Abstract
To explore the incidence, MR imaging findings, dynamic developing process of delayed leukoencephalopathy (DLE) in non-small cell lung cancer (NSCLC) patients with brain metastases patients who undergone whole brain radiation (WBRT) therapy, we retrospectively reviewed 48 NSCLC patients who underwent WBRT for brain metastases from January 2010 through June 2015 and had evaluable magnetic resonance imaging after treatment. The DLE were graded using a scale to evaluate T2-FLAIR (fluid attenuated image recovery) images: grade 1 = little or no white matter hyperintensity, grade 2 = limited periventricular hyperintensity and grade 3 = diffuse white matter hyperintensity. 48 NSCLC patients with brain metastases were enrolled. The median age of these patients was 55.7 years (range 33–75 years). The median follow-up was 12 months. The characteristic MR imaging of DLE in those patients was bilaterally diffuse white matter T2 hyperintensity around the periventricular areas without enhancement, sparing from U-fiber, callosum and gray matter structure. The incidence of DLE developed 6.25 % (3/48), 30.00 % (12/40), 48.39 % (15/31), 61.90 % (13/21), 85.71 % (6/7), 100 % (3/3) in those patients who were followed up for 3, 6, 9, 12, 24, 36 months, respectively. Through increased understanding of it, it may be possible to help clinicians develop further therapeutic strategies to maximize benefit while limiting potential long term toxicities. These data supplement existing reports regarding the late effects of WBRT in NSCLC patients with brain metastasis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Whole-brain radiation therapy (WBRT) is frequently used in the treatment of non-small cell lung cancer (NSCLC) patients to cure or palliate brain metastases. In the past, those patients with brain metastases had a short life expectancy, who did not survive long enough to suffer from late side effects of WBRT. However, with earlier cancer detection and advanced systemic treatments, the median survival has improved [1, 2]. Long-term survivors of NSCLC patients with brain metastases who have received WBRT are at risk for developing delayed leukoencephalopathy (DLE) which can result in dementia, cognitive dysfunction, urinary incontinence and mood [3].
A growing emphasis in cancer therapy has been on quality of life, not merely disease control, making DLE being a significant issue. DLE is related to white matter changes, which can be qualitatively shown on brain MRI images. Small cerebral vasculature and neuropil are damaged resulting in oligodendrocytic death and demyelination in white matter injury [4, 5].
DLE occurring in NSCLC patients with brain metastases patients who undergone WBRT hasn’t been studied intensively. And recognition of DLE progression is critically important to any oncologist, here we explore the incidence, MR imaging findings, dynamic developing process of DEL in an attempt to better understand the treatment-associated leukoencephalopathy.
Methods
Patient selection
With Institutional Review Board approval, we retrospectively reviewed data from 220 consecutive NSCLC patients with brain metastases who underwent WBRT between January 2010 and June 2015. 48 NSCLC patients with brain metastases met the following criteria were enrolled: (a) histologically or cytologically proven NSCLC, with brain metastases identified by MRI. (b) had undergone WBRT once. (c) had evaluable imaging obtained every 3 month in the first year, and every 6 month after one year. (d) the first MR images showing no or less white matter hyperintensities. (e) had no neurosurgery history or prophylactic cranial irradiation (PCI). (f) free of any neurologic, psychiatric, or major systemic illnesses in the initial diagnosis.
WBRT
Cranial irradiation was given by lateral opposed fields to the whole brain delivered by Varian (6MV). Total dose used was 30 Gy in 10 fractions of 3 Gy in 2 weeks. No patient had repeat WBRT. No radiosensitizer was given during WBRT.
MR Imaging protocol
Brain MRI was performed on 1.5T or 3.0T scanner (Signa Excite HD GE Healthcare, Milwaukee, WI, USA). The parameters on 1.5T were as followed: image acquisition was performed with a fast spin-echo technique, including T2-weighted imaging, repetition time (TR)/echo time (TE) = 4300/102 ms; fluid attenuated inversion recovery (FLAIR) T2-weighted imaging, TR/TE = 8400/120 ms, inversion time = 2100 ms; T1WI contrast (axial), TR/TE = 500/12 ms. The parameters on 3.0T were as followed: image acquisition was performed with a spin-echo technique, including T2-weighted imaging, repetition time (TR)/echo time (TE) = 5100/130 ms; fluid attenuated inversion recovery (FLAIR) T2-weighted imaging, TR/TE = 9600/110 ms, inversion time = 2400 ms; T1WI, FLAIR, TR/TE = 2705/24 ms, inversion time = 860 ms; T1WI contrast (axial), TR/TE = 580/17 ms. Section thickness of all images was 5 mm and the interslice gap was 1 mm. All axial scans were parallel to the anteroposterior commissure (AC-PC) line. The gadopentetate dimeglumine was injected through ulnar vein with .1 mmol/kg.
