Abstract
Temozolomide is a standard chemotherapy agent for malignant gliomas, but the efficacy is still not satisfactory. Therefore, combination chemotherapy using temozolomide with other anti-tumor compounds is now under investigation. Here we studied the mechanism of the synergistic anti-tumor effect achieved by temozolomide and doxorubicin, and elucidated the inhibitory effect of temozolomide on P-glycoprotein (P-gp). Temozolomide significantly enhanced sensitivity to P-gp substrate in glioma cells, particularly in P-gp-overexpressed cells. Synergetic effects, as determined by isobologram analysis, were observed by combining temozolomide and doxorubicin. Subsequently, flow cytometry was utilized to assess the intracellular retention of doxorubicin in cells treated with doxorubicin with or without temozolomide. Temozolomide significantly increased the accumulation of doxorubicin in these cells. The P-gp adenosine triphosphatase (ATPase) assay showed that temozolomide inhibited the ATPase activity of P-gp. In addition, temozolomide combined with doxorubicin significantly prolonged the survival of 9L intracranial allografted glioma-bearing rats compared to single agent treatment. Collectively, our findings suggest that temozolomide can reverse doxorubicin resistance by directly affecting P-gp transport activity. Combination chemotherapy using temozolomide with other agents may be effective against gliomas in clinical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma is the most common and malignant primary brain tumor in adults. The current standard care consists of surgical resection followed by radiotherapy and chemotherapy. However, the median survival time for patients diagnosed with glioblastoma is a meager 12–18 months, with only ~3 % of patients surviving longer than 5 years [1]. These statistics highlight the urgency of developing novel and effective therapeutic strategies against this devastating and uniformly fatal disease.
Temozolomide is an orally administered alkylating agent which is used in the standard care for glioblastoma. To improve the efficacy of temozolomide treatment, combination chemotherapy with other agents has also been extensively studied. Doxorubicin is one of the candidate agents. In vitro studies have demonstrated a synergistic effect when temozolomide is used in association with doxorubicin [2]. Temozolomide and polyethylene glycol-coated liposomal doxorubicin (PLD; doxorubicin hydrochloride liposome injection) have been used in combination in phase 2 clinical studies of the treatment of brain metastases from solid tumors [3] and glioblastoma following concurrent radiotherapy and chemotherapy [4]. Moreover, our group previously showed that local intratumoral infusion of PLD via a method of convection-enhanced delivery (CED) is a promising chemotherapy for the treatment of malignant gliomas [5].
Doxorubicin is known to induce expression of members of the ATP–binding superfamily of transporter proteins, such as P-glycoprotein (P-gp; also known as ATP-binding cassette sub-family B member 1 [ABCB1] or multidrug resistance protein 1 [MDR1]) [6] and multiple resistance protein (MRP) [7], which are known to play major roles in the development of cellular resistance to chemotherapeutic agents [8]. MRP is strongly expressed in up to 70 % of central nervous system tumor specimens, and P-gp expression has been detected in 18 % of high-grade gliomas [9]. P-gp and other transporters actively pump substrates out of the brain, thereby limiting their effects in the central nervous system [10–12]. The function of P-gp at the blood–brain barrier is now actively studied.
The present study investigated the hypothesis that temozolomide might inhibit the function of P-gp, by studying the mechanisms of the synergistic effect obtained using temozolomide in combination with doxorubicin.
Materials and methods
Materials
Monoclonal antibody C-219 (against P-gp) was obtained from EMD Millipore (#517310, Billerica, MA), and beta-actin from Santa Cruz Biotechnology, Inc. (#sc-81178, Dallas, TX). Temozolomide was obtained from Schering-Plough (Osaka, Japan), and was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich Corp., St. Louis, MO) at 200 × 103 µM as stock solution and diluted with medium at use. Doxorubicin hydrochloride was purchased from Sigma-Aldrich Japan K.K. (Tokyo, Japan). Stock solutions of free doxorubicin were prepared by diluting DMSO to a concentration of 50 mg/mL. Infusion solutions of free doxorubicin were prepared by diluting the stock solution with PBS. PLD was purchased from Janssen Pharmaceutical K.K. (Tokyo, Japan). The commercial PLD solutions contained 2 mg/mL of doxorubicin. Infusion solution was prepared by diluting the stock solution with 5 % glucose solution. Verapamil hydrochloride (V4629) was purchased from Sigma-Aldrich Japan K.K.
