Abstract
The average survival time for patients with recurrent glioblastoma is between 5 and 9 months. Phase I and II trials have shown a modest survival benefit with combination temozolomide and other chemotherapeutics. We conducted a phase I trial of dose-escalating temozolomide with bevacizumab and the proteasome inhibitor bortezomib for patients with recurrent disease. Three groups of three patients were scheduled to receive daily doses of temozolomide at 25, 50, and 75 mg/m2. Fixed doses of bortezomib and bevacizumab were given at standard intervals. Patients were monitored for dose-limiting toxicities (DLT) to determine the maximum-tolerated dose (MTD) of temozolomide with this regimen. No DLT were seen in the first two groups (25 and 50 mg/m2 temozolomide). One patient in the 75 mg/m2 group experienced a grade 4 elevation of ALT and three more patients were accrued for a total of six patients at that dose level. No other DLT occurred, thus making 75 mg/m2 the MTD. Progression-free survival was 3.27 months for all patients and mean overall survival was 20.75 months. The MTD of temozolomide was 75 mg/m2 in combination with bevacizumab and bortezomib for recurrent glioblastoma. Only one patient experienced a severe (Grade 4) elevation of ALT. This study will provide the framework for further studies to elicit effectiveness and better determine a safety profile for this drug combination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma carries a devastating prognosis, with median survival of only 12.1–18.9 months after diagnosis [1, 2]. Temozolomide administered concomitant with radiation and as adjunct chemotherapy for newly diagnosed glioblastoma has been shown to improve survival compared to radiation alone [2, 3]. Recurrence typically occurs shortly after initial treatment and tends to be resistant to therapy, necessitating the development of novel chemotherapeutic agents and dosing schemes.
Multiple phase II studies have attempted to treat recurrent disease with temozolomide monotherapy using metronomic and dose-dense regimens. These studies have yielded median progression free survival (PFS) from 2.1 to 7.0 months and median overall survival (OS) from 5.4 to 11 months [4–7]. Furthermore, one prospective randomized trial comparing temozolomide to procarbazine for progressive disease demonstrated superior 6-month PFS rates with temozolomide (21 %) compared to procarbazine (8 %) [8]. Although these studies are heterogeneous in terms of the dosing schemes and inclusion criteria, their combined evidence suggests that temozolomide provides a small benefit for patients with progressive disease [9].
Bevacizumab, a monoclonal antibody targeting vascular endothelial growth factor (VEGF) has also been studied extensively as monotherapy for recurrent glioblastoma, and was granted accelerated approval by the FDA in May 2009 as single agent for recurrent disease [10]. Phase II trials have reported outcomes superior to historical controls, with median PFS of 3.7–4.2 months and median OS of 6.5–9.2 months [11–13].
Another targeted agent under trial for progressive glioblastoma is bortezomib (VelcadeTM), a potent, highly selective inhibitor of the 20S subunit of the 26S proteasome complex, which is responsible for degrading multiple proteins involved in cell-cycle regulation, apoptosis, and angiogenesis [14]. In vitro studies indicate that bortezomib is active against glioblastoma cell lines, decreasing cell growth and increasing apoptosis with minimal effects on neural stem cells [15–18]. One of the proteins affected by proteasome inhibition is O6-methylguanine methyltransferase (MGMT), a DNA repair enzyme thought to be involved in temozolomide resistance. In vitro studies have shown down-regulation of MGMT in glioblastoma cell lines treated with combination temozolomide and bortezomib, as well as enhanced anti-tumor activity with the combination compared to either drug alone [19–21]. Further preclinical work has shown decreased ability of bortezomib to penetrate the intact blood–brain barrier and provide therapeutic levels in vivo [22–24], and to overcome this, separate studies have evaluated drugs which have the ability to increase its CNS penetrance and thus provide therapeutic effect. A recent Phase II trial by Raizer et al. evaluating the penetration of bortezomib intra-tumorally showed excellent uptake of the drug into the tissue in recurrent glioblastoma, where the blood–brain barrier is already disrupted and reinforces the decision to evaluate its ability to provide treatment effect [25]. While this trial failed to show a delay in progression of glioblastoma when initiated, their work demonstrated nicely a direct effect of bortezomib on proteasome inhibition in vivo.