MR Imaging evaluation
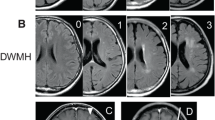
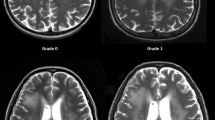
DLE were scored according to Monaco et al. [6]: grade 1, little or no white matter hyperintensity; grade 2, limited periventricular hyperintensity; and grade 3, diffuse white matter hyperintensity. All images were reviewed independently by two neuroradiologists, who had more than 10-year work experience in neuroradiology. They were blinded to the patients’ clinical findings and history. All discrepancies were rechecked and consensus was achieved by discussion.
Figure 1 shows representative MRIs.
Statistical analysis
Descriptive statistics such as mean with standard deviation and median with range were used for continuous data. The cumulative incidence was calculated using the Kaplan–Meier method. Categorical data were compared using χ2 test. Normally distributed continuous data were compared using appropriate t test (SPSS 13.0). A P value of .05 was considered statistically significant.
Results
Patient characteristics
48 NSCLC patients with brain metastases were enrolled, with 22 men and 26 females. The median age of the patients was 55.7 years (range 33–75 years,SD 10.6). The median follow-up was 12 months. At diagnosis, the median Karnofsky Performance Score (KPS) was 90 (range 70–100). Symptoms developed a median of 6 months (range 3–12 months) after WBRT. 34 patients (70.8 %) presented with headache, 20 (41.7 %) with cognitive dysfunction, 11 (22.9 %) with motor deficits and 2 (4.2 %) with confusion. The mean number of brain metastases lesions was 5.8 (SD 3.4) at diagnosis. After WBRT, 29 (60.4 %) patients had a favorable effect of brain metastases.
MR imaging of delayed leukoencephalopathy
DLE showed low signal intensity on T1-weighted imaging and high signal on T2-weighted imaging around the periventricular areas, reflecting the increase in water content and loss of myelin. It became sporadic patchy T2-FLAIR hyperintensities in the semiovale areas at the beginning, and developed progressively bilaterally diffuse white matter T2-FLIAR hyperintensities over months, with irregular border, sparing from U-fibra, corpus callosum and gray matter (Fig. 2). No enhancements or any mass effects were found. It revealed as well cortical-subcortical atrophy, with variable degrees of ventriculoectasis.
A 51-year-old woman with brain metastases from NSCLC underwent whole-brain radiation therapy (WBRT). a–d T2-FLAIR images show patchy white matter changes in the periventricular areas and develop to diffuse white matter changes over months. e–h No enhancements of those white matter changes were found
Incidence of delayed leukoencephalopathy
Among 48 patients, 24 patients (50 %) developed treatment-related DLE a median of 6 months after WBRT (range 3–9 months). The patients had similar initial white matter grades, which both are ≤grade 1. Among 24 patients, man/female was 10/14 and the median age was 59. The incidence of DLE developed 6.25 % (3/48), 30.00 % (12/40), 48.39 % (15/31), 61.90 % (13/21), 85.71 % (6/7), 100 % (3/3) in these patients who were followed up for 3, 6, 9, 12, 24, 36 months, respectively (Fig. 3).
Discussion
WBRT has been advised as the primary treatment for metastatic brain cancer, as it can reduce metastatic tumor recurrence [2, 7]. We found high incidence of treatment-related delayed leukoencephalopathy (≥grade 2) that have correlated with white matter changes in those NSCLC patients with brain metastases. The incidence of DLE developed 6.25 % (3/48), 30.00 % (12/40), 48.39 % (15/31), 61.90 % (13/21), 85.71 % (6/7), 100 % (3/3) in these patients who were followed up for 3, 6, 9, 12, 24, 36 months, respectively, which were varied in other studies. Ebi et al. found that the incidence were 34.4 % (11/32), 42.9 % (6/14), 66.7 % (2/3), and 100 % (2/2) of the patients with brain metastases who were followed up for 6, 12, 24, and 36 months, respectively [8]. Stokes et al. found that the incidence were 25.7, 31.4, 50.1 and 69.5 % in breast cancer brain metastases patients who received WBRT plus SRS for 6, 9, 12 and 15 month, respectively [9]. Fujii et al. detected it in 83 % of long-term survivors treated with WBRT for brain metastases, but not before 6 months [10]. Steven et al. declared that diffuse white matter changes can also occur in normal aging people, but it had a more slower progress [11]. Among the possible reasons for the high incidence of our study might be the generally older age of NSCLC patients with brain metastases after WBRT, which was similar with Ebi et al.
The complications of radiation-leukoencephalopathy are usually divided into acute effects that can occur during the course of radiation therapy, early-delayed effects that appear 2–4 months after radiation therapy, and late effects that can develop >90 days after the initiation of radiation therapy, which have been reviewed in detail in previous publications [12, 13]. We found that, the DLE of NSCLC patients with brain metastases developed a median of 6 months (range 3–9 months) after WBRT. Patients typically exhibit memory problems, motor disorders, urinary incontinence and personality changes, which impact their quality of life. To improve its efficacy, several randomized trials attempted to modify WBRT via different fractionation schedules or the use of radiation sensitizers but failed [14–16]. Some radioprotectants like memantine are being explored [17].
Among 48 patients who underwent WBRT, 24 patients developed DLE compared with a normal pretreatment MR. At the first 3 months after WBRT, there were near-normal MR images. At the median month of 6, MRI revealed various degrees of cerebral atrophy and diffuse white matter T2-FLAIR hyperintensity sparing of the U-fiber and gray matter, which developed spot, patchy white matter T2-FLAIR hyerintensity around the periventricular areas at the beginning and progressed to diffuse white matter changes over months or years. The cerebral atrophy and ventricular dilatation are more likely secondary to the white matter process. The pathophysiological mechanisms include damage to oligodendrocytes, thereby creating axonal demyelination and disruption of vascular endothelial cells, contributing to coagulative necrosis, vessel thickening, and focal mineralization [18, 19]. DLE was a progressive development over time since it occurred, which was a classic time-dependent course. It was irreversible and treatment with steroids had modest benefit.
A growing emphasis have been linked to neurocognitive dysfunction and white matter changes in brain metastases patients after WBRT. Correal et al. systematically reviewed studies and found that comparisons between patients treated with combined modality therapy versus chemotherapy alone indicated greater cognitive impairments in patients who also had WBRT [20]. In a secondary analysis of a randomized trial revealed that WBRT had nonnegligible effect on neurocognitive function despite of metastatic tumor recurrence [21]. And Chang et al. also demonstrated that patients treated with stereotactic radiosurgery (SRS) plus WBRT were at a greater risk of a significant decline in learning and memory function by 4 months compared with the group that received SRS alone [22]. White matter changes have been associated with cognitive dysfunction, in addition, impaired hippocampal neurogenesis after WBRT was also related to neurocognitive functions decline. Therefore, hippocampal-avoidance whole brain radiation therapy (HA-WBRT) as an innovative approach has been investigated [23]. Gondi et al. reported the results from the phase III RTOG 0933 trial of HA-WBRT might mitigate the neurocognitive effects of WBRT [24]. And two cooperative group trials such as NRG (National Surgical Adjuvant Breast and Bowel Project, Radiation Therapy Oncology Group, and Gynecologic Oncology Group) CC (Cancer Control) 003 and NRG-CC001 to elucidate the effects of HA-WBRT will be performed.
Our study had several limitations. It was retrospective and lacked pathologies. Long-term survivors of NSCLC patients with brain metastases remain the minority. Prolongation of survival for our patients was not analyzed, as the main object of our study was to demonstrate the incidence and MR imaging of DLE in a different cancer history. And we lacked of association between white matter abnormalities and cognitive and motor performance. Despite these limitations, the results in our study suggested the high incidence of DLE in NSCLC patients after WBRT was true. This work added to mounting evidence demonstrating the delayed effects of WBRT in those brain metastatic patients. Through increased understanding of it, it may be possible to develop further therapeutic strategies to maximize benefit while limiting potential long term toxicities.
References
NSCLC Meta-analysis Collaborative Group (2014) Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 383:1561–1571
Postmus PE, Haaxma-Reiche H, Smit EF et al (2000) Treatment of brain metastases of small-cell lung cancer: comparing teniposide and teniposide with whole-brain radiotherapy-a phase III study of the European Organization for the Research and Treatment of Cancer. Lung Cancer Cooperative Group. J Clin Oncol 18:3400–3408
Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD (2005) Radiosurgery with or without whole-brain radiotherapy for brain metastases: the patients’ perspective regarding complications. Am J Clin Oncol 28:173–179
Burger PC, Mahley MS Jr, Dudka L, Vogel FS (1979) The morphologic effects of radiation administered therapeutically for intracranial gliomas: a postmortem study of 25 cases. Cancer 44:1256–1272
Panagiotakos G, Alshamy G, Chan B et al (2007) Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS ONE 2:e588
Monaco EA, Faraji AH, Berkowitz O et al (2013) Leukoencephalopathy after whole-brain radiation therapy plus radiosurgery versus radiosurgery alone for metastatic lung cancer. Cancer 1:226–232
Mornex F, Thomas L, Mohr P et al (2003) A prospective randomized multicentre phase III trial of fotemustine plus whole brain irradiation versus fotemustine alone in cerebral metastases of malignant melanoma. Melanoma Res 13:97–103
Ebi J, Sato H, Nakajima M et al (2012) Incidence of leukoencephalopathy after whole-brain radiation therapy for brain metastases. Int J Radiat Oncol Biol Phys 85:1212–1217
Stokes TB, Niranjan A, Kano H et al (2015) White matter changes in breast cancer brain metastases patients who undergo radiosurgery alone compared to whole brain radiation therapy plus radiosurgery. J Neurooncol 121:583–590
Fujii O, Tsujino K, Soejima T et al (2006) White matter changes on magnetic resonance imaging following whole-brain radiotherapy for brain metastases. Radiat Med 24:345–350
Kohama Steven G, Rosene Douglas L, Sherman Larry S (2012) Age-related changes in human and non-human primate white matter: from myelination disturbances to cognitive decline. AGE 34:1093–1110
Tofilon PJ, Fike JR (2000) The radioresponse of the central nervous system: a dynamic process. Radial Res 53:357–370
Schultheiss TE, Kun LE, Ang KK, Stephens LC (1995) Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys 31:1093–1112
Komarnicky LT, Phillips TL, Martz K, Asbell S, Isaacson S, Urtasun R (1991) A randomized phase III protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (RTOG-7916). Int J Radiat Oncol Biol Phys 20:53–58
Phillips TL, Scott CB, Leibel SA, Rotman M, Weigensberg IJ (1995) Results of a randomized comparison of radiotherapy and bromodeoxyuridine with radiotherapy alone for brain metastases: report of RTOG trial 89-05. Int J Radiat Oncol Biol Phys 33:339–348
Meyers CA, Smith JA, Bezjak A et al (2004) Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol 22:157–165
Brown PD, Pugh S, Laack NN et al (2013) Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 15:1429–1437
Wefel JS, Kayl AE, Meyers CA (2004) Neuropsychological dysfunction associated with cancer and cancer therapies: a conceptual review of an emerging target. Br J Cancer 90:1691–1696
Kutita H, Kawahara N, Asai A et al (2001) Radiation-induced apoptosis of oligodendrocytes in the adult rat brain. Neurol Res 23:869–874
Correal DD, Maron L, Harder H et al (2007) Cognitive functions in primary central nervous system lymphoma: literature review and assessment guidelines. Ann Oncol 18:1145–1151
Aoyama H, Tago M, Kato N et al (2007) Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys 68:1388–1395
Chang EL, Wefel JS, Hess KR et al (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037–1044
Suh JH (2014) Hippocampal-avoidance whole-brain radiation therapy: a new standard for patients with brain metastases? J Clin Oncol 32:3789–3791
Gondi V, Pugh SL, Tome WA et al (2014) Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 32:3810–3816
Acknowledgments
This work was supported in part by Grand No. 81471654 from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Zhong, X., Huang, B., Feng, J. et al. Delayed leukoencephalopathy of non-small cell lung cancer patients with brain metastases underwent whole brain radiation therapy. J Neurooncol 125, 177–181 (2015). https://doi.org/10.1007/s11060-015-1888-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1888-9