Cell lines and cell culture
The human glioblastoma cell lines T98G and U251MG, and the rat gliosarcoma cell line 9L (American Type Culture Collection, Rockville, MD) were cultured in essential medium containing 10 % FBS at 37 °C in the presence of 5 % CO2. All cells were grown in drug-free culture medium for more than 2 weeks before assay. P-gp-overexpressing 9L/ADR cell line was derived as follows [13]. 9L cells were continuously exposed to stepwise increasing concentrations of doxorubicin, with repeated two-fold increments in drug concentration from 100 to 1600 µM. This procedure was continued over a period of 3–4 months. The obtained 9L/ADR cells were cultured in the absence of doxorubicin for >2 weeks in the growth medium before use.
ABCB1 plasmids and transfection
U251MG cells were seeded in 3-cm dishes in Opti-MEM (Invitrogen, Calsbad, CA) and were transfected at a confluency of 70–85 % with 2 µg cDNA, ABCB1 plasmid DNA (#RC216080, Origene, Rockville, MD) with 25 µL Lipofectamine 2000 (Invitrogen) in a final volume of 5 mL. After transfection of human ABCB1 cDNA transcripts into U251MG cells, the cells were trypsinized and plated for G418 (Invitrogen, Calsbad, CA) selection (1.5 mg/mL). The cells were cultured with fresh G418 containing medium every 4 days. G418-resistant colonies were visible after 14 days in ABCB1 plasmid DNA transfected cells. No colonies developed in nontransfected cells treated with G418 with the same method.
In vitro cytotoxicity assay
Cells were plated in 6-well plates in triplicate, allowed to attach for 48 h, and then exposed to temozolomide, doxorubicin, or both, in complete medium and harvested after 96 h. In every assay, the concentration of DMSO was lower than 0.2 %. The trypan blue dye-exclusion assay was used to assess cytotoxicity. Synergism, as determined by isobologram analysis [14, 15], was tested between the two agents.
Intracellular doxorubicin accumulation
The intracellular accumulation of doxorubicin was analyzed using flow cytometry. The logarithmically growing cells were treated with indicated concentrations of temozolomide and doxorubicin at 37 °C for 6 h. Then, the cells were collected and washed twice with cold PBS containing 10 µM verapamil. Cells were resuspended in 200 µL PBS and then analyzed using a FACS Canto 2 (Becton–Dickinson, CA), with an excitation wavelength of phycoerythrin for the mean fluorescence intensity of intracellular doxorubicin. A minimum of 20,000 events was analyzed for each histogram generation. The mean value of drug accumulation was identified by dividing the mean fluorescence intensity for each measurement by that obtained in the absence of temozolomide.
Western blot analysis
To determine the protein expression of P-gp, cells were incubated with different concentrations of temozolomide for 96 h. Then, the cells were harvested and rinsed twice with PBS. The proteins were extracted from the cells, separated by SDS-PAGE, and transferred by electrophoresis onto polyvinylidene difluoride membranes. After incubation in blocking solution containing 5 % nonfat milk in TBST buffer (10 mmol/L Tris–HCl [pH 8.0], 150 mmol/L NaCl, 0.1 % Tween 20) for 1 h at room temperature, the membranes were incubated with primary antibodies against P-gp (mAb C219, 1:100 dilution; Calbiochem, San Diego, CA) and beta-actin (clone 6C5, 1:500; Chemicon, Temecula, CA) overnight at 4 °C. Then the membranes were incubated for 1 h with horseradish peroxidase-conjugated secondary antibody at 1:1000 dilution at room temperature. The immunoreactive bands were visualized using a Light Capture system (BIO-RAD, Hercules, CA) with an enhanced chemiluminescent substrate for horseradish peroxidase detection (ECL Western Blotting system; GE Healthcare UK Ltd.).