In a pharmacodynamic study within the 9L gliosarcoma in vivo, bortezomib has been shown to preferentially reach the tumor in comparison to adjacent and contralateral cerebrum [26]. In vivo studies comparing combination treatment with bortezomib and bevacizumab have demonstrated significantly greater reductions in tumor volume with the combination compared to bevacizumab alone, and also found that the combination increased survival to a greater degree than bevacizumab monotherapy (44.7 vs. 38.7 days, p = 0.038) [16]. The effects of bortezomib on temozolomide-resistant cells in vitro and in combination with temozolomide or bevacizumab in vitro and in vivo support the assessment of this drug in combined therapy for glioblastoma.
In clinical trials, bortezomib has been well tolerated by GBM patients when administered with temozolomide and radiotherapy [14, 27, 28]. In a phase I trial for recurrent glioblastoma, bortezomib was well tolerated at doses used for multiple myeloma; median PFS was 5.8–6.0 months and the rate of response was 3 %, leading the authors to suggest a role for the drug in combination therapy [28].
Considering the distinct mechanisms of action of bortezomib, bevacizumab, and temozolomide, we elected to perform a phase I trial to assess the safety of these drugs in combination and the feasibility of advancement to a phase II trial for efficacy. Our primary objective was to determine the maximum tolerated dose (MTD) of temozolomide combined with fixed doses of bortezomib and bevacizumab in patients with progressive glioblastoma. Secondary measures included time to tumor progression, survival from enrollment, overall survival, and best radiographic response in patients who completed at least one cycle of therapy.
Methods
Patient population
Patients were enrolled and treated at Emory University Winship Cancer Institute, beginning with written informed consent per the IRB-approved protocol (#14595). Eligible patients were ≥18 years of age with histologically proven WHO grade IV glioblastoma and evidence of progression or recurrence on MRI within 2 weeks prior to enrollment. Further inclusion criteria included: initial surgical resection (≥6 weeks prior), radiation therapy (≥3 months), and/or chemotherapy with regimens including nitrosurea agents (≥6 weeks) or not including nitrosurea agents (≥4 weeks), no history of bortezomib treatment, and no more than three prior biologic agents. Exclusion criteria included the use of enzyme-inducing antiepileptic drugs (EIAEDs), standard systemic and hematologic parameters, a Karnofsky Performance Status (KPS) of at least 60, and no more than 3 previous episodes of GBM progression.
Drug administration and dose escalation
Patients were treated with one of three escalating doses of temozolomide. The plan was to enroll at least three patients per group, up to six if dose-limiting toxicities (DLT) were encountered at that dose. The maximum dose of 75 mg/m2 was chosen based on clinical experience with toxicities at dosages above this level [2].
The three agents were administered in 42 day cycles as shown in Fig. 1. Bortezomib was given at 1.3 mg/m2 on day 1 of weeks 1–4. Bevacizumab was given at a dose of 10 mg/kg on day 1 of weeks 1, 3, and 5. Temozolomide was given at doses of 25 mg/m2 (Group 1), 50 mg/m2 (Group 2), or 75 mg/m2 (Group 3) on days 1–28 of each cycle.
Temozolomide doses were escalated from 25 to 50 mg/m2 after a minimum of three patients had received the lowest dose, and similarly to 75 mg/m2 after three patients had received 50 mg/m2. Any DLT prompted addition of three patients at that dose level, and if two or more of the six patients receiving that dose experienced DLT, that dose was defined as the MTD.
Evaluation of dose limiting toxicity and establishment of maximum tolerated dose
Monthly evaluations included physical exam, vital signs, and blood work. Reductions in temozolomide were required for nonhematologic toxicities of grade 3 or 4 in severity according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0, excluding nausea and vomiting or neurotoxicity that was attributed to the CNS malignancy. These toxicities were considered resolved once severity reached grade 2 or lower. Dose limiting hematologic toxicities included ANC ≤500/mm3, platelets ≤25,000/mm3, and febrile neutropenia. In the event of a hematologic toxicity, CBC was obtained twice weekly until ANC rose to 1500/mm3 and platelets were ≥100,000/mm3, and was considered resolved at grade 1 or baseline. Failure to recover from toxicity within 2 weeks was an indication to remove the patient from treatment and consider the event a DLT.
