Abstract
Glioma cells rely on glycolysis to obtain energy and sustain their survival under microenvironmental stress in vivo. The mechanisms of regulation of glycolysis in glioma cells are unclear. Signaling pathway mediated by the transcription factor X box-binding protein 1 (XBP1) is one of the most important pathways of unfolded protein response which is comprehensively activated in cancer cells upon the microenvironmental stress. Here we showed that XBP1 was significantly activated in glioma tissues in vivo. XBP1 silencing resulted in decreasing of glioma cell viability and ATP/lactate production under hypoxia, which is possibly mediated by inhibition of Hexokinase II (HK2)’s expression. More importantly, XBP1 silenced glioma cells showed the decrease of tumor formation capacity. Our results revealed that XBP1s activation was involved in glioma glycolysis regulation and might be a potential molecular target for glioma treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioma cells grow in the microenvironment where is hypoxia and lack of nutrients. To accommodate to the microenvironmental stress, glioma cells are forced to obtain enough energy via particular metabolism and gene expression styles [1, 2]. The shift from oxidative phosphorylation (OXPHOX) to glycolysis is the classic glucose metabolism adaptation [3]. The molecular mechanism of this shift needs further investigation. Some recent studies showed that HK2 was the first key enzyme for glycolysis and abundantly expressed in gliomas, moreover, expression level of HK2 was negatively correlated with glioma (WHO grade IV) patients’ prognosis, while inhibition of HK2’s expression decreased the glioma cell viability [4]. The report of HK2’s function on glioma provided new perspectives for scientists to better understand the special metabolic characteristics of glioma. However, the molecular mechanism of glioma metabolism regulation is still not clear.
IRE1α-XBP1 signaling is one of the most important downstream pathways of unfolded protein response (UPR) which is comprehensively activated in cancer cells under microenvironment stress [5]. Under endoplasmic reticulum (ER) stress conditions, active IRE1α processes the mRNA encoding the transcription factor X box-binding protein 1 (XBP1), excising a 26-nucleotide-long intron that shifts the coding reading frame of this mRNA [6–8]. This results in the expression of an active and stable transcription factor, which is termed as spliced XBP1 (XBP1s). XBP1s translocate to the nucleus to induce the upregulation of a variety of target genes [9–11]. Hypoxia induces XBP1 at the transcriptional level and activates the splicing of its mRNA, leading to increased levels of activated XBP1s protein. After exposure to hypoxia, Glioma undergoes increased apoptosis and decreased clonogenic survival in XBP1-deficient cells [12]. Loss of XBP1 significantly inhibited tumor growth due to a reduced capacity for these transplanted tumor cells to survive in a hypoxic microenvironment [12, 13].
Several studies demonstrated that UPR pathways played critical role in cancer cell survival and tumor growth in vivo by activating adaptive strategies against the microenvironment stress [14, 15]. Blockage of UPR signals including IRE1-XBP1 arm results in tumor growth arrest and cancer cell apoptosis in different malignancies, which suggests that XBP1 is a potential molecular target for cancer therapy [16]. Recently Chen et al.’s study demonstrated that XBP1 participated in controlling the HiF1a-mediated hypoxia response pathway genes including HK2 under hypoxia, which was verified by gene-set enrichment analysis [14]. Considering the tight cross-relationship among UPR signals, cancer microenvironmental stress and energy metabolism, it is reasonable to speculate that UPR molecules may be involved in cancer cell metabolism regulation.
In our previous study [17], we reported that XBP1 was highly expressed, more importantly, XBP1s was clearly activated in glioma tissues. However, the role of XBP1 controlling metabolism in human glioma is not clear. In this study, we showed that XBP1s was significantly activated in glioma tissues and glioma cells under hypoxia. Moreover, XBP1 silencing inhibited glioma cell viability and tumor growth by inhibiting HK2 expression and glycolysis under hypoxia. These data suggested XBP1 could be a molecular target for glioma treatment.
Methods
Cell culture
C6, U87 and U251 cell lines were obtained from Cell Resource Center, IBMS, CAMS/PUMC where they were characterized by mycoplasma detection, short tandem repeat (STR) and cell vitality detection. These cell lines were immediately expanded and frozen so that they could be restarted every 3–4 months from a frozen vial of the same batch of cells. Cells cultured in DMEM high glucose medium (Invitrogen, USA) with 10 % fetal bovine serum (Invitrogen, USA) and 1 % streptomycin/penicillin solution (Beyotime, China), at 37 °C with 5 % CO2.