Adenosine triphosphatase (ATPase) assay of P-gp
The ATPase activities of P-gp were determined using the luminescent ATP detection kit (Pgp-Glo Assay Systems, Promega, Madison, WI) according to the manufacturer’s instructions. Briefly, 1.25 mg/mL P-gp membranes and 25 mM MgATP were incubated in the absence or presence of temozolomide at 37 °C for 40 min, and the remaining ATP was detected as a luciferase-generated luminescent signal. Basal P-gp ATPase activities were determined as the difference between the ATP hydrolysis in the presence or absence of sodium orthovanadate. Temozolomide-stimulated P-gp ATPase activity was measured in the presence of varying concentrations of temozolomide.
Animals and intracranial tumor implantation
Twelve-week-old male Fischer 344 rats were purchased from Japan SLC, Inc. (Hamamatsu, Shizuoka, Japan). The protocol used in the animal study was approved by the Institute for Animal Experimentation of Tohoku University Graduate School of Medicine. The intracranial allograft rat 9L tumor model was prepared as described previously [15]. Briefly, 9L cells were harvested by trypsinization and resuspended in cold PBS for implantation. A cell suspension containing 5 × 105 cells per 10 μL of PBS was used for implantation into the striatum of the rat brain. Under deep isoflurane anesthesia, the rat was placed in a small animal stereotactic frame (David Kopf Instruments, Tujunga, CA). A sagittal incision was made through the skin to expose the cranium, and a burr hole was made in the skull positioned at 0.5 mm anterior and 3 mm lateral from the bregma using a small dental drill. 5 μL of cell suspension was injected at a depth of 4.5 mm from the brain surface. After a wait of 2 min, another 5 μL was injected at a depth of 4 mm. After a final wait of 2 min, the needle was removed, and the wound was closed with sutures.
Convection-enhanced delivery
Infusion was performed using the CED method as described previously [7, 17]. Briefly, a reflux-free step-design infusion cannula connected to a 1-mL syringe mounted on a microinfusion pump (BeeHive; Bioanalytical System, West Lafayette, IN) was used to control the infusion rate. The tip of the cannula was guided to the tumor using the same coordinates as tumor implantation. The following ascending infusion rates were applied to achieve 20 μL total infusion volume: 0.2 μL/min for 15 min, 0.5 μL/min for 10 min, and 0.8 μL/min for 15 min.
Combination therapy against the intracranial allograft model
Thirty-nine rats that received 9L tumor cell implants were randomly divided into four groups: control group (n = 9), temozolomide group (n = 10), PLD group (n = 10), and combination group (n = 10). Seven days after tumor implantation, CED infusion of 4 μg of PLD in 20 μL of PBS was performed in the PLD and combination groups. CED infusion of 20 μL of PBS was performed as a control in the control and temozolomide groups. Temozolomide (350 mg/(m2 days)) in a solution of 10 % DMSO in 0.9 % saline at a dose of 90 mL/m2 was given systemically (intraperitoneal administration) daily for 5 days, starting on day 5 after tumor implantation, in the temozolomide and combination groups. All other rats were monitored for survival. Survival between the treatment groups was compared using a log-rank test. Estimated survival was expressed as a Kaplan–Meier curve.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 for Windows (GraphPad Software, Inc., San Diego, CA). All experiments were repeated three times and the differences for two sample comparisons were determined using the Student t test; one-way ANOVA was used for multiple comparisons. Survival analyses were carried out using Kaplan–Meier curves and the log-rank test. Significance was determined at P < 0.05.