In the case of bortezomib, any previously established or new toxicity other than neuropathic pain or peripheral sensory neuropathy were managed as follows: if Grade 4 hematologic toxicity, or Grade 3–4 non-hematologic toxicity occurred and was considered by the investigator to be related to bortezomib, this agent was held. For non-hematologic toxicities, bortezomib would be held for up to 2 weeks until the toxicity returned to Grade 1 or better. If toxicity failed to resolve, the drug was discontinued and the patient removed from the study. If the toxicity resolved, bortezomib was restarted at a reduced dose (0.9 mg/m2). For peripheral neuropathy attributed to bortezomib, the drug was reduced to 0.9 mg/m2 (grade 2), held until symptoms resolved to grade 1 (grade 3), or discontinued (grade 4).
Dose reductions were not performed because of bevacizumab’s long half-life (2–3 weeks); instead, doses were held for toxicities meeting certain criteria. Bevacizumab was held for the following events: (a) grade 1 pulmonary or CNS hemorrhage or grade 3 non-pulmonary non-CNS hemorrhage in patients not on anticoagulation, (b) grade ≥3 venous thrombosis, (c) grade 3 congestive heart failure, (d) grade 3 proteinuria, (e) grade ≥2 bowel obstruction, or (f) any other grade 3 event considered to be related to bevacizumab. The drug could be restarted after resolution of toxicity once other contributors to the event were controlled. Bevacizumab was to discontinued for (a) any grade 4 toxicity, (b) grade 3 hypertension if blood pressure could not be medically controlled to ≤150/100, (c) hemorrhage (specified above) in a patient on anticoagulation or occurring more than once, (d) grade ≥2 pulmonary or CNS hemorrhage, (e) arterial thrombosis (including unstable angina, myocardial infarction, and cerebrovascular events), (f) gastrointestinal fistula, (g) wound dehiscence, or (h) MRI-confirmed reversible posterior leukoencephalopathy.
Secondary outcome measures
Overall survival (OS) was measured from date of histologic diagnosis of glioblastoma to date of death, and survival from enrollment (SFE) was measured from date of enrollment in the study to date of death. Time to progression (TTP) was measured from date of enrollment to the date of progression on MRI. Disease progression and response were determined by the primary investigator (JJO) according to the Modified MacDonald Criteria [29].
Results
Patient characteristics
Twelve patients participated in the study which started accrual from August 2012 to February 2015. Three patients were enrolled in each of the first two groups (25 and 50 mg/m2 of TMZ) and six were enrolled in the third group (75 mg/m2 of TMZ). Patient characteristics are listed in Table 1, including results of molecular profiling.
Ten out of 12 patients had undergone primary surgical resection or debulking while the remaining 2 patients had biopsy alone. All 12 patients were treated with temozolomide and radiotherapy before study enrollment, and all were naïve to bortezomib. One patient had prior treatment with bevacizumab, receiving a single cycle of temozolomide plus bevacizumab the month prior to enrollment in group 3 of the study.
Dose escalation and toxicities
All 12 patients were evaluable for toxicity. Initially, three patients were enrolled in each group and completed the first 6-week cycle without evidence of DLT. In treatment Group 3 (75 mg/m2), one of the first three patients suffered an elevation of alanine transaminase (ALT) to a maximum value of 242 (grade 3) while receiving therapy. Per protocol, temozolomide was held. 1 week later, ALT had decreased to 104 (grade 2), so temozolomide was restarted at a reduced dose (50 mg/m2). Over the next month, the patient progressed clinically with worsening right hand weakness, aphasia, and slurred speech; MRI demonstrated progressive disease, indicating removal from the study. 4 weeks after discontinuation of investigational therapy, liver enzymes increased dramatically, from an ALT of 43 on the day she was taken off the study to 780 (grade 4), with her only treatment at that time being biweekly bevacizumab. Considering that hepatotoxicity is listed as one of the primary warnings for temozolomide [30], this event was considered a DLT even though the investigational therapy had already been discontinued.
Per study protocol, an additional three patients were enrolled in Group 3. None of the 5 other patients in Group 3 experienced liver enzyme elevation above grade 1, and no other DLT were observed in any of the treatment groups. Common low-grade toxicities experienced in all groups included: hyperglycemia, hypertension, hypocalcemia, AST increase, and thrombocytopenia. The frequency of individual toxicities and their incidence in each group are elaborated in Table 2.
