Abstract
In the present study we assessed the activity of the next-generation anaplastic lymphoma kinase (ALK)-tyrosine kinase inhibitor (-TKI) alectinib, in patients with ALK-postive, advanced non-small cell lung cancer (NSCLC) and central nervous system (CNS) metastases. NSCLCs with ALK-positive disease, as assessed by fluorescence in situ hybridization, and CNS metastases were treated with alectinib 600 mg BID. Included patients were followed prospectively in order to evaluate the efficacy of the drug, with particular emphasis on activity in the CNS. Eleven consecutive patients were enrolled. The majority of them were pretreated with crizotinib (n = 10, 90.9 %), and cranial radiotherapy (n = 8, 72.7 %). Six of the seven patients with measurable CNS disease experienced a CNS response, including three patients who were naïve for cranial radiation. Median duration of response was 8 months. For the whole population, median CNS-progression-free survival (-PFS), systemic-PFS, overall-PFS, overall survival, and 1-year survival were 8, 11, 8, 13 months, and 31.1 %, respectively. Two patients experiencing a CNS response were assessed for alectinib’s concentrations in serum and cerebro-spinal fluid (CSF), and showed a CSF-to-serum ratio ranging from 0.001 to 0.003 ng/mL. Alectinib is highly active against CNS metastases from ALK-positive NSCLCs, irrespective of prior treatment(s) with ALK-TKI(s) and/or cranial radiotherapy. The low CSF-to-serum ratio of alectinib suggests that measuring the concentrations of the drug in the CSF may not be a reliable surrogate of its distribution into the CNS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC) represents a molecularly defined subtype of lung cancer that exhibits exquisite sensitivity to treatment with an ALK-tyrosine kinase inhibitor (-TKI) [1]. Crizotinib, was the first ALK-TKI to be approved by both FDA and EMA for use in first-line treatment of ALK-positive advanced NSCLCs, a disease in which it demonstrated superiority over platinum-based chemotherapy in terms of response rate, progression-free survival (PFS) and quality of life in a randomized phase III trial [2]. Nevertheless, resistance to crizotinib is virtually inevitable in all treated patients, usually occurring within 1 year of treatment. However, despite biological mechanisms of resistance to crizotinib are being progressively elucidated, it is rather common for crizotinib-treated patients to eventually relapse in the central nervous system (CNS) [3]. This phenomenon occurs in approximately 70 and 20 % of patients with and without pre-existing CNS metastases, respectively, and has been largely ascribed to the low cerebro-spinal fluid (CSF) penetration rate of crizotinib, which has been shown to be in the range of 0.06–0.26 % [4, 5].
Based on their higher potency against native ALK, next-generation ALK-TKIs such as ceritinib and alectinib have shown to be active in patients resistant to crizotinib, including those individuals with disease progression in the CNS [1]. Particularly, alectinib is emerging as one of the most promising agents for patients with ALK-positive NSCLC and CNS metastases, having demonstrated in a preclinical study not to be a substrate of P-glycoprotein (P-gp), a key efflux transporter that may impair drug penetration through the blood–brain barrier (BBB) [6]. In the present observational study, we report on the efficacy of alectinib in a series of 11 ALK-positive NSCLC patients with CNS metastases, also assessing the CSF levels of alectinib in two of the six patients with measurable CNS disease who responded in the brain.
Methods
Study population
Consecutive patients with histologically-proven ALK-positive NSCLC, as assessed by fluorescence in situ hybridization (FISH) analysis (using dual-color break-apart assays, and standardized criteria), and newly diagnosed or progressive CNS metastases were treated with alectinib. Patients were eligible regardless of whether they had received prior treatment with an ALK-TKI other than alectinib. Patients provided written informed consent for the collection and analysis of clinical information.
Study drug
All patients received alectinib 600 mg BID with a meal. Patients continued treatment until progressive disease (PD), unacceptable toxicity, or withdrawal of consent. Based on investigator’s decision, patients who were deemed to experience prolonged clinical benefit from treatment, were allowed to continue alectinib beyond PD. Minimum washout period between the last dose of prior ALK-TKI and the first dose alectinib was 7 days.
Serum and CSF concentration of alectinib
The alectinib concentrations were measured in serum and CSF at steady state (after day 7 of treatment) 5 h after the morning dose of alectinib. A validated high performance liquid chromatography with tandem mass spectrometry (LC-MS/MS) method was used. Briefly, CFS and serum samples were precipitated in plain methanol and diluted in ultrapure water before injection in the LC-MS/MS system. Limit of quantification was 0.1 ng/mL.