Westernblot analysis
Cell and tissue proteins were collected with RIPA lysis buffer containing protease and phosphatase inhibitors (Roche, Swiss) according to the manufacturer instructions, westernblot was achieved as previously described. Membranes were incubated with the following antibodies: anti- XBP1 (1:1000, GeneTex, USA), anti- XBP1s (1:1000, Biolegend, USA), anti-HK2 (1:1000, Cell Signaling Technology, USA) and anti-β-actin (1:1000, Santa Cruz, USA). Protein expression levels were evaluated by quantified gray density of westernblot bands with Odyssey V1.2 software and normalized to internal controls.
Cell infection and construction of lentiviral vector
XBP1 and negative control (NC) shRNAs were first constructed according to effective XBP1’s siRNA (5′ CCAGUCAUGUUCUUCAAAU 3′) and NC sequences (5′ UUCUCCGAACGUGUCACGU 3′) which were confirmed in this study (Supplementary Fig. 1). XBP1 and negative control (NC) shRNAs were used for transient transfection experiments in this study. For stable transfection, XBP1 and NC shRNAs were embedded into pENTR/U6-GFP vector with GFP, then recombination reaction was processed between pENTR/U6-shRNA-GFP and pLenti6/Block-it-DEST vector, after that, the combined vector and package plasmid were co-transfected into HEK 293T cells and then virus particles were collected after 48 h. U87 cells were then infected with lenti-Xbp1 shRNA or NC vector. U87 cells which expressed GFP were chose for further culture.
Trypan blue staining
12 h or 24 h after treatment the cell suspension was mixed with 0.4 % trypan blue solution (Beyotime, China) at a 1:1 ratio. After 1–2 min incubation at room temperature, the mixture was loaded onto one chamber of Neubauer hemocytometer and squares of the chamber were observed under a light microscope. The viable/live (clear) and non-viable/dead (blue) cells were counted and the viability was calculated using the formula (number of live cells counted/total number of cells counted) × 100.
ATP production, lactate production and glucose uptake assays
Cells were treated by Oligomycin A (OA, 1 mol/L, Cayman, USA) or grew in 1 % O2. ATP and Lactate production were respectively detected by ATP kit (Beyotime, China) and Lactate assay kit (Eton Bioscience, USA) on the basis of manufacturer’s instructions and normalized to live glioma cell numbers. For glucose uptake, 1 million cells were stained with fluorescent D–glucose analogue 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D–glucose (2-NBDG; 100 µM; Invitrogen, USA) for 30 min, washed with PBS, and analyzed by flow cytometry.
O2 consumption
106 U87 or U251 cells were resuspended in 1 mL of fresh medium prewarmed to 37 °C and pregassed with 95 % air and 5 % CO2. The cell suspension was placed in a sealed respiration chamber equipped with a temperature control, a microstirring device, and a Clark-type oxygen electrode. Oxygen consumption in the cell suspension was measured using a Mitocell MT200 respirometer and an oxygen electrode (Warner Instruments). The oxygen content was periodically monitored with an MT200 respirometer, and the oxygen consumption rate was measured over 1 min.
In vivo tumor formation
C6 cell suspensions (containing 5 × 105 cells) were injected at a depth of 5.0 mm into the caudate nucleus of three male Wistar Rats weighing 200–250 g (Vital River Laboratory, Beijing, China). Rats were sacrificed at 21-day after C6 cell transplantation. At the same time, glioma and normal adjacent tissues were dissected and immediately frozen at −80 °C for use.
Ten female BALB/c nude mice used in this study (4 weeks old) were purchased from Slac Laboratory Animal Company, Shanghai, China. 2 × 106 lenti-NC or lenti-XBP1 shRNA stable transfected glioma cells were respectively injected into right shoulders subcutaneous of two groups nude mice, in addition, each group contained five nude mice. The mice were then sacrificed at 28-day. The glioma tissues were immediately resected and stored at −80 °C for use. All the procedures were approved by the Ethical Committee of the First Affiliated Hospital of Harbin Medical University.
Statistical analysis
All the experiments were repeated three times at least and the statistical results were expressed as mean ± SEM. The significance was evaluated by Student’s t test or one way ANOVA using GraphPad Prism software.