Results
Synergistic cytotoxic effects of temozolomide and doxorubicin in vitro and increased accumulation of doxorubicin
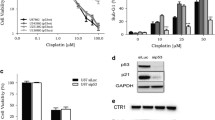
Synergistic effects were confirmed between the two agents in 9L cells (Fig. 1a) and U251MG cells (Fig. 1b). In contrast, only an additive effect was observed in T98G cells (Fig. 1c). Searching for the mechanism of the observed synergistic effects, we examined doxorubicin accumulation in 9L cells, U251MG cells, and T98G cells in the presence or absence of temozolomide. Doxorubicin accumulation was significantly higher in the presence of 50, 100, 200, and 400 µM temozolomide in 9L cells (Fig. 1e). As U251MG cells are sensitive to temozolomide, U251MG cells were treated with lower concentration of temozolomide (0, 50, 100 µM) and doxorubicin (50 nM) at 37 °C for 6 h. Intracellular accumulation of doxorubicin was significantly enhanced in the presence of 100 µM temozolomide in U251MG cells (Fig. 1f). However, in T98G cells, intracellular doxorubicin was significantly higher only in the presence of 200 and 400 µM temozolomide (Fig. 1g). These results show that temozolomide caused higher intracellular accumulation of doxorubicin in glioma cells.
a–c Cellular cytotoxicity in a culture of 9L cells, U251MG cells, and T98G cells, respectively, treated with combination chemotherapy of TMZ and DXR in different concentrations. Data are expressed as isobologram analyses. d Western blotting detected the expression of P-gp protein in U251MG cells but hardly detected in T98G cells. p.c, positive control. Flow cytometric analysis showing the intracellular accumulation of doxorubicin with or without temozolomide (TMZ) in 9L cells (e), in U251MG cells (f), and in T98G cells (G). Columns show means of triplicate determinations; bars, SD. *P < 0.05; **P < 0.01; ***P < 0.001, versus control group. Experiments were repeated at least three times, and a representative experiment is shown
Doxorubicin-selected derivative P-gp-overexpressing 9L/ADR cell line and ABCB1 transfected U251MG cell line
After step by step increased doxorubicin treatment, P-gp overexpressing 9L/ADR cell were obtained. Cell viability study showed the sensitivity of 9L/ADR to doxorubicin is distinctly lower than of 9L parental cells and expression level of P-gp greatly increased in 9L/ADR cells (Fig. 2a). Isobologram analysis showed synergistic effect of temozolomide with doxorubicin (Fig. 2b). Flow cytometric analysis presented doxorubicin accumulation was significantly higher in the presence of 50, 100, 200, and 400 µM temozolomide (Fig. 2c).
a Sensitivity of 9L parental cells and doxorubicin-selected subline 9L/ADR to doxorubicin (DXR). Expression of P-gp in 9L and 9L/ADR cells examined by Western blot analysis. b Cell viability assay of 9L/ADR in culture treated with combination chemotherapy of temozolomide (TMZ) and DXR in different concentrations. Isobologram analyses show the synergistic effect of TMZ with DXR. c Flow cytometric analysis showing the significant increase of intracellular doxorubicin accumulation. Columns show means of triplicate determinations; bars, SD. ***P < 0.001, versus control group. d Expression of P-gp in U251MG and U251/ABCB1 cells examined by Western blot analysis. e Cell viability assay of U251/ABCB1 cells in culture treated with combination chemotherapy of temozolomide (TMZ) and DXR in different concentrations. Isobologram analyses show the synergistic effect of TMZ with DXR. f Flow cytometric analysis showing the significant increase of intracellular doxorubicin accumulation. Columns show means of triplicate determinations; bars, SD. ***P < 0.001, versus control group. Experiments were repeated at least three times, and a representative experiment is shown
To test if the similar findings observed in P-gp gene transfected cells, ABCB1 plasmid was infected to U251MG cells. Expression level of P-gp greatly increased in U251/ABCB1 cells (Fig. 2d). Cell viability study showed the sensitivity of U251/ABCB1 cells to doxorubicin was distinctly lower than of U251MG parental cells. Isobologram analysis showed synergistic effect of temozolomide with doxorubicin (Fig. 2e). Flow cytometric analysis presented doxorubicin accumulation was significantly higher in the presence of 50, 100, 200, and 400 µM temozolomide (Fig. 2f).