Assessments
Both CNS and systemic responses were assessed according to RECIST version 1.1 [7]. All patients had tumor imaging at baseline, including CT scan of the chest and abdomen, as well as brain imaging (either CT scan or MRI). CNS brain imaging was performed prospectively every 8 weeks.
Adverse events (including alterations in laboratory parameters) considered to be potentially related to alectinib were graded according to Common Terminology Criteria for Adverse Events, version 4.0 [8], and were evaluated once every 3 or 4 weeks or before if clinically indicated.
Statistical analysis
CNS-objective response rate (-ORR), classifying as complete response (CR) + partial response (PR), CNS-disease control rate (-DCR), classifying as CR + PR + stable disease (SD), and CNS-duration of response (-DOR), namely the time elapsing since the first observation of CNS-objective response until the first evidence of CNS progression or death from any cause, were assessed only in the population with measurable CNS disease at baseline. For this purpose, patients were considered as having measurable CNS disease if all the following statements were satisfied: (a) CNS PD had been documented prior to the start of alectinib (b) MRI was the only method employed for the assessment of CNS response (c) at least one parenchymal brain metastases ≥10 mm in its largest diameter was present (d) cranial radiotherapy had been completed ≥6 months prior to the start of alectinib. Patients with CNS measurable disease were also assessed for systemic-ORR outside the brain.
On the other hand, regardless of the presence of measurable CNS disease, all patients were assessed for CNS-PFS, systemic-PFS (S-PFS), and overall-PFS (O-PFS), which were measured from the start of alectinib to the occurrence of either CNS progression or death for CNS-PFS, to the development of systemic progression or death for S-PFS, and to the occurrence of CNS and/or systemic progression or death for O-PFS. Overall survival (OS) was calculated from the start of alectinib to the time of death from any cause.
Results
Patients characteristics
From December 2013 to August 2015, 11 ALK-positive patients with CNS metastases received alectinib 600 mg BID. Alectinib was administered within a clinical study (NCT02075840, n = 1; NCT01801111, n = 2) or on a compassionate use basis (n = 8). Table 1 lists patients’ characteristics. Ten patients (90.9 %) had received prior crizotinib, while 4 (36.3 %) had been previously treated with both crizotinib and ceritinib in sequence. The median duration of prior ALK-TKIs for all patients was 426 days (0–1003), and the median time from the last dose of prior ALK-TKI to the first dose of alectinib was 25 days (7–99). Seven patients (63.6 %) had measurable CNS metastases, three of which had not received any prior cranial radiation. Only two of seven the patients with measurable CNS metastases had received the sequence of crizotinib and ceritinib, the rest being treated with alectinib following crizotinib (n = 4) or as upfront therapy (n = 1).
Efficacy of alectinib
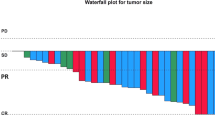
The median follow-up for all patients was 9.5 months (3–25). At the time of this analysis (January 2016) six patients were alive, five of which were still on alectinib treatment (one patient on alectinib beyond PD). Six of the seven patients with measurable CNS disease experienced a CNS-ORR (85.7, 95 % CI 53.5–100) (Fig. 1a). Among them, two CNS CRs and one CNS PR were observed in the three patients with no prior brain radiation, while in the two patients treated with crizotinib and ceritinib in sequence one CNS PR and one CNS PD were observed. The only patient with CNS-PD experienced also concomitant systemic PD. Median CNS-DOR was 8 months (95 % CI 2–11). Figure 1b shows the duration of CNS response in each patient. Of the seven patients with measurable CNS disease, three patients had a systemic response (42.8, 95 % CI 0–71.1), while three systemic SDs were observed. Median CNS-PFS, S-PFS, and O-PFS were 8 months (95 % CI 2–14), 11 months (95 % CI 7–15) and 8 months (95 % CI 3–13), respectively, while median OS was 13 months (95 % CI 7–19) (Fig. 2a–d).
a Waterfall plot of best CNS-ORR. b Duration of CNS response in each patient. Asterisk indicates patients with prior crizotinib; double dollor indicates patients with prior crizotinib and ceritinib; hash indicates patients with prior WBRT; arrows indicate patients who are still responding. CNS-ORR central nervous system-objective response rate
Kaplan–Meier estimates of a CNS-PFS, b S-PFS, c O-PFS and d OS. 1-year survival probability was 23.3 % for CNS-PFS, 29.2 % for S-PFS, 23.3 % for O-PFS, and 31.1 % for OS. CNS-PFS central nervous system-progression-free survival, S-PFS systemic-progression-free survival, O-PFS overall-progression-free survival, OS overall survival
Safety
No cases of dose interruptions and withdrawals due to adverse events occurred. Treatment-related adverse events (all grades, maximum toxicity per patient reported) were more commonly grade 1 (Table 2). Only one grade three adverse event was reported, consisting of nausea/vomiting which was well managed with the use of proactive metoclopramide without dose interruption and/or reduction. Of note, no cases of diarrhoea were reported. One patient died as a result of an adverse event, namely upper gastrointestinal hemorrhage. This patient was a 46-year old woman who was experiencing a CNS CR to alectinib. As she had a history of gastroduodenal ulcer and was receiving concomitant prophylactic low-molecular weight heparin for the prevention of recurrent deep venous thrombosis, her death was considered to be unrelated to study treatment.