Results
XBP1 silencing suppressed glioma cell viability and metabolism under hypoxia condition
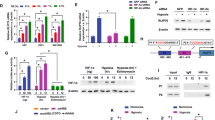
XBP1s activation is an important marker of UPR and protects cancer cells against different kinds of stress such as hypoxia and nutrients deprivation inside solid tumors in vivo [18, 19]. However, little is known about its effects in glioma. To investigate the effect of in vivo microenvironmental stress on XBP1s activation, we compared the XBP1s level between C6 glioma cells in vitro and C6 intracranial glioma tissues in vivo. The results showed that XBP1s was slightly activated in C6 cells cultured in vitro while strongly activated in C6 intracranial glioma tissue (Fig. 1a).
XBP1 silencing suppressed glioma cell viability and metabolism under hypoxia. a Westernblot analysis was performed to detect XBP1s and XBP1 protein expression in C6 cells cultured in vitro and intracerebral glioma tissues formed by C6 cells. b U87 and U251 cells were cultured in 1 % O2 for 12 or 24 h. Westernblot analyses were performed to detect XBP1s, XBP1 and actin protein expression. c U87 and U251 cells were transfected with NC or xbp1 shRNA, and then 48 h later the cells were cultured in 1 % O2 for 24 h. Westernblot analyses were performed to detect XBP1s, XBP1 and actin protein expression. d, e U87 and U251 cells were transfected with NC or xbp1 shRNA, and then 48 h later the cells were cultured in 1 % O2 for 12 or 24 h. Cell viability was assessed using trypan blue exclusion assay. The results were repeated three times and the data was shown represent mean ± SEM of three independent experiments. **P < 0.01; ***P < 0.001. f, g U87 and U251 cells were transfected with NC or XBP1 shRNA, and then 48 h later the cells were cultured in 1 % O2 for 12 h. ATP production was examined by ATP kit. The results repeated last three times and the data shown represent mean ± SEM of three independent experiments. *P < 0.05; **P < 0.01. h, i: U87 and U251 cells were transfected with NC or XBP1 shRNA, and then 48 h later the cells were cultured under 1 % O2 for 12 h. Lactate production was examined by lactate kit. The results were repeated three times and the data was shown represent mean ± SEM of three independent experiments. *P < 0.05. j, k U87 and U251 cells were transfected with NC or XBP1 shRNA, and then 48 h later the cells were cultured under 1 % O2 for 12 h. Glucose uptake in cells was measured by incubation with the glucose analogue 2-NBDG followed by flow cytometry. The results were repeated three times and the data was shown represent mean ± SEM of three independent experiments. **P < 0.01
In order to clarify the function of XBP1s activation in glioma cells, we mimicked hypoxia microenvironment in glioma tissues by culturing glioma cells in 1 % O2. As shown in Fig. 1b, U87 and U251 cells were cultured in 1 % O2 for 12 h or 24 h. XBP1s protein levels were strongly activated in 1 % O2 for 12 or 24 h, while XBP1 protein levels were not clearly changed. We then used XBP1 shRNA to knock down XBP1 expression in glioma cells. It was shown that XBP1s activation under hypoxia was inhibited in XBP1 silenced cells compared with NC transfection (Fig. 1c). Notably, Trypan blue assay showed that XBP1 silencing alone had little effect on glioma cell viability, while effectively inhibited cell viability under hypoxia condition for 24 h (Fig. 1d, e). To further investigate the effect of XBP1 silencing on glioma cell metabolism, we detected ATP level, lactate production and glucose uptake under hypoxia. To eliminate the effect of cell death on metabolism assays, we selected 12 h treatment under hypoxia as the time point since very minor cell death happened at this time (Fig. 1d, e). The results showed that although XBP1 silencing alone had little effect under normoxia, it significantly blocked the ATP and Lactate production and glucose uptake of glioma cells under hypoxia (Fig. 1f–k, Supplementary Fig. 2A-C). These data suggested that XBP1 silencing inhibited glioma cell glycolysis followed by cell death under hypoxia.