Effect of temozolomide on the expression of protein levels and ATPase activity of P-gp
The effect of temozolomide on the expression levels of P-gp protein was investigated using western blot analysis. Our results showed no marked difference in P-gp expression at the protein level in 9L cells and 9L-ADR cells treated with temozolomide for 96 h compared with untreated cells (Fig. 3a). These results suggest that temozolomide inhibits the function of P-gp without affecting the expression level of ABCB1.
a Effect of temozolomide (TMZ) on the expression of P-gp protein. 9L and 9L/ADR cells were treated with TMZ of various concentrations for 96 h. Western blotting detected the expression of P-gp protein. b ATPase activity of P-gp. After 40 min incubation with various concentrations of TMZ, the remaining unmetabolized ATP was detected as a luciferase-generated luminescent signal. P-gp-dependent decreases in luminescence reflected ATP consumption by P-gp. Means of three independent experiments are shown. Bars, SD. All experiments were repeated at least three times, and a representative experiment is shown in each panel
The drug efflux function of P-gp is coupled to ATP hydrolysis, which is stimulated in the presence of P-gp substrates. To assess the effect of temozolomide on the ATPase activity of P-gp, P-gp-mediated ATP hydrolysis was measured using various concentrations of temozolomide. As shown in Fig. 3b, temozolomide affected the ATPase activity of P-gp in a concentration-dependent manner, indicating that temozolomide is an inhibitor of P-gp.
Combined effect of temozolomide and doxorubicin on 9L brain tumor allografts in vivo
All rats from the control group, which received CED infusion of PBS and intraperitoneal injection of 10 % DMSO solution (the vehicle for temozolomide), had to be euthanized because of tumor progression between 14 and 21 days after implantation. Single treatment with CED of the PLD group (CED infusion of PLD and intraperitoneal injection of 10 % DMSO solution) showed no significant difference in survival (Fig. 4). In contrast, the temozolomide group (CED infusion of PBS and intraperitoneal injection of temozolomide) survived significantly longer (P < 0.05, log-rank test). Furthermore, rats from the combination group receiving temozolomide and PLD lived significantly longer than the rats in the single-therapy groups (P < 0.05 compared with temozolomide group, P < 0.001 compared with PLD group, log-rank test).
Effect of combination therapy of temozolomide (TMZ) with PLD on the intracranial 9L tumor model. a Experimental design of the survival study. Daily intraperitoneal injections of TMZ (350 mg/m2/d) were performed 5–9 days after tumor implantation, and local infusions of PLD (4 μg in 20 μL of PBS) were performed on day 7. b Kaplan–Meier survival curves for combination chemotherapy in the tumor model. Rats from the group receiving combination treatment of TMZ and PLD lived significantly longer than the animals in the single-therapy groups (P < 0.05 compared with TMZ group, P < 0.001 compared with PLD group, log-rank test)
Discussion
Recently, a preclinical study demonstrated a synergistic effect for temozolomide used in association with doxorubicin [2]. Moreover, clinical trials to evaluate the combination therapy of doxorubicin with temozolomide in patients with solid tumors have observed higher response rates and longer response duration compared to single agent temozolomide, and indicated the safety and moderate efficacy of combined strategy [3, 4]. However, the possible mechanisms were not elaborated further. It is generally accepted that the principal limitation of the prototypical anthracycline doxorubicin in treating patients with cancer is the high incidence of innate anthracycline resistance, and the rapid emergence of acquired drug resistance, which is associated with the multidrug resistance (MDR) phenotype.
Many different mechanisms of MDR-induced chemoresistance have been elucidated, including alterations in cell cycle checkpoints, failure of apoptotic cascades, repair of damaged cellular targets, and reduced drug accumulation [16, 17]. Of these mechanisms, reduced drug accumulation has been studied in most detail, and appears to be a very common mechanism of chemoresistance both in vitro and in vivo [18]. One of the most intensively studied multidrug transporters is P-gp (also known as ABCB1 or MDR1), which is central in drug absorption and distribution in many organisms. P-gp functions as an ATP-driven efflux pump of substrates ranging from approximately 300–4000 Da in mass, including some human immunodeficiency virus protease inhibitors, antibiotics, immunosuppressive agents, and many other prescribed drugs [19–21]. Our in vitro cytotoxicity study evaluating combinational effect of temozolomide and doxorubicin revealed that this combination worked synergistic in 9L and U251MG, but additive in T98G. Studying the expression of P-gp protein, we found that P-gp was expressed in 9L and U251MG cells but was scarcely detected in T98G (Fig. 1d). Judging from all these facts, we hypothesized that temozolomide may affect the function of P-gp and attempted to elucidate the possible mechanisms in the present study.
We first investigated if addition of temozolomide affects the intracellular retention of doxorubicin after doxorubicin treatment. As doxorubicin has its own autofluorescence, intracellular accumulation of doxorubicin was able to detect by measuring fluorescence signal. Compared to cells those were not treated with temozolomide, cells treated with temozolomide contained higher amount of intracellular doxorubicin indicating the effect of temozolomide on drug efflux pump. Focusing on P-gp, we then analyzed if there is any change in P-gp protein expression. Western blot examination detected no difference in protein expression before and after temozolomide treatment. Subsequently, P-gp ATPase assay was utilized to test if temozolomide directly inhibit the drug efflux activity of P-gp. With addition of temozolomide, there was an apparent decrease in P-gp ATPase activity indicating the direct inhibitory effect of temozolomide on P-gp. Present study showed for the first time that temozolomide inhibits P-gp function through inhibiting P-gp ATPase activity, resulting in increased intracellular accumulation of the substrates of P-gp in malignant glioma cells.
On the other hand, clinical studies have also suggested the interaction of temozolomide and ATP binding cassette transporters. A subset of patients with low-grade glioma experienced improvement in seizure frequency controlled with a combination of antiepileptic drugs and temozolomide [22], and almost all antiepileptic drugs act as substrates for P-gp [23]. Moreover, one of the potential mechanisms of antiepileptic drug resistance is overexpression of ATP binding cassette transporters in the capillary endothelial cells of the blood–brain barrier in patients with drug refractory epilepsy. Since antiepileptic drugs must traverse the blood–brain barrier to enter the brain and exert their desired effects, overexpression of the ATP binding cassette transporters in the endothelial cells of the blood–brain barrier may contribute to drug resistance [24, 25]. Our present findings suggest the possibility that temozolomide enhances the effects of antiepileptic drugs in brain tissue through inhibition of the ATP binding cassette transporters.
Overcoming P-gp has the potential to greatly enhance the activity of many relevant anticancer agents. Our group previously reported that PLD delivered locally with CED demonstrated significant survival prolongation in U251MG and U87MG intracranial xenograft models [5], however the survival benefit was limited in 9L intracranial allograft model. Based on the in vitro data acquired in this study, we determined to evaluate the efficacy of combination of systemic temozolomide and local CED of PLD on 9L brain tumor allografts in vivo. The present data demonstrated that although CED of PLD failed to prolong the survival of 9L tumor-bearing rats, combination treatment of temozolomide and PLD achieved a significant survival benefit compared to the temozolomide treated group.
The present study confirmed the synergistic anti-tumor effect of temozolomide and doxorubicin against malignant gliomas. Direct inhibition of P-gp ATPase activity was found to be one of the mechanisms in this synergistic effect. This effect was more prominent when tested in P-gp overexpressing doxorubicin highly resistant 9L/ADR and U251/ABCB1 cells. Translating this finding, we observed synergistic effect of systemic temozolomide and local CED of PLD against 9L intracranial allografted brain tumor model which was refractory to CED of PLD alone. This strategy can be a promising novel therapeutic approach against malignant gliomas.
References
Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J (2010) Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res 16(8):2443–2449
Balzarotti M, Ciusani E, Calatozzolo C, Croci D, Boiardi A, Salmaggi A (2004) Effect of association of temozolomide with other chemotherapic agents on cell growth inhibition in glioma cell lines. Oncol Res 14(7–8):325–330
Caraglia M, Addeo R, Costanzo R, Montella L, Faiola V, Marra M, Abbruzzese A, Palmieri G, Budillon A, Grillone F, Venuta S, Tagliaferri P, Del Prete S (2006) Phase II study of temozolomide plus pegylated liposomal doxorubicin in the treatment of brain metastases from solid tumours. Cancer Chemother Pharmacol 57(1):34–39
Ananda S, Nowak AK, Cher L, Dowling A, Brown C, Simes J, Rosenthal MA (2011) Phase 2 trial of temozolomide and pegylated liposomal doxorubicin in the treatment of patients with glioblastoma multiforme following concurrent radiotherapy and chemotherapy. J Clin Neurosci 18(11):1444–1448
Kikuchi T, Saito R, Sugiyama S, Yamashita Y, Kumabe T, Krauze M, Bankiewicz K, Tominaga T (2008) Convection-enhanced delivery of polyethylene glycol-coated liposomal doxorubicin: characterization and efficacy in rat intracranial glioma models. J Neurosurg 109(5):867–873
Twentyman PR (1992) MDR1 (P-glycoprotein) gene expression-implications for resistance modifier trials. J Natl Cancer Inst 84(19):1458–1460
Cole SP, Deeley RG (1998) Multidrug resistance mediated by the ATP-binding cassette transporter protein MRP. BioEssays 20(11):931–940
Bredel M, Zentner J (2002) Brain-tumour drug resistance: the bare essentials. Lancet Oncol 3(7):397–406
Abe T, Mori T, Wakabayashi Y, Nakagawa M, Cole SP, Koike K, Kuwano M, Hori S (1998) Expression of multidrug resistance protein gene in patients with glioma after chemotherapy. J Neurooncol 40(1):11–18
Chen C, Hanson E, Watson JW, Lee JS (2003) P-glycoprotein limits the brain penetration of nonsedating but not sedating H1-antagonists. Drug Metab Dispos 31(3):312–318
Schinkel AH (1999) P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv Drug Deliv Rev 36(2–3):179–194
Sun H, Dai H, Shaik N, Elmquist WF (2003) Drug efflux transporters in the CNS. Adv Drug Deliv Rev 55(1):83–105
Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, Ashby CR Jr, Huang Y, Robey RW, Liang YJ, Chen LM, Shi CJ, Ambudkar SV, Chen ZS, Fu LW (2008) Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of atp-binding cassette subfamily B member 1 and G member 2. Cancer Res 68(19):7905–7914
Berenbaum MC (1981) Criteria for analyzing interactions between biologically active agents. Adv Cancer Res 35:269–335
Saito R, Bringas JR, Panner A, Tamas M, Pieper RO, Berger MS, Bankiewicz KS (2004) Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model. Cancer Res 64(19):6858–6862
Gottesman MM (2002) Mechanisms of cancer drug resistance. Annu Rev Med 53:615–627
Borst P, Elferink RO (2002) Mammalian ABC transporters in health and disease. Annu Rev Biochem 71:537–592
Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM (2003) P-glycoprotein: from genomics to mechanism. Oncogene 22(47):7468–7485
Juliano RL, Ling V (1976) A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 455(1):152–162
Gottesman MM, Pastan I (1988) The multidrug transporter, a double-edged sword. J Biol Chem 263(25):12163–12166
Hennessy M, Spiers JP (2007) A primer on the mechanics of P-glycoprotein the multidrug transporter. Pharmacol Res 55(1):1–15
Sherman JH, Moldovan K, Yeoh HK, Starke RM, Pouratian N, Shaffrey ME, Schiff D (2011) Impact of temozolomide chemotherapy on seizure frequency in patients with low-grade gliomas. J Neurosurg 114(6):1617–1621
Zhang C, Kwan P, Zuo Z, Baum L (2012) The transport of antiepileptic drugs by P-glycoprotein. Adv Drug Deliv Rev 64(10):930–942
Loscher W, Potschka H (2005) Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci 6(8):591–602
Loscher W, Sills GJ (2007) Drug resistance in epilepsy: why is a simple explanation not enough? Epilepsia 48(12):2370–2372
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan to R.S. (#26293319).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Zhang, R., Saito, R., Shibahara, I. et al. Temozolomide reverses doxorubicin resistance by inhibiting P-glycoprotein in malignant glioma cells. J Neurooncol 126, 235–242 (2016). https://doi.org/10.1007/s11060-015-1968-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1968-x