is mass effect causing herniation [23].
While we have identified several selection factors for resection in our series, surgical decision-making is complex. Indeed, results of surveys on surgeons’ surgical plans correlates much more strongly with extent of resection than known predictive variables and clinical scales [47], suggesting that the decision-making process is incompletely captured with standard analyses such as ours. Moreover, Orringer et al. found 28.3% disagreement between academic tumor surgeons about the feasibility of gross total resection for given lesions, suggesting that further highlighting the complexity of the decision and its inability to be reduced to simple binary variables [48].
We found that the complication rates from biopsy were statistically similar and numerically higher than complication rates from resection. Our findings are consistent with Tanaka et al’s recent series of glioblastoma patients, which also found a higher rate of complications after biopsy using the GOP method of classification (18.9% after resection, 30.8% after biopsy) [42]. This finding likely results from the selection factors that distinguished biopsy patients from resection patients: our biopsy patients had lower KPS scores, and age predicted selection for biopsy on multivariable regression. Age and KPS have both been shown to predict systemic and regional complications [25, 34], which result in a higher rate of complications in the biopsy group. Of note, our finding that age and KPS were different between resection and biopsy groups is not consistent with a recent series on PCNSL from Weller et al. [2], which could reflect differences in institutional patterns of care, or bias introduced by a clinical trial inclusion criteria in the study of Weller et al.
The proportion of patients who underwent surgical resection for PCNSL in our series (45%) appears high, likely due to multiple factors. The patients included in our series were identified using neuropathology records, which would only have included patients who underwent a cranial procedure from which tissue could have been obtained. Furthermore, as we only studied PCNSL, patients with a history of lymphoma who developed a CNS lesion were not included, and may have contributed to the disproportionately low numbers in the biopsy group. Additionally, our methodology could have excluded patients who received radiological workup that identified extra-axial lesions, particularly if such lesions were amenable to biopsy. Finally, local practice may have contributed to a higher rate of resection in the PCNSL population. Current guidelines recommend resection when there is mass effect causing herniation [23], and our institution is a referral center that may receive a disproportionate number of these high-risk cases.
Our study has several important limitations. It was conducted retrospectively and it spanned a wide time period, during which patterns of care and surgical management may have changed. While our series is larger than many existing surgical series on PCNSL, it is relatively small compared to surgical series for other intra-axial tumors. While we have examined the safety of surgical resection for PCNSL, it remains to be determined whether there is therapeutic benefit to undergoing resection, or achieving a greater extent of resection for PCNSL. Notably, the standard of care for PCNSL consists of radiotherapy and chemotherapy [1], and postoperative recovery from surgical resection may delay time to initiation of these other modalities whose benefits are well known. Unfortunately, our data is limited in this regard, and future investigations should examine whether such a delay occurs, and if that delay affects outcomes.
Conclusion
Surgical resection of PCNSL is safe for select patients, with complication rates comparable to rates for other intracranial neoplasms. Whether there is a clinical benefit to resection cannot be concluded.
References
Bataille B, Delwail V, Menet E, Vandermarcq P, Ingrand P, Wager M, Guy G, Lapierre F (2000) Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg 92:261–266
Weller M, Martus P, Roth P, Thiel E, Korfel A (2012) Surgery for primary CNS lymphoma? Challenging a paradigm. Neuro Oncol 14:1481–1484
Bierman PJ (2014) Surgery for primary central nervous system lymphoma: is it time for reevaluation? Oncology (Williston Park, NY) 28
Siasios I, Fotiadou A, Fotakopoulos G, Ioannou M, Anagnostopoulos V, Fountas K (2015) Primary diffuse large B-cell lymphoma of central nervous system: is still surgery an unorthodox treatment? J Clin Med Res 7:1007
Henry JM, Heffner RR, Dillard SH, Earle KM, Davis RL (1974) Primary malignant lymphomas of the central nervous system. Cancer 34:1293–1302
DeAngelis LM, Yahalom J, Heinemann MH, Cirrincione C, Thaler HT, Krol G (1990) Primary CNS lymphoma combined treatment with chemotherapy and radiotherapy. Neurology 40:80–80
Murray K, Kun L, Cox J (1986) Primary malignant lymphoma of the central nervous system: results of treatment of 11 cases and review of the literature. J Neurosurg 65:600–607
Abrey LE, Yahalom J, DeAngelis LM (2000) Treatment for primary CNS lymphoma: the next step. J Clin Oncol 18:3144–3150
Tomlinson FH, Kurtin PJ, Suman VJ, Scheithauer BW, O’Fallon JR, Kelly PJ, Jack Jr CR, O’Neill BP (1995) Primary intracerebral malignant lymphoma: a clinicopathological study of 89 patients. J Neurosurg 82:558–566
Frank G, Ferracini R, Spagnolli F, Frank F, Gaist G, Lorenzini P, Ricci R (1985) Primary intracranial lymphomas. Surg Neurol 23:3–8
Gonzalez DG, Schuster-Uitterhoeve ALJ (1983) Primary non-Hodgkin’s lymphoma of the central nervous system. Results of radiotherapy in 15 cases. Cancer 51:2048–2052
Helle TL, Britt RH, Colby TV (1984) Primary lymphoma of the central nervous system: clinicopathological study of experience at Stanford. J Neurosurg 60:94–103
Mendenhall NP, Thar TL, Frank O, Harty-Golder B, Ballinger WE, Million RR (1983) Primary lymphoma of the central nervous system. Computerized tomography scan characteristics and treatment results for 12 cases. Cancer 52:1993–2000
Marcus R, Hodson D, Coupland S, Bessell E, Mead B, Pettitt AR (2009) Guidelines on the diagnosis and management of adult patients with primary CNS lymphoma (PCNSL) and primary intra-ocular lymphoma (PIOL)
Bellinzona M, Roser F, Ostertag H, Gaab RM, Saini M (2005) Surgical removal of primary central nervous system lymphomas (PCNSL) presenting as space occupying lesions: a series of 33 cases. Eur J Surg Oncol (EJSO) 31:100–105
Finks JF, Osborne NH, Birkmeyer JD (2011) Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med 364:2128–2137. doi:10.1056/NEJMsa1010705
Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG (2010) Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 303:1259–1265
Patil PG, Turner DA, Pietrobon R (2005) National trends in surgical procedures for degenerative cervical spine disease: 1990–2000. Neurosurgery 57:753–758
Cheney FW, Posner KL, Lee LA, Caplan RA, Domino KB (2006) Trends in anesthesia-related death and brain damage. Anesthesiology 105:1081–1086
Smith-Bindman R, Miglioretti DL, Johnson E, Lee C, Feigelson HS, Flynn M, Greenlee RT, Kruger RL, Hornbrook MC, Roblin D (2012) Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996–2010. JAMA 307:2400–2409
Gumprecht HK, Widenka DC, Lumenta CB (1999) BrainLab VectorVision Neuronavigation System: technology and clinical experiences in 131 cases. Neurosurgery 44:97–104
Schebesch K-M, Hoehne J, Hohenberger C, Acerbi F, Broggi M, Proescholdt M, Wendl C, Riemenschneider MJ, Brawanski A (2015) Fluorescein sodium-guided surgery in cerebral lymphoma. Clin Neurol Neurosurg 139:125–128
Hoang-Xuan K, Bessell E, Bromberg J, Hottinger AF, Preusser M, Rudà R, Schlegel U, Siegal T, Soussain C, Abacioglu U (2015) Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European Association for Neuro-Oncology. Lancet Oncol 16:e322–e332
Karnofsky DA (1949) The clinical evaluation of chemotherapeutic agents in cancer. Evaluation of chemotherapeutic agents
Chang SM, Parney IF, McDermott M, Barker FG 2nd, Schmidt MH, Huang W, Laws ER Jr, Lillehei KO, Bernstein M, Brem H, Sloan AE, Berger M (2003) Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma outcome project. J Neurosurg 98:1175–1181. doi:10.3171/jns.2003.98.6.1175
Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KK, Ludwig LE, Pedan A, Goldman L (1999) Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 100:1043–1049
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
D’Amico RS, Cloney MB, Sonabend AM, Zacharia B, Nazarian MN, Iwamoto FM, Sisti MB, Bruce JN, McKhann GM (2015) The safety of surgery in elderly patients with primary and recurrent glioblastoma. World Neurosurg. doi:10.1016/j.wneu.2015.05.072.
Patil CG, Lad SP, Santarelli J, Boakye M (2007) National inpatient complications and outcomes after surgery for spinal metastasis from 1993 to 2002. Cancer 110:625–630
Curry WT, McDermott MW, Carter BS, Barker FG 2nd (2005) Craniotomy for meningioma in the United States between 1988 and 2000: decreasing rate of mortality and the effect of provider caseload. J Neurosurg 102:977–986. doi:10.3171/jns.2005.102.6.0977
Barker FG 2nd, Curry WT Jr, Carter BS (2005) Surgery for primary supratentorial brain tumors in the United States, 1988 to 2000: the effect of provider caseload and centralization of care. Neuro Oncol 7:49–63. doi:10.1215/S1152851704000146
Zada G, Du R, Laws Jr ER (2011) Defining the “edge of the envelope”: patient selection in treating complex sellar-based neoplasms via transsphenoidal versus open craniotomy: clinical article. J Neurosurg 114:286–300
Brell M, Ibanez J, Caral L, Ferrer E (2000) Factors influencing surgical complications of intra-axial brain tumours. Acta Neurochir (Wien) 142:739–750
Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi W-M, Wildrick DM (1998) Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 42:1044–1055
Hoover JM, Nwojo M, Puffer R, Mandrekar J, Meyer FB, Parney IF (2013) Surgical outcomes in recurrent glioma. J Neurosurg. doi:10.3171/2013.2.JNS121731
Moiyadi AV, Shetty PM (2012) Perioperative outcomes following surgery for brain tumors: objective assessment and risk factor evaluation. J Neurosci Rural Pract 3:28
Talacchi A, Turazzi S, Locatelli F, Sala F, Beltramello A, Alessandrini F, Manganotti P, Lanteri P, Gambin R, Ganau M, Tramontano V, Santini B, Gerosa M (2010) Surgical treatment of high-grade gliomas in motor areas. The impact of different supportive technologies: a 171-patient series. J Neurooncol 100:417–426. doi:10.1007/s11060-010-0193-x
Malone HR, Cloney M, Yang J, Neugut AI, Bruce JN (2015) Hospital surgical volume as a source of variation in failure to rescue and inpatient mortality following craniotomy for resection of intracranial neoplasms. 2015 congress of neurological surgeons annual meeting. Congress of Neurological Surgeons, New Orleans, LA
Benz RJ, Ibrahim ZG, Afshar P, Garfin SR (2001) Predicting complications in elderly patients undergoing lumbar decompression. Clin Orthop Relat Res 384:116–121
Birim Ö, Maat A, Kappetein AP, Van Meerbeeck JP, Damhuis RAM, Bogers A (2003) Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardio-thorac Surg 23:30–34
Singh B, Bhaya M, Stern J, Roland JT, Zimbler M, Rosenfeld RM, Har-El G, Lucente FE (1997) Validation of the Charlson comorbidity index in patients with head and neck cancer: a multi-institutional study. Laryngoscope 107:1469–1475
Tanaka S, Meyer FB, Buckner JC, Uhm JH, Yan ES, Parney IF (2013) Presentation, management, and outcome of newly diagnosed glioblastoma in elderly patients. J Neurosurg 118:786–798 doi:10.3171/2012.10.JNS112268
Hochberg FH, Miller DC (1988) Primary central nervous system lymphoma. J Neurosurg 68:835–853
Michalski JM, Garcia DM, Kase E, Grigsby PW, Simpson JR (1990) Primary central nervous system lymphoma: analysis of prognostic variables and patterns of treatment failure. Radiology 176:855–860
Pollack IF, Dade Lunsford L, Flickinger JC, Lee Dameshek H (1989) Prognostic factors in the diagnosis and treatment of primary central nervous system lymphoma. Cancer 63:939–947
Iwamoto FM, Cooper AR, Reiner AS, Nayak L, Abrey LE (2009) Glioblastoma in the elderly: the memorial Sloan-Kettering cancer center experience (1997–2007). Cancer 115:3758–3766 doi:10.1002/cncr.24413
Sonabend AM, Zacharia BE, Cloney MB, Sonabend A, Showers C, Ebiana V, Nazarian M, Swanson KR, Baldock A, Brem H (2016) Defining glioblastoma resectability through the wisdom of the crowd: a proof-of-principle study. Neurosurgery
Orringer D, Lau D, Khatri S, Zamora-Berridi GJ, Zhang K, Wu C, Chaudhary N, Sagher O (2012) Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg. doi:10.3171/2012.8.jns12234
Funding
NIH office of the Director, Award Number 1DP5OD021356-01, and Diversity Recruitment Award by the Office of Senior Associate Provost for Faculty Diversity and Inclusion, Columbia University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Michael Brendan Cloney and Adam Sonabend contributed equally to this manuscript, and are co-first authors.
Rights and permissions
About this article
Cite this article
Cloney, M.B., Sonabend, A.M., Yun, J. et al. The safety of resection for primary central nervous system lymphoma: a single institution retrospective analysis. J Neurooncol 132, 189–197 (2017). https://doi.org/10.1007/s11060-016-2358-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2358-8