Glioma cells glycolysis under hypoxia relies on XBP1s activation
A classical metabolic adaptation of tumor cells is a shift to aerobic glycolysis instead of oxidative phosphorylation (OXPHOS), a phenomenon referred as the Warburg effect [20, 21]. To investigate the effect of XBP1s on glioma cell metabolism, we detected the oxygen consumption level in XBP1 silenced glioma cells. The results showed that XBP1 shRNA transfected glioma cells had a much higher oxygen consumption level compared to NC glioma cells (Fig. 2a, b). Then we treated NC or XBP1 shRNA glioma cells with a specific OXPHOS inhibitor, oligomycin A (OA) [22], to examine whether the glioma cell viability and metabolism could be restrained by XBP1 silencing. The Trypan blue assays showed that OA slightly inhibited cell viability in NC cells and ATP production was also inhibited in NC cells, while XBP1 silencing significantly augmented OA’s effects (Fig. 2c–f). Since OA inhibited OXPHOS in glioma cells, it was possible that glycolysis could be increased by OA treatment. We then investigated ATP and lactate production and glucose uptake after OA treatment for 12 h since at this time clear cell death happened in neither NC nor XBP1 shRNA transfected glioma cells. The results showed that OA clearly elevated ATP and lactate production and glucose uptake in NC cells but failed in XBP1 silenced cells (Fig. 2g–j). Interestingly, western blotting results showed that OA treatment induced XBP1s expression in glioma cells (Fig. 2k). Taken together, these data suggested that metabolism of XBP1 silenced cells relied more on OXPHOS, and XBP1s was activated upon metabolism stress such as hypoxia or OXPHOS inhibition and was essential for the shift of glioma cell metabolism to glycolysis.
Glioma cells glycolysis under hypoxia relied on XBP1 s activation. a, b 106 NC or XBP1 transfected glioma cells including U87 and U251 cells were resuspended in 1 mL of fresh medium prewarmed to 37 °C and pregassed with 95 % air and 5 % CO2. O2 consumption were analyzed. Data represent mean +SEM of 3 independent experiments. *P < 0.05. c–h U87 and U251 cells were transfected with NC or xbp1 shRNA, and then 48 h later the cells were treated by Oligomycin A (OA, 1 mol/L) for 12 h or 24 h. Cell viability was assessed using trypan blue exclusion assay at 12 h and 24 h (c, d). ATP production was examined by ATP kit at 12 h (e, f). Lactate production was examined by lactate kit at 12 h (g, h). Glucose uptake in cells measured by incubation with the glucose analogue 2-NBDG followed by flow cytometry at 12 h(I-J).The results were repeated three times and the data was shown represent mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01. k U87 and U251 cells were transfected with NC or XBP1 shRNA, and then 48 h later, the cells were treated by OA for 24 h. Westernblot analyses were performed to detect XBP1s, XBP1 and actin protein expression
XBP1 silencing inhibited HK2 induction
Previous study showed that hypoxia induced HK2 was essential for glioma cell glycolysis and growth in vivo [4, 21]. Here we investigated the role of XBP1s activation on HK2 expression. It was revealed that UPR inducer Tunicamycin (TM) [23], hypoxia and OA all induced HK2 expression (Fig. 3a, b). Importantly, XBP1 silencing clearly inhibited HK2 elevation induced by hypoxia or TM treatment (Fig. 3c–f, Supplementary Fig. 2D). More interestingly, in Fig. 3c–f, XBP1 silencing slightly inhibited HK2 expression under normoxia. These data suggested that XBP1 silencing mediated glioma cell glycolysis suppression was possibly due to HK2’s inhibition.
XBP1 silencing inhibited HK2 induction. a, b U87 and U251 cells were respectively treated by TM(2 g/L) or OA(1 mol/L) for 24 h, in addition, cultured in 1 % O2 for 24 h. Western-blot analyses were performed to detect HK2, XBP1s and actin protein expression. c, d U87 and U251 cells were transfected with NC or xbp1 shRNA, then 48 h later the cells were treated under hypoxia. Westernblot analyses were performed to detect HK2 and actin protein expression. e, f U87 and U251 cells were transfected with NC or XBP1 shRNA, then 48 h later the cells were treated by TM. Westernblot analyses were performed to detect HK2 and actin protein expression
XBP1 silencing suppressed glioma growth in vivo
To investigate the effect of XBP1 silencing on glioma growth, we performed tumor formation assay in vivo. Our results indicated that lenti-XBP1 shRNA U87 glioma cells formed smaller volume of glioma compared to lenti-NC glioma cells (Fig. 4a, b). Moreover, westernblot results showed that tumors formed by lenti-XBP1 shRNA glioma cells had much lower HK2 and XBP1s expression levels than those formed by lenti-NC glioma cells (Fig. 4c). Our results suggested that XBP1s activation played an important role on glioma carcinogenesis possibly by regulation of HK2 expression.