Despite the lack of a second DLT at 75 mg/m2 to fit the predefined definition of the MTD, we chose not to attempt higher doses because 75 mg/m2 is the highest dose previously reported for dosing regimens similar to this [5, 31] and the cumulative dose of 2100 mg/m2 per cycle has not been exceeded in studies of temozolomide using alternative dosing strategies [13]. Thus, the MTD of temozolomide for this combination was determined to be 75 mg/m2.
Secondary outcome measures
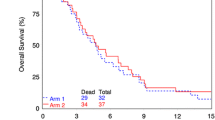
Time to progression (TTP), survival from enrollment (SFE), and overall survival (OS) for all patients and per group are shown in Table 3. In total, 10 of the 12 patients had died at the writing of this manuscript (8 months after closure of the study) and the remaining living patients were excluded from the SFE and OS calculations. Kaplan–Meier survival curves are presented for OS and PFS in Fig. 2.
According to the modified MacDonald criteria, MRI demonstrated partial responses in 2 patients (16.7 %) with a 78 % reduction in one patient in Group 3 and a 74 % reduction in one patient in Group 1. Two patients in Groups 1 and 2 demonstrated 60.7 and 68 % reductions in tumor size that did not persist for the 4 weeks required to qualify as response to treatment. Three patients had reductions of less than 50 % after their first cycle of therapy which also did not persist to qualify as treatment response. MRI response data is shown in Table 4.
Discussion
Recurrent glioblastoma remains difficult to treat despite modern treatments. Multi-agent drug therapy has long been used investigatively for progressive disease with the belief that their combined effects will maximize survival benefits, but no specific regimen has consistently demonstrated superiority over single-agent therapy when subjected to prospective scrutiny. For this trial, three drugs were chosen for investigational treatment based on their distinct mechanisms of action, preclinical research indicating additive effects for the drugs in combination, and evidence for each drug’s activity against progressive glioblastoma.
No previously reported trials have combined temozolomide, bevacizumab, and bortezomib for recurrent glioblastoma. We therefore embarked on this phase I clinical trial and evaluated the safety of dose-escalating temozolomide with fixed doses of bevacizumab and bortezomib for recurrent glioblastoma, using the standard tolerated doses previously reported for the latter two drugs [27, 32, 33]. Considering the maximum doses of temozolomide reported previously, both in terms of daily doses in metronomic regimens [5] and cumulative per cycle doses in dose-dense regimens [13], the maximum-tolerated dose of temozolomide co-administered with bevacizumab and bortezomib was determined to be 75 mg/m2 despite the lack of a second dose limiting toxicity.
Metronomic dosing schedules for temozolomide were chosen in this study as the side effects from the three-drug combination were unknown at the time of study creation and enrollment. This dosing schedule has been reported as efficacious in some patients even after failure of standard dose temozolomide given at the time of initial diagnosis [6, 34–36]. Previous trials showed response with low-dose metronomic therapy at 50 mg/m2/day and thus this was the basis testing doses below, at and above this level to assess tolerability of this drug within this combination.
A previous Phase II study by Bota et al. evaluating 6 month PFS and OS for patients with recurrent glioblastoma showed the combination of bevacizumab and bortezomib to be an effective treatment combination with a benefit of partial radiographic response in patients on non-EIAEDs [37]. While subgroup analysis of patients showed a benefit, their results indicated that this treatment was no better than single-agent bevacizumab alone for recurrent disease and any demonstrable result may have been blunted by EIAEDs. In our study, EIAEDs were part of our exclusion criteria in order to eliminate this finding in an attempt to isolate the true treatment effect of the chemotherapy.
With regard to TTP and SFE in our study, there was no significant difference in survival between groups. Median TTP was comparable to previously reported PFS for bevacizumab monotherapy (3.43 months compared to 3.7–4.2 months), as was median SFE (7.03 months compared to 6.5–9.2 months) [11, 12]. Additionally, at this writing, 2 patients in Group 3 were still alive and doing well. However, comparison of outcomes is in this case purely speculative, since this study was not powered nor intended to draw conclusions about the efficacy of this drug combination.
This phase 1 trial demonstrates the tolerability of temozolomide at metronomic dosing schedules combined with bevacizumab and bortezomib for recurrent glioblastoma. Our results provide evidence to proceed with a phase II trial to evaluate efficacy and determine a more accurate safety profile. This study also provides guidance for further studies using combination agents with consideration of mechanisms of action and potential synergy.
Limitations
At the initiation of the IRB and subsequent approval of the clinical study the modified MacDonald criteria for assessing radiological response was the standard of care. Shortly after initiation of the trial the RANO criteria [38] was developed as a standardized method for assessing response on MRI. Further studies by our group, including a possible Phase II trial with this regimen, would include assessment by the RANO criteria.
Additionally, previous studies looking at bortezomib for recurrent glioblastoma have evaluated response with biweekly dosing schedules (e.g., days 1, 4, 8, 11, etc.) [25, 28, 37, 39] as opposed to the once-weekly schedule we used in our study. This investigation was designed to be administered over a 4 week cycle. In the study of Phuphanich et al. the twice weekly administration for 4 weeks was curtailed, in favor of biweekly doses for the first two of every 3 weeks, over concern for toxicity [28]. To address this safety issue and match the planned 4-week period of administration (followed by 2 weeks off) we sought with the other treatment agents we decided to pursue a once a week regimen with bortezomib. Given the overall positive safety profile in our regimen, further studies could possibly include an escalated dose of bortezomib in line with previously reported dosing schedules.
Conclusion
The combination of temozolomide, bevacizumab, and bortezomib is well-tolerated by patients with recurrent glioblastoma. Further studies are needed to fully assess the safety profile and to establish the degree of efficacy of this chemotherapy regimen against malignant glioma.
References
Grossman SA, Ye X, Piantadosi S et al (2010) Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res 16(8):2443–2449
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466
Brada M, Hoang-Xuan K, Rampling R et al (2001) Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol 12(2):259–266
Brandes AA, Ermani M, Basso U et al (2001) Temozolomide as a second-line systemic regimen in recurrent high-grade glioma: a phase II study. Ann Oncol 12(2):255–257
Kong DS, Lee JI, Kim WS et al (2006) A pilot study of metronomic temozolomide treatment in patients with recurrent temozolomide-refractory glioblastoma. Oncol Rep 16(5):1117–1121
Wick A, Felsberg J, Steinbach JP et al (2007) Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol 25(22):3357–3361
Yung WK, Albright RE, Olson J et al (2000) A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 83(5):588–593
Olson JJ, Nayak L, Ormond DR, Wen PY, Kalkanis SN, Committee ACJG (2014) The role of cytotoxic chemotherapy in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol 118(3):501–555
Cohen MH, Shen YL, Keegan P, Pazdur R (2009) FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist 14(11):1131–1138
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740
Kreisl TN, Kim L, Moore K et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27(5):740–745
Olson JJ, Nayak L, Ormond DR et al (2014) The role of targeted therapies in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol 118(3):557–599
Styczynski J, Olszewska-Slonina D, Kolodziej B, Napieraj M, Wysocki M (2006) Activity of bortezomib in glioblastoma. Anticancer Res 26(6B):4499–4503
Koschny R, Holland H, Sykora J et al (2007) Bortezomib sensitizes primary human astrocytoma cells of WHO grades I to IV for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Clin Cancer Res 13(11):3403–3412
Bota DA, Alexandru D, Keir ST, Bigner D, Vredenburgh J, Friedman HS (2013) Proteasome inhibition with bortezomib induces cell death in GBM stem-like cells and temozolomide-resistant glioma cell lines, but stimulates GBM stem-like cells’ VEGF production and angiogenesis. J Neurosurg 119(6):1415–1423
Unterkircher T, Cristofanon S, Vellanki SH et al (2011) Bortezomib primes glioblastoma, including glioblastoma stem cells, for TRAIL by increasing tBid stability and mitochondrial apoptosis. Clin Cancer Res 17(12):4019–4030
Gong X, Schwartz PH, Linskey ME, Bota DA (2011) Neural stem/progenitors and glioma stem-like cells have differential sensitivity to chemotherapy. Neurology 76(13):1126–1134
Vlachostergios PJ, Hatzidaki E, Befani CD, Liakos P, Papandreou CN (2013) Bortezomib overcomes MGMT-related resistance of glioblastoma cell lines to temozolomide in a schedule-dependent manner. Invest New Drugs 31(5):1169–1181