CSF-to-serum ratio of alectinib
Two patients with measurable CNS metastases consented for lumbar puncture in order to assess the CSF-to-serum ratio of alectinib.
Case three was a 45-year-old never smoker man with stage IV ALK-positive adenocarcinoma pretreated with platinum-based chemotherapy and crizotinib, from which he achieved a partial response lasting for 14 months. Upon the development of multiple asymptomatic brain metastases he was treated with whole brain radiotherapy (WBRT) and continuation of crizotinib beyond progression. After a further clinical benefit of 7 months, he complained acute urinary retention associated with a marked motor deficit in his left leg. A MRI of the brain and spinal cord showed progression of brain metastases as well as diffuse meningeal enhancement with cytological confirmation of leptomeningeal carcinomatosis. Also, multiple intramedullary lesions at dorsal level were detected. Therefore the patient underwent symptomatic dorsal radiotherapy and initiated alectinib 600 mg BID, with full recovery of neurological symptoms 2 weeks after the initiation of treatment. At that time a repeat MRI of the brain and spinal cord showed a CNS PR, which lasted for approximately 11 months. The serum and CSF sampling performed at month 2 of alectinib showed clearance of leptomeningeal carcinomatosis and revealed a concentration of alectinib of 694 and 2.1 ng/mL, respectively, for a CSF-to-serum ratio of 0.003 (CSF penetration rate 0.3 %).
Case six was a 44-year-old woman with stage IV adenocarcinoma initially treated with cisplatin/pemetrexed followed by nivolumab. Because of newly developed multiple brain metastases she underwent WBRT and, upon documentation of ALK rearrangement, she received crizotinib 250 mg BID with both CNS and systemic SD. After 8 months of crizotinib the patient developed asymptomatic progression in the brain for which she initiated alectinib 600 mg BID. Six weeks after the start of alectinib, a MRI of the brain showed a marked CNS PR (Fig. 3a, d). Serum and CSF sampling at month 2 of treatment revealed a drug concentration of 707 and 0.70 ng/mL, respectively, for a CSF-to-serum ratio of 0.001 (CSF penetration rate 0.1 %). After 4 months of treatment CNS PR is still ongoing.
Discussion
CNS metastases represent a relevant oncological issue in ALK-positive advanced NSCLCs, especially for those patients pretreated with crizotinib. With regard to this, next-generation ALK-TKIs offer a chance to effectively tackle newly developed or progressive CNS disease pretreated or not with crizotinib. Our case series confirms that alectinib is a highly active next-generation ALK-TKI against CNS metastases from ALK-positive NSCLC. In fact, an activity of 85.7 % was reported in the seven patients with measurable CNS disease, with a median CNS-DOR of 8 months (Fig. 1a, b). Interestingly, a pooled analysis of a crizotinib-pretreated CNS population coming from two alectinib phase II studies reported similar results, as a CNS-ORR of 64 % was observed in patients who had measurable CNS disease (n = 50), with a median CNS-DOR of 11.1 months for all patients with measurable and/or non-measurable CNS disease (n = 136) [9]. Also, we confirm that a CNS CR to alectinib is not an uncommon event, being 28.5 % in our series, as opposed to 22 % in the pooled analysis.
In addition, we showed that CNS response can occur regardless of prior treatment(s) with ALK-TKI(s) and/or cranial radiation. At the present time, most of the data about CNS activity of alectinib refer to patients pretreated with crizotinib only [10, 11]. However, we reported a CNS response in one of the two patients who had received prior crizotinib and ceritinib in sequence (Fig. 1a, b). In line with this, another recent case series, reported a CNS response to alectinib after both crizotinib and ceritinib in four ALK-positive NSCLCs who had symptomatic leptomeningeal carcinomatosis [12], which reinforces the hypothesis that a CNS response to alectinib can be obtained in patients who have been pretreated with more than one ALK-TKI. On the other hand, within patients with CNS measurable disease, we reported a CNS response in three of four patients (75.0 %) treated with prior WBRT, as well as in three of three patients (100 %) who had not received any prior cranial radiotherapy. Similarly, a CNS DCR well above 80 % was reported for all patients with measurable and/or non-measurable CNS disease from the pooled analysis of alectinib phase II studies, regardless of whether they had received prior cranial radiation or not [9].