XBP1 silencing suppressed glioma growth in vivo. a, b lenti-XBP1 shRNA and lenti-NC U87 cells were injected into right shoulders subcutaneous respectively of BALB/c nude female mice (n = 5). The typical images of mice bearing glioma were pictured 4 weeks later and the tumor volumes were compared at day 28. The data was showed represented mean ± SEM of tumor volumes. *P < 0.05. c Westernblot analysis was performed to detect HK2, XBP1s and actin in indicated cells or glioma tissues respectively
Disscusion
In this study, we showed that the microenvironmental stress activated XBP1s in C6 glioma cells, and more importantly, XBP1 silencing in human glioma cells resulted in decrease of cell viability, ATP production and glycolysis under hypoxia. We for the first time demonstrated that XBP1 silencing strongly decreased the induction of HK2 under hypoxia and inhibited glioma tumorigenesis in vivo, thus suggesting XBP1 activation was involved in glycolysis regulation in glioma.
Although both HK1 and HK2 express in GBMs and catalyze glucose phosphorylation, only HK2 is the major regulator of glycolysis in GBMs since HK2 depletion clearly induced glioma cell apoptosis and only HK2 depletion, not HK1, significantly reduced lactate production [4]. It is revealed that HK2 can be induced under hypoxia, which is necessary for glioma cells to adapt to the microenvironmental stress. Our results also showed that hypoxia induced HK2 expression in glioma cells, while XBP1 silencing clearly compromised this induction, which suggested that XBP1 was necessary for HK2’s induction under hypoxia. Previous studies showed that XBP1 indirectly activated expression of a variety of genes including HK2 by enhancing HiF1a’s transcriptional activity [14, 24–26], which suggested a possible mechanism by which XBP1 regulated HK2’s induction under hypoxia by a HiF1a dependent manner. Moreover, our results also demonstrated that TM, a UPR inducer, induced HK2 expression, which implied that XBP1 alone may activate HK2’s expression independent of HiF1a. The mechanism of XBP1′s role on HK2 induction still needs further investigation.
Cancer cells utilize glycolysis rather than OXPHOS to generate energy to survive, which was named as “Warburg effect” [21]. Our data showed that under normoxia XBP1 silencing resulted in elevation of oxygen consumption, which implied that XBP1 silencing lead to dependence of glioma cells on OXPHOS. Moreover, upon exposure to hypoxia or OA, cells could not utilize OXPHOS for energy generation, thus XBP1 silenced cells showed decrease of cell viability, loss of ATP production and reduced tumor formation capacity due to inhibition of HK2’s induction and glycolysis.
In summary, this is the first report that XBP1 silencing decreased glioma cell viability and ATP/lactate production under hypoxia, more importantly, reduced glioma formation capacity in vivo via inhibition of HK2’s induction. Silencing of XBP1 may act as a promising way for glioma therapy is worthy of further investigation.
References
Kathagen A, Schulte A, Balcke G, Phillips HS, Martens T, Matschke J, Günther HS, Soriano R, Modrusan Z, Sandmann T, Kuhl C, Tissier A, Holz M, Krawinkel LA, Glatzel M, Westphal M, Lamszus K (2013) Hypoxia and oxygenation induce a metabolic switch between pentose phosphate pathway and glycolysis in glioma stem-like cells. Acta Neuropathol 126(5):763–780
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Lai JH, Jan HJ, Liu LW, Lee CC, Wang SG, Hueng DY, Cheng YY, Lee HM, Ma HI (2013) Nodal regulates energy metabolism in glioma cells by inducing expression of hypoxia-inducible factor 1alpha. Neuro-oncology 15(10):1330–1341
Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, Hawkins C, Guha A (2011) Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med 208(2):313–326
Wang M, Kaufman RJ (2014) The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer 14(9):581–597
Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415(6867):92–96
Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ (2002) IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev 16(4):452–466
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107(7):881–891
Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13(2):89–102
Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23(21):7448–7459
Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD (2007) XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27(1):53–66
Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H, Mori K, Glimcher LH, Denko NC, Giaccia AJ, Le QT, Koong AC (2004) XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res 64(17):5943–5947
Koumenis C (2006) ER stress, hypoxia tolerance and tumor progression. Curr Mol Med 6(1):55–69
Chen X, Iliopoulos D, Zhang Q, Tang Q, Greenblatt MB, Hatziapostolou M, Lim E, Tam WL, Ni M, Chen Y, Mai J, Shen H, Hu DZ, Adoro S, Hu B, Song M, Tan C, Landis MD, Ferrari M, Shin SJ, Brown M, Chang JC, Liu XS, Glimcher LH (2014) XBP1 promotes triple-negative breast cancer by controlling the HiF1alpha pathway. Nature 508(7494):103–107
Hart LS, Cunningham JT, Datta T, Dey S, Tameire F, Lehman SL, Qiu B, Zhang H, Cerniglia G, Bi M, Li Y, Gao Y, Liu H, Li C, Maity A, Thomas-Tikhonenko A, Perl AE, Koong A, Fuchs SY, Diehl JA, Mills IG, Ruggero D, Koumenis C (2012) ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J Clin Investig 122(12):4621–4634
Li H, Chen X, Gao Y, Wu J, Zeng F, Song F (2015) XBP1 induces snail expression to promote epithelial- to-mesenchymal transition and invasion of breast cancer cells. Cell Signal 27(1):82–89
Liu Y, Zhang X, Liang Y, Yu H, Chen X, Zheng T, Zheng B, Wang L, Zhao L, Shi C, Zhao S (2011) Targeting X box-binding protein-1 (XBP1) enhances sensitivity of glioma cells to oxidative stress. Neuropathol Appl Neurobiol 37(4):395–405
Hu R, Warri A, Jin L, Zwart A, Riggins RB, Fang HB, Clarke R (2015) NF-kappaB signaling is required for XBP1 (unspliced and spliced)-mediated effects on antiestrogen responsiveness and cell fate decisions in breast cancer. Mol Cell Biol 35(2):379–390
Koong AC, Chauhan V, Romero-Ramirez L (2006) Targeting XBP-1 as a novel anti-cancer strategy. Cancer Biol Ther 5(7):756–759
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324(5930):1029–1033
Warburg O (1956) On respiratory impairment in cancer cells. Science 124(3215):269–270
Lee CH, Wu SB, Hong CH, Liao WT, Wu CY, Chen GS, Wei YH, Yu HS (2011) Aberrant cell proliferation by enhanced mitochondrial biogenesis via mtTFA in arsenical skin cancers. Am J Pathol 178(5):2066–2076
Jiang S, Yan C, Fang QC, Shao ML, Zhang YL, Liu Y, Deng YP, Shan B, Liu JQ, Li HT, Yang L, Zhou J, Dai Z, Liu Y, Jia WP (2014) Fibroblast growth factor 21 is regulated by the IRE1α-XBP1 branch of the unfolded protein response and counteracts endoplasmic reticulum stress-induced hepatic steatosis. J Biol Chem 289(43):29751–29765
Rouault-Pierre K, Lopez-Onieva L, Foster K, Anjos-Afonso F, Lamrissi-Garcia I, Serrano-Sanchez M, Mitter R, Ivanovic Z, de Verneuil H, Gribben J, Taussig D, Rezvani HR, Mazurier F, Bonnet D (2013) HIF-2alpha protects human hematopoietic stem/progenitors and acute myeloid leukemic cells from apoptosis induced by endoplasmic reticulum stress. Cell Stem Cell 13(5):549–563
Margariti A, Li H, Chen T, Martin D, Vizcay-Barrena G, Alam S, Karamariti E, Xiao Q, Zampetaki A, Zhang Z, Wang W, Jiang Z, Gao C, Ma B, Chen YG, Cockerill G, Hu Y, Xu Q, Zeng L (2013) XBP1 mRNA splicing triggers an autophagic response in endothelial cells through BECLIN-1 transcriptional activation. J Biol Chem 288(2):859–872
Cheung EC, Ludwig RL, Vousden KH (2012) Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proc Natl Acad Sci USA 109(50):20491–20496
Acknowledgments
The funding of this study was supported by the National Natural Science Foundation of China (No. 81172388 and 81372701 to Yaohua Liu, No. 81272788 to Shiguang Zhao).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exist.
Additional information
Yaohua Liu, Xu Hou and Min Liu have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Y., Hou, X., Liu, M. et al. XBP1 silencing decreases glioma cell viability and glycolysis possibly by inhibiting HK2 expression. J Neurooncol 126, 455–462 (2016). https://doi.org/10.1007/s11060-015-2003-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-2003-y