Vlachostergios PJ, Hatzidaki E, Stathakis NE, Koukoulis GK, Papandreou CN (2013) Bortezomib downregulates MGMT expression in T98G glioblastoma cells. Cell Mol Neurobiol 33(3):313–318
Vlachostergios PJ, Papandreou CN (2015) Efficacy of low dose temozolomide in combination with bortezomib in U87 glioma cells: a flow cytometric analysis. Arch Med Sci 11(2):307–310
Lu S, Chen Z, Yang J et al (2010) The effects of proteasome inhibitor bortezomib on a P-gp positive leukemia cell line K562/A02. Int J Lab Hematol 32(1 Pt 1):e123–131
Rumpold H, Salvador C, Wolf AM, Tilg H, Gastl G, Wolf D (2007) Knockdown of PgP resensitizes leukemic cells to proteasome inhibitors. Biochem Biophys Research Communications 361(2):549–554
Nakamura T, Tanaka K, Matsunobu T et al (2007) The mechanism of cross-resistance to proteasome inhibitor bortezomib and overcoming resistance in Ewing’s family tumor cells. International journal of oncology 31(4):803–811
Raizer JJ, Chandler JP, Ferrarese R, Grimm SA (2016) A phase II trial evaluating the effects and intra-tumoral penetration of bortezomib in patients with recurrent malignant gliomas. J Neurooncol 129(1):139–146
Olson JJ, Bowers G, Zhang Z (2004) Protease inhibitors in a brain tumor model. In: Adams J (ed) Totowa: Humana Press Inc. pp 161–170
Kubicek GJ, Werner-Wasik M, Machtay M et al (2009) Phase I trial using proteasome inhibitor bortezomib and concurrent temozolomide and radiotherapy for central nervous system malignancies. Int J Radiat Oncol Biol Phys 74(2):433–439
Phuphanich S, Supko JG, Carson KA et al (2010) Phase 1 clinical trial of bortezomib in adults with recurrent malignant glioma. J Neurooncol 100(1):95–103
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8(7):1277–1280
Sarganas G, Orzechowski HD, Klimpel A et al (2012) Severe sustained cholestatic hepatitis following temozolomide in a patient with glioblastoma multiforme: case study and review of data from the FDA adverse event reporting system. Neuro Oncol 14(5):541–546
Perry JR, Belanger K, Mason WP et al (2010) Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol 28(12):2051–2057
Jagannath S, Barlogie B, Berenson J et al (2004) A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol 127(2):165–172
Chamberlain MC, Johnston SK (2010) Salvage therapy with single agent bevacizumab for recurrent glioblastoma. J Neurooncol 96(2):259–269
Reynes G, Martinez-Sales V, Vila V et al (2016) Phase II trial of irinotecan and metronomic temozolomide in patients with recurrent glioblastoma. Anticancer Drugs 27(2):133–137
Reynes G, Balana C, Gallego O, Iglesias L, Perez P, Garcia JL (2014) A phase I study of irinotecan in combination with metronomic temozolomide in patients with recurrent glioblastoma. Anticancer Drugs 25(6):717–722
Clarke JL, Iwamoto FM, Sul J et al (2009) Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol 27(23):3861–3867
Bota ZE DA, Reardon DA, Fu BD, Norfleet J, Desjardins A, Linskey ME, Peters K, Friedman HS, Vredenburgh JJ (2011) Phase II clinical trial of bortezomib and bevacizumab combination in recurrent glioblastoma. ASCO Annual Meeting. J Clin Oncol 29:2011 (suppl; abstr 2056)
Wen P, Macdonald D, Reardon D et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972
Friday BB, Anderson SK, Buckner J et al (2012) Phase II trial of vorinostat in combination with bortezomib in recurrent glioblastoma: a north central cancer treatment group study. Neuro Oncol 14(2):215–221
Acknowledgments
D.J.M. wrote the manuscript and analyzed the data, E.C.C. performed all chart reviews, collected data, assisted in manuscript creation, A.D.V. and W.L.R. were co-investigators for the clinical trial, J.J.O. served as the Primary Investigator for the clinical trial and oversaw all aspects related to its implementation.
Funding
Funding for trial provided through Genetech, Takeda, and Merck.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None of the authors have any conflicts-of-interest to report with respect to this manuscript.
Rights and permissions
About this article
Cite this article
McCracken, D.J., Celano, E.C., Voloschin, A.D. et al. Phase I trial of dose-escalating metronomic temozolomide plus bevacizumab and bortezomib for patients with recurrent glioblastoma. J Neurooncol 130, 193–201 (2016). https://doi.org/10.1007/s11060-016-2234-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2234-6