Poor prognostic factors were predominant in patients from this case series. In fact, our population was largely preatreated with ALK-TKI(s) and extracranial metastases were present in all patients, two factors that have been recently associated with poor prognosis in ALK-positive NSCLCs with CNS metastases [13]. In addition, 54.5 % had symptomatic CNS metastases prior to alectinib. Notwithstanding, a median survival and 1-year survival of 13 months and 31.1 %, respectively, were reported. This suggests that offering an ALK-TKI with good CNS activity is crucial in ALK-positive NSCLCs with CNS metastases, particularly if neurologically symptomatic and with no other option of local therapy if re-treatment with radiotherapy is not feasible because of concerns of radiation-induced toxicity.
A previous phase I/II study found measurable CSF concentrations of alectinib in all of the five patients that were analyzed, with a linear relationship noted between CSF and paired unbound systemic concentrations of alectinib (assuming a 0.3 % fraction of unbound alectinib in plasma) [14]. Nevertheless, a CSF concentration of about 1.29 ng/mL was found with alectinib 600–900 mg BID, which is very close to the mean 1.4 ng/mL reported in the two patients from the present study. Although these values appear to be slightly higher than the mean 0.59 ng/mL reported for crizotinib [4, 5], they result into a low CSF penetration rate of alectinib (0.1–0.3 %), which is consistent with a poor solubility of alectinib into the watery CSF enviroment. On the other hand, given its relevant activity, it cannot be excluded that other mechanisms could prevail in determining the efficacy of alectinib in the CNS, such as higher potency against native ALK and/or activity against resistance mutations, as well as and avoidance of P-gp efflux transporter [6, 15]. In any case, the poor CSF concentrations of alectinib suggest that measuring drug levels in the CSF could not reliably predict the CNS activity of the drug, and alternative methods (e.g. positron emission tomography with measurement of the uptake of radiolabeled drug in cerebral metastases as opposed to normal brain) should be sought for this purpose.
The present study has some limitations, among which the small sample size and the fact that no centralized review of brain scans was performed. However, it adds to current evidence as more than half of our patients had symptomatic CNS metastases on steroids, suggesting that alectinib is effective in a CNS population mostly taken from daily clinical practice, in which alectinib also showed good tolerability and no safety concerns (Table 2). Finally, it suggests that, despite slightly higher CSF concentration of alectinib were reported as compared with crizotinib, CSF drug concentration of alectinib does not reflect its distribution into the CNS.
References
Iacono D, Chiari R, Metro G et al (2015) Future options for ALK-positive non-small cell lung cancer. Lung Cancer 87:211–219
Solomon BJ, Mok T, Kim DW et al (2014) First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371:2167–2177
Costa DB, Shaw AT, Ou SH (2015) Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol 33:1881–1888
Costa DB, Kobayashi S, Pandya SS et al (2011) CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 29:e443–e445
Metro G, Lunardi G, Floridi P et al (2015) CSF concentration of crizotinib in two ALK-positive non-small-cell lung cancer patients with CNS metastases deriving clinical benefit from treatment. J Thorac Oncol 10:e26–e27
Kodama T, Hasegawa M, Takanashi K et al (2014) Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol 74:1023–1028
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 1 Jan 2016
Gadgeel S, Shaw T, Govindan R et al (2015) Pooled analysis of CNS response to alectinib in two studies of pre-treated ALK + NSCLC. J Thorac Oncol 10(9Suppl 2):S238 (abstract)
Shaw AT, Gandhi L, Gadgeel S et al (2016) Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 17:234–242
Ou SH, Ahn JS, De Petris L et al (2016) Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol 34:661–668
Gainor JF, Sherman CA, Willoughby K et al (2015) Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol 10:232–236
Johung KL, Yeh N, Desai NB et al (2016) Extended survival and prognostic factors for patients with ALK-rearranged non-small-cell lung cancer and brain metastasis. J Clin Oncol 34:123–129
Gadgeel SM, Gandhi L, Riely GJ et al (2014) Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 15:1119–1128
Sakamoto H, Tsukaguchi T, Hiroshima S et al (2011) CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 17:679–690
Acknowledgments
Supported by the Italian Association for Cancer Research (AIRC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Metro, G., Lunardi, G., Bennati, C. et al. Alectinib’s activity against CNS metastases from ALK-positive non-small cell lung cancer: a single institution case series. J Neurooncol 129, 355–361 (2016). https://doi.org/10.1007/s11060-016-2184-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2184-z