Abstract
The term “biomarker” historically refers to a single parameter, such as the expression level of a gene or a radiographic pattern, used to indicate a broader biological state. Molecular indicators have been applied to several aspects of cancer therapy: to describe the genotypic and phenotypic state of neoplastic tissue for prognosis, to predict susceptibility to anti-proliferative agents, to validate the presence of specific drug targets, and to evaluate responsiveness to therapy. For glioblastoma (GBM), immunohistochemical and radiographic biomarkers accessible to the clinical lab have informed traditional regimens, but while immunotherapies have emerged as potentially disruptive weapons against this diffusely infiltrating, heterogeneous tumor, biomarkers with strong predictive power have not been fully established. The cancer immunotherapy field, through the recently accelerated expansion of trials, is currently leveraging this wealth of clinical and biological data to define and revise the use of biomarkers for improving prognostic accuracy, personalization of therapy, and evaluation of responses across the wide variety of tumors. Technological advancements in DNA sequencing, cytometry, and microscopy have facilitated the exploration of more integrated, high-dimensional profiling of the disease system—incorporating both immune and tumor parameters—rather than single metrics, as biomarkers for therapeutic sensitivity. Here we discuss the utility of traditional GBM biomarkers in immunotherapy and how the impending transformation of the biomarker paradigm—from single markers to integrated profiles—may offer the key to bringing predictive, personalized immunotherapy to GBM patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Targeting Immunosuppression in GBM
Glioblastoma (GBM) is a WHO grade IV malignant glioma which invariably results in recurrence and mortality despite the current standard of care—maximal safe surgical resection, fractionated radiation, and systemic temozolamide chemotherapy [1]. Patient treatment failure is attributed to GBM cellular heterogeneity and the aggressive diffuse infiltration observed at the tumor margins, which allows resistance to radiotherapy and the cytotoxic agents to propagate under selective pressure [2–5], especially upon tumor recurrence [6], and is further complicated by local and systemic tumor derived immunosuppression. While not yet fully elucidated, the causal mechanisms that link the physiology of the tumor to the dysfunction of the infiltrating and peripheral immune cells are complex and interdependent. They include: direct cell–cell inhibition, exposure to immunosuppressive cytokines, intermediate cell death signaling [7, 8], persistent or self-antigen mediated tolerance [9], and exhaustion from chronic exposure to tumor antigens [10, 11]. Underexpression of immunostimulatory MHC class I [12] and overexpressesion of suppressive surface proteins (e.g., FasL and PD-1L) and cytokines (e.g., TGF-β, IL-10, and CCL2) fosters the accumulation of regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC), while impairing the proliferation and functional activation of the cytotoxic lymphocyte (CTL) population. Meanwhile, accumulation of natural killer T (NKT) cells and Tregs in the peripheral circulation of GBM patients leaves them immunocompromised and leukopenic [13, 14].
Establishing effective, predictive immunotherapy regimens in GBM requires alleviating glioma-associated immune suppression while instigating, targeting, protecting, and resourcing specific responsiveness. Despite the obvious benefits of such a strategy due to difficulty targeting residual tumor cells and accumulating examples of therapeutic success, consistent biomarkers providing guidance in the design and prescription of regimens in GBM have lagged far behind the ability to administer the major classes of immunotherapy: effector cell therapy, antigen-presenting cell therapy, defined and complex vaccines, and monoclonal antibodies.
Effector cell therapies utilize adoptive immune function and employ ex vivo activation of potential anti-tumor effector cells—with non-specific lymphokines and/or potential tumor antigens—followed by re-introduction of these cells into the patient either via systemic injection or directly into the tumor site, guaranteeing some degree of cytotoxic function. Early trials using Lymphokine-activated killer cells (LAKs) administered peripherally with IL-2 as an adjuvant for recurrent glioma produced anti-tumor responses in a subset of patients, but also dose-limiting systemic and neurological adverse events, such as aseptic meningitis, increased intracranial pressure, and fever following infusion [15–18]. Lillehei et al. delivered LAKs, along with IL-2, directly to the resection cavities of 20 recurrent GBM patients, finding much better tolerance of therapy, but no significant improvement in survival [19]. Subsequently, a small local delivery trial decreased tumor volume in two of four patients [20], while trials by Dillman et al. reported a modest survival benefit (median 20.5 months, 1-year survival rate 75 %) among 36 patients [21, 22]. None of these studies employed biomarkers for enrollment, nor conducted follow-up analysis of molecular correlates of the clinical outcomes.
Autologous CTLs, stimulated with tumor-derived antigens, offer the advantage of glioma-directed cytotoxic function. Following preclinical studies confirming the survival, localization, and persistent anti-tumor specificity of autologous stimulated glioma-infiltrating lymphocytes [23], Holladay et al. tested the ability of autologous monocytes pre-loaded with irradiated autologous tumor cells and stimulated with IL-2 to selectively expand CD4+ and CD8+ T cells, compared to simple vaccination with such autologous irradiated tumor cells and adjuvant, first in a rodent model, then human patients. Although treatment was well tolerated in both arms of the clinical trial, and tumor regression and improved survival were observed among a subset of patients, there was no retrospective analysis of potential immune biomarkers associated with treatment responses [24, 25].
Chimeric Antigen Receptor (CAR) cells are CTLs, generally autologous, engineered ex vivo using recombinant DNA to express tumor antigen-specific proteins, most commonly chimeras of antigen-specific antibodies and T cell receptor (TCR) signaling domains, then reintroduced into the patient. CAR targeting the EGFRvIII mutated protein was first demonstrated in glioma cell culture [26], then by in vivo localization in pre-clinical murine studies, leading to tumor cell infiltration and increased survival [27, 28]. Currently, a single-group pilot clinical study of an optimized EGFRvIII-CAR [29] in GBM (NCT02209376) includes pre-screening for expression of EGFRvIII (31 % of GBM [30]) as an enrollment criterion, utilizing the artificially-targeted nature of these constructs as a biomarker to ensure targeting potential in light of the severity of off-target, adverse events. The more general tumor antigens HER2 and EphA2 have been successfully targeted by CARs in glioma cell culture and preclinical models [31, 32], leading to a phase I clinical trial of autologous generated HERT-CD28 fusion receptor cells (NCT01109095).
Vaccination strategies rely on multi-step activation of adaptive immune cells to yield a viable population of tumor-specific CTLs, thus igniting fewer adverse events caused due to spurious cytolytic activity than effector cell therapies, but providing less predictable targeting of antigens, especially in immunoprivileged tissues, such as the CNS. The latter concern was alleviated by the success of amyloid-β vaccination in Alzheimers [33], and recombinant peptides, the most accessible and scalable vaccine inoculum, were first proven effective against tumor antigens in melanoma [34]. However, single peptide vaccination may lead to selection for tumor mutations, epitope masking, and ultimately immune escape, and are also limited by peptide degradation, MHC presentation efficiency, and recognition by the endogenous TCR repertoire [35]. Therefore, multi-peptide vaccines in GBM have employed mixtures of several potentially immunogenic, HLA-A02-restricted peptides representing tumor markers: WT-1, HER2, MAGE-A3, and MAGE-A1 or gp100 [36] (NCT02149225). Although this study includes the collection of longitudinal peripheral blood samples for evaluation of overall immunological response and their correlation with tumor progression, and to compare responses to each of the individual vaccine components, results are not available to date.
Vaccination with a non-defined, lysate-derived protein extract utilizing the immunogenicity of heat shock protein peptide complex 96 (HSPPC96) has provided a shift toward a stimulatory peripheral and intratumoral immune response, marked by increases in CD4+ , CD8+ , and CD56+ T cells and IFNγ production. [37] Early phase success has led to a 3-arm phase III clinical trial (NCT01814813) evaluating both HSPPC96 vaccination and its combination with the anti-VEGF agent bevacizumab [37]. Although initial experiments retrospectively associated a decreased peripheral Treg abundance and increased CD8+ T cell functionality with survival benefit, these biomarkers are not used directly in regimen design or assessment.
Ex vivo instruction of antigen presenting cells circumvents the need to achieve sufficient immune activation in vivo, while maintaining the lower risk profile of vaccination. Autologous dendritic cells (DC) are harvested from peripheral blood, stimulated with tumor-specific antigens—from tumor lysate, recombinant peptides, or tumor mRNA transfection [38] —and reintroduced, specifically activating endogenous CTLs. In phase I/II studies, DC-treated patients achieved decreased tumor size, increases tumor-infiltrating CD8+ cells, and reversal of prevaccination CD4:CD8 ratios. Significantly, adverse events were limited to transient elevations in liver transaminases with no secondary autoimmune disease [39–43]. In a similar trial with 25 randomized patients, DC vaccination with heat shocked autologous tumor lysate delayed tumor recurrence compared to a control cohort, increased levels of peripheral T cells (CD3+, CD4+, CD8+), and NK cells, and restored CD4:CD8 ratios [44]. Response of peripheral lymphocytes to cytokine stimulation via pSTAT signaling was later identified as a potential predictor of two-year survival and therapeutic efficacy [45].
Monoclonal antibody therapy, stimulatory or inhibitory, targets critical receptors that are markers or regulators of immune cell functional states. Early pre-clinical studies aimed at tumor-mediated immunosuppression, targeted Tregs through peripheral injection of monoclonal antibodies against CD25 (IL2Rα receptor) in a GL261 mouse model, resulting in Treg depletion, increased CTL activity and immunoactivating cytokines [46–48]. The importance of the EGFR pathway in GBM motivated the use of cetuximab (anti-EGFR) blockade, which strongly curbs tumor growth except in the presence of compensatory mutations and expression of alternative receptors (i.e. k-Ras, erbB1/2, [49, 50]), turning such features into contra-indicative biomarkers for this antibody therapy.Three antibodies with distinct immune targets showed efficacy in various glioma mouse models: anti-CCL2, targeting suppressive monocytes, provided a survival benefit when paired with temozolamide [51]; anti-PD1, blockading T cell exhaustion, provided survival with localized radiotherapy [51]; and anti-CTLA4 prevented T cell exhaustion when combined with the inflammatory cytokine IL-12 [52]. In human trials, Sampson et al. administered dacluzimab (anti-CD25) to patients receiving the PEPvIII vaccine. Positive response and depletion of circulating Tregs in the dacluzimab-treated patients supported potential therapeutic synergy [53, 54], as well as the use of peripheral Treg as a surrogate marker for response for these and other immunotherapeutic regimens in GBM.
Profiling, prediction, and personalization in immunotherapy
In 2013, over two decades of contributions by innumerable scientists and clinicians toward demonstrating the clinical power of cancer immunotherapy were recognized by Science Magazine (AAAS) as the breakthrough of the year. Durable and curative responses from, most notably, “checkpoint-blockading” antibodies against CTLA-4 [55] and PD-1 [56–60] in several cancers, alone and in combination (for review, see [61]), and genetically engineered chimeric antigen receptors (CAR) cells, in both leukemia and solid tumors [62, 63], have motivated the rapid expansion of clinical trials to new targets, mechanisms, and delivery systems. Yet, the potential to synergize these technologies with each other [64] and with more traditional immune interventions [65] depends heavily on clearing what the World Immunotherapy Council outlined in 2011 as “critical hurdles” [66]. Among these, re-evaluation of the mainly radiographic and survival-based response evaluation criteria in solid tumors (RECIST) [67] was a priority, given their failure to capture molecular and cellular correlates of subclinical outcomes or to adequately stratify responders prospectively or retrospectively.
Presently, the expression of immunotherapeutic target proteins at the tumor site or among the circulating immune cells is commonly monitored by flow cytometry or RNA expression. However, the only ones formally utilized in clinical trials as prospective biomarkers are the T cell “exhaustion marker” PD-1 and its tumor-expressed ligand PD-L1 for predicting efficacy of the anti-PD-1 monoclonal antibody [68] and other antibodies targeting that pathway. Even in this case, the fidelity of PD-1 as an “exhaustion” marker, compared to other T cell surface markers (such as LAG-3, TIM-3, and TIGIT, associated exclusively with specific subsets of exhausted T cells [69, 70], remains disputed, and the biological mechanism by which these antibodies restore functionality of the CTL population (specifically, blockade of exhaustion signaling versus depletion of exhausted cells [71] is not completely resolved). For anti-CTLA-4 therapy, a clinical biomarker has not yet been ratified, though retrospective analysis of clinical data has suggested clusters of diagnostic correlates (for a review see [72]), and subsequent laboratory work on patient samples has validated its dependence on related immune receptors [73]. Meanwhile, the list of single factors with direct and indirect influence on the evolution of the immune response continues to grow—particularly small molecule metabolites and the pathways regulating them (e.g. indoleamine dioxygenase [74], arginase [75], and α-galactosylceramide [76]), “danger” pathways (e.g., DAMPs, TLRs, and high-mobility group box-1 release [77], for a review see [78]), and innate immune, wound-healing, and inflammatory signals (e.g. STING pathway ligands, inflammasome expression, TGF-β family signaling, and NFκB induction).
Despite the benefits of simple biomarkers, achieving prognostic and therapeutic predictability through any single expression-based marker beyond the targeted pathway has proved an expanding challenge, especially across tumor types. However, this need no longer constitutes a roadblock. Evolving technology in gene expression profiling, flow cytometry, and histopathology has allowed these assay paradigms to achieve depth and accessibility, leveraging “whole profile” metrics with the prognostic potential, rather than singular indicators associated with these underlying states.
Quantitative, high-sensitivity resolution of rare populations and functional markers by flow cytometry has drastically increased the amount data obtained per sample. Multi-parameter profiles, including the relative abundance of T cells (CD3+), B cells (CD19+), NK cells (CD56+CD16+), Treg (CD4+CD25+CD127lo), total monocytes (CD86+) and immunosuppressive monocytes (CD14+HLA-DRlo/neg) have been associated with survival across GBM, non-Hodgkins lymphoma, and renal cell carcinoma, with particular significance in the relationship between CD4+ T cells and immunosuppressive monocytes in circulating blood [79].
Histopathology, meanwhile, has leveraged improvements in high-throughput automated microscopy of tumor infiltrating lymphocytes in situ. In particular, the recently commercialized Immunoscore system utilizes relative abundance of functionally marked T cell populations in the histologically identified tumor core vs. infiltrating margin, achieving survival-significant prognostic resolution beyond standard clinical staging of colorectal cancer [80].
With strong evidence that tumor-associated immunosuppression originates locally, characterization of gene expression patterns in the tumor microenvironment became an early focus. Microarray and later next-generation sequencing (RNAseq) of biopsied tissue have provided simultaneous whole-transcriptome profiling of tumor and immune genes, and given rise to several sub-transcriptomic technologies aimed at making high-content, abbreviated profiling widely accessible to clinical samples. For example, the nanostring [81] nCounter platform (quantifying cell type markers, tumor antigens, and a suite of >400 immune genes) has been applied to both cryopreserved and FFPE tissue from several tumor types, bringing mid-throughput gene expression profiling to archival samples. These profiles were successfully correlated with risk of recurrence (ROR) [82], intrinsic subtype, and trastuzumab responsiveness in breast cancer, OS and PFS neuroblastoma [83], and with subtype in diffuse large B cell lymphoma (DLBCL) [84]. The expression of other smaller-scale panels of genes and miRNA have been correlated with clinical and pathological outcomes in different tumor types [85], including a recent computational study of TCGA samples further reduced prognostic immunoprofiling to a minimal geneset reflecting cytolytic activity [86].
Gene expression profiling, combined with in situ pathology, has given rise to the broad proposition of an inflamed vs. non-inflamed tumor state [87] characterized by the balance and activation of the immune pathways driving them. Importantly, such a model offers a context in which to triage the needs to elicit an anti-tumor response (through vaccines, adjuvants, antigen presenting cell therapies, or effector cell therapies) and to alleviate immunosuppression (by depleting Tregs, MDSCs, and immunosuppressive cytokines, chemokines, and small metabolites, or blockading tolerance/exhaustion pathways), and predict synergistic combinations of therapies depending on that state. However, like the orthogonally informative model of immunoediting [88], this framework is broad and does not yet resolve known confounding factors such as the organ-specificity of baseline and tumor-associated immune activity, and what drives transitions between states.
Two new platforms with great disruptive potential in immunological profiling of cancer and other diseases have emerged in the last five years. Time-of-flight mass cytometry (CyTOF) of cells using antibodies conjugated to heavy metal molecular tags in place of fluorophores allows quantification up to 25+ dimensions [89, 90], allowing unprecedented detection of specific populations, informing the relationship between immune cell development, function, and perturbation in disease [91]. Whole-repertoire amplification and high-throughput sequencing of the antigen-specific receptors of whole lymphocyte populations (TCRseq and BCRseq) provides a link between antigen specificity and function, as well as novel statistical indicators of clonal selection and bias during immunosuppression, treatment response, and residual disease in cancer, infection [92–94], autoimmunity [95], and response to immunotherapy [96, 97]. Both technologies are rapidly expanding into new clinical paradigms, and developing tools for analysis and integration of these “big data” readouts with their corresponding clinical and lower-dimensional phenotypes promises enrich immunological biomarker profiles in both infectious disease [10, 98, 99] and cancer.
GBM immunotherapy and the biomarkers of the future
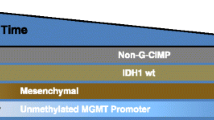
Motivated in part by the characteristics that make GBM a therapeutic challenge [2, 100–102], glioma research has already seen the clinical impact of high-dimensional, “big data” profiling of the tumor tissue and its immune components on our interpretation of classical biomarkers (Table 1). For example, the cytologically diagnosable 10q23 deletion [103] as a biomarker of tumor etiology and prognosis [104–107] preceded elucidation of the tumor suppressor functions of PTEN and their cross-talk with glioma-specific growth pathways. In the broadest synthesis to date, comparative sequencing of genomic rearrangements [108] and gene expression profiling through the TCGA collaborative produced a cohesive set of >800 genes [109] by which to classify GBM tissue into subtypes (proneural, classical, and mesenchymal). These subtypes stratified prognoses, drug and radio-sensitivity [110, 111], as well as the expression of many other known biomarkers. EGFR, for which overexpression [112], genomic amplification [113, 114], and truncation [115–117] were each associated with differential prognosis [118, 119] and therapeutic response [6, 120–122], was found to have a pleiotropic role in tumor cell growth and angiogenesis [123], concomitant with these variations [113, 116] [124]. The “classical” subtype, which captures this EGFR-active state, is predictive of sensitivity to PEPvIII-KLH DC vaccination [125] (see above) and therapeutic targeting of the pathway [126]. Hypermethylation of O 6-methylguanine DNA methyltransferase (MGMT) [127] predicted increased sensitivity to temozolamide and radiation [128] before the “hypermethylator” phenotype was well-established in GBM and other tumors [129, 130]. Isocitrate dehydrogenase-1 mutation (IDH1-R132H), was a known biomarker of longer OS in GBM [108, 131] before its association with transcriptome-wide expression alterations characteristic profile of the “proneural” subtype, and functional contribution to genome-wide hypermethylation [132] were appreciated.
Beyond functioning as biomarkers, high-dimensional profiling have rendered new targets and informed diagnostic and therapeutic options directly. Inference of regulatory networks from transcriptomic data has further elucidated these profiles [133–135], motivating clinical and pre-clinical attempts to synergistically target subtype-specific regulatory nodes [134]. Thus, use of the IDH1-R132H peptide vaccine [136] and EGFRvIII-targeting therapies can be guided not only by the single target epitopes but by the broader phenotypes of proneural and classical GBM. Meanwhile, in silico [137, 138] and experimental efforts have begun to capture the diversity of new, potentially immunogenic GBM antigens [139]. A mass spectrometry-based study [140] yielded 11 candidate peptides now included in the IMA950 vaccine trial (NCT01929191). Gene expression profiling of stereotactically localized biopsies, combined with novel computational analyses, has uncovered cell type-specific signatures at the non-contrast enhancing infiltrated margins with the potential to inform post-surgical therapy targeting the residual tissue from which recurrence arises [61, 141].
Fundamental, outstanding questions in experimental GBM treatment and immunotherapy paradigms likely require the combination of gene expression, tissue pathology, and immunological profiles to resolve. The largest randomized Phase III clinical trial in GBM (PRECISE) leverages dysregulated immune signaling (overexpression of IL-4 and IL-13 receptors [142, 143]) to target high local concentrations of the protein toxin-conjugated IL13-PE38QQR (cintredekin besudotox) to the tumor stroma [142, 144, 145]. Despite distinct drug distribution profiles [146], no difference in median OS nor adverse events were observed compared to carmustine wafer delivery of BCNU (Gliadel) in recurrent GBM [144, 147]. Microarray analysis of treated cultured cells [148] confirmed cytotoxicity (tumor cell apoptosis) as well as differential expression of immune-related genes (e.g. IL-8). Yet, confirmation of this phenotype and its relationship to subclinical efficacy in patient samples has not been established. Meanwhile, bevacizumab, a neutralizing antibody against vascular endothelial growth factor A (VEGF-A) [149]—both a biomarker and effector of angiogenesis [150–154, 155]—presents a conflict between reported efficacy by PFS [156, 157] and OS [158], with an apparent negative impact on concurrent or subsequent therapies [159, 160]. Both the similar shortfall of radiographic metrics as progression criteria and known cross-talk between VEGF and immune signaling [161–163] suggest that combined tumor-immune profiling may be key to interpreting these findings. Reconciling such conflicting or occult therapeutic outcomes requires a profile-wide understanding of the role of the targets, the metrics used to evaluate responses, and confounding factors in the tumor microenvironment.
High-dimensional profiling now allows both animal models [164] and experimentally treated tissue [165], to be compared to their human counterpart or source and validated in unprecedented detail, producing higher-fidelity translational platforms for pre-clinical experiments. Perhaps more importantly, the recent technological advances being integrated into the biomarker strategies of the cancer immunotherapy field at large allow unprecedented volumes of molecular and immunological data to be extracted from clinical samples which are relatively straightforward to procure—such as tissue cryopreserved during resection, and reasonable quantities of peripheral blood obtained throughout care—directly from patients in immunotherapy trials. Although currently no single biomarkers adequately stratify responses in GBM, tumorigenesis research and immunotherapy research are converging, and have begun to use high-dimensional profiles to capture tumor and immune states predictive of disease and therapeutic response [166], promising synergy for these two fields in the development of a new generation of biomarkers for immunotherapy.
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi:10.1056/NEJMoa043330
Moskwa P, Zinn PO, Choi YE, Shukla SA, Fendler W, Chen CC, Lu J, Golub TR, Hjelmeland A, Chowdhury D (2014) A functional screen identifies miRs that induce radioresistance in glioblastomas. Mol Cancer Res. doi:10.1158/1541-7786.MCR-14-0268
Xiao S, Yang Z, Lv R, Zhao J, Wu M, Liao Y, Liu Q (2014) miR-135b contributes to the radioresistance by targeting GSK3beta in human glioblastoma multiforme cells. PLoS ONE 9:e108810. doi:10.1371/journal.pone.0108810
Wu L, Yang L, Xiong Y, Guo H, Shen X, Cheng Z, Zhang Y, Gao Z, Zhu X (2014) Annexin A5 promotes invasion and chemoresistance to temozolomide in glioblastoma multiforme cells. Tumour Biol. doi:10.1007/s13277-014-2545-1
Garrido W, Rocha JD, Jaramillo C, Fernandez K, Oyarzun C, Martin RS, Quezada C (2014) Chemoresistance in high-grade gliomas: relevance of adenosine signalling in stem-like cells of glioblastoma multiforme. Curr Drug Targets 15:931–942
Brada M, Stenning S, Gabe R, Thompson LC, Levy D, Rampling R, Erridge S, Saran F, Gattamaneni R, Hopkins K, Beall S, Collins VP, Lee SM (2010) Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. J Clin oncology : official journal of the American Society of Clinical Oncol 28:4601–4608. doi:10.1200/JCO.2009.27.1932
Ch’en IL, Tsau JS, Molkentin JD, Komatsu M, Hedrick SM (2011) Mechanisms of necroptosis in T cells. J Exp Med 208:633–641. doi:10.1084/jem.20110251
Gaiha GD, Brass AL (2014) Immunology. The fiery side of HIV-induced T cell death. Science 343:383–384. doi:10.1126/science.1250175
Ohlen C, Kalos M, Hong DJ, Shur AC, Greenberg PD (2001) Expression of a tolerizing tumor antigen in peripheral tissue does not preclude recovery of high-affinity CD8+ T cells or CTL immunotherapy of tumors expressing the antigen. Journal of immunology 166:2863–2870
Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R (1998) Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 188:2205–2213
Wherry EJ (2011) T cell exhaustion. Nature immunology 12:492–499
Yang I, Kremen TJ, Giovannone AJ, Paik E, Odesa SK, Prins RM, Liau LM (2004) Modulation of major histocompatibility complex Class I molecules and major histocompatibility complex-bound immunogenic peptides induced by interferon-alpha and interferon-gamma treatment of human glioblastoma multiforme. J Neurosurg 100:310–319. doi:10.3171/jns.2004.100.2.0310
Mahindra AK, Grossman SA (2003) Pneumocystis carinii pneumonia in HIV negative patients with primary brain tumors. J Neurooncol 63:263–270
Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S, Consortium NC (2011) Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res 17:5473–5480. doi:10.1158/1078-0432.CCR-11-0774
Jacobs SK, Wilson DJ, Kornblith PL, Grimm EA (1986) Interleukin-2 and autologous lymphokine-activated killer cells in the treatment of malignant glioma. Preliminary report. J Neurosurg 64:743–749. doi:10.3171/jns.1986.64.5.0743
Merchant RE, Grant AJ, Merchant LH, Young HF (1988) Adoptive immunotherapy for recurrent glioblastoma-multiforme using lymphokine activated killer cells and recombinant interleukin-2. Cancer 62:665–671. doi:10.1002/1097-0142(19880815)62:4<665:Aid-Cncr2820620403>3.0.Co;2-O
Yoshida S, Tanaka R, Takai N, Ono K (1988) Local administration of autologous lymphokine-activated killer cells and recombinant interleukin 2 to patients with malignant brain tumors. Cancer Res 48:5011–5016
Barba D, Saris SC, Holder C, Rosenberg SA, Oldfield EH (1989) Intratumoral LAK cell and interleukin-2 therapy of human gliomas. J Neurosurg 70:175–182. doi:10.3171/jns.1989.70.2.0175
Lillehei KO, Mitchell DH, Johnson SD, McCleary EL, Kruse CA (1991) Long-term follow-up of patients with recurrent malignant gliomas treated with adjuvant adoptive immunotherapy. Neurosurgery 28:16–23
Tsurushima H, Liu SQ, Tuboi K, Matsumura A, Yoshii Y, Nose T, Saijo K, Ohno T (1999) Reduction of end-stage malignant glioma by injection with autologous cytotoxic T lymphocytes. Jpn J Cancer Res 90:536–545
Dillman RO, Duma CM, Ellis RA, Cornforth AN, Schiltz PM, Sharp SL, DePriest MC (2009) Intralesional lymphokine-activated killer cells as adjuvant therapy for primary glioblastoma. J Immunother 32:914–919. doi:10.1097/CJI.0b013e3181b2910f
Dillman RO, Duma CM, Schiltz PM, DePriest C, Ellis RA, Okamoto K, Beutel LD, De Leon C, Chico S (2004) Intracavitary placement of autologous lymphokine-activated killer (LAK) cells after resection of recurrent glioblastoma. J Immunother 27:398–404
Saris SC, Spiess P, Lieberman DM, Lin S, Walbridge S, Oldfield EH (1992) Treatment of murine primary brain tumors with systemic interleukin-2 and tumor-infiltrating lymphocytes. J Neurosurg 76:513–519. doi:10.3171/jns.1992.76.3.0513
Holladay FP, Heitz T, Chen YL, Chiga M, Wood GW (1992) Successful treatment of a malignant rat glioma with cytotoxic T lymphocytes. Neurosurgery 31:528–533
Holladay FP, Heitz-Turner T, Bayer WL, Wood GW (1996) Autologous tumor cell vaccination combined with adoptive cellular immunotherapy in patients with grade III/IV astrocytoma. J Neurooncol 27:179–189
Morgan RA, Johnson LA, Davis JL, Zheng Z, Woolard KD, Reap EA, Feldman SA, Chinnasamy N, Kuan CT, Song H, Zhang W, Fine HA, Rosenberg SA (2012) Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum Gene Ther 23:1043–1053. doi:10.1089/hum.2012.041
Miao H, Choi BD, Suryadevara CM, Sanchez-Perez L, Yang S, De Leon G, Sayour EJ, McLendon R, Herndon JE 2nd, Healy P, Archer GE, Bigner DD, Johnson LA, Sampson JH (2014) EGFRvIII-specific chimeric antigen receptor T cells migrate to and kill tumor deposits infiltrating the brain parenchyma in an invasive xenograft model of glioblastoma. PLoS ONE 9:e94281. doi:10.1371/journal.pone.0094281
Sampson JH, Choi BD, Sanchez-Perez L, Suryadevara CM, Snyder DJ, Flores CT, Schmittling RJ, Nair SK, Reap EA, Norberg PK, Herndon JE 2nd, Kuan CT, Morgan RA, Rosenberg SA, Johnson LA (2014) EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res 20:972–984. doi:10.1158/1078-0432.CCR-13-0709
Johnson LA, Scholler J, Ohkuri T, Kosaka A, Patel PR, McGettigan SE, Nace AK, Dentchev T, Thekkat P, Loew A, Boesteanu AC, Cogdill AP, Chen T, Fraietta JA, Kloss CC, Posey AD Jr, Engels B, Singh R, Ezell T, Idamakanti N, Ramones MH, Li N, Zhou L, Plesa G, Seykora JT, Okada H, June CH, Brogdon JL, Maus MV (2015) Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med 7(275):275ra22. doi:10.1126/scitranslmed.aaa4963
Faulkner C, Palmer A, Williams H, Wragg C, Haynes HR, White P, DeSouza RM, Williams M, Hopkins K, Kurian KM (2014) EGFR and EGFRvIII analysis in glioblastoma as therapeutic biomarkers. Br J Neurosurg. doi:10.3109/02688697.2014.950631
Ahmed N, Salsman VS, Kew Y, Shaffer D, Powell S, Zhang YJ, Grossman RG, Heslop HE, Gottschalk S (2010) HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res 16:474–485. doi:10.1158/1078-0432.CCR-09-1322
Chow KK, Naik S, Kakarla S, Brawley VS, Shaffer DR, Yi Z, Rainusso N, Wu MF, Liu H, Kew Y, Grossman RG, Powell S, Lee D, Ahmed N, Gottschalk S (2013) T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther 21:629–637. doi:10.1038/mt.2012.210
Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JAR (2008) Long-term effects of Aβ42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet 372:216–223. doi:10.1016/s0140-6736(08)61075-2
Chianese-Bullock KA, Pressley J, Garbee C, Hibbitts S, Murphy C, Yamshchikov G, Petroni GR, Bissonette EA, Neese PY, Grosh WW, Merrill P, Fink R, Woodson EMH, Wiernasz CJ, Patterson JW, Slingluff CL (2005) MAGE-A1-, MAGE-A10-, and gp100-derived peptides are immunogenic when combined with granulocyte-macrophage colony-stimulating factor and montanide ISA-51 adjuvant and administered as part of a multipeptide vaccine for melanoma. J Immunol 174:3080–3086. doi:10.4049/jimmunol.174.5.3080
Slingluff CL Jr (2011) The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination? Cancer J 17:343–350. doi:10.1097/PPO.0b013e318233e5b2
Akiyama Y, Oshita C, Kume A, Iizuka A, Miyata H, Komiyama M, Ashizawa T, Yagoto M, Abe Y, Mitsuya K, Watanabe R, Sugino T, Yamaguchi K, Nakasu Y (2012) alpha-type-1 polarized dendritic cell-based vaccination in recurrent high-grade glioma: a phase I clinical trial. BMC Cancer 12:623. doi:10.1186/1471-2407-12-623
Crane CA, Han SJ, Ahn B, Oehlke J, Kivett V, Fedoroff A, Butowski N, Chang SM, Clarke J, Berger MS, McDermott MW, Prados MD, Parsa AT (2013) Individual patient-specific immunity against high-grade glioma after vaccination with autologous tumor derived peptides bound to the 96 KD chaperone protein. Clin Cancer Res 19:205–214. doi:10.1158/1078-0432.CCR-11-3358
Kobayashi T, Yamanaka R, Homma J, Tsuchiya N, Yajima N, Yoshida S, Tanaka R (2003) Tumor mRNA-loaded dendritic cells elicit tumor-specific CD8(+) cytotoxic T cells in patients with malignant glioma. Cancer Immunol Immunother 52:632–637. doi:10.1007/s00262-003-0408-5
Chang CN, Huang YC, Yang DM, Kikuta K, Wei KJ, Kubota T, Yang WK (2011) A phase I/II clinical trial investigating the adverse and therapeutic effects of a postoperative autologous dendritic cell tumor vaccine in patients with malignant glioma. J Clin Neurosci 18:1048–1054. doi:10.1016/j.jocn.2010.11.034
Marsh JC, Goldfarb J, Shafman TD, Diaz AZ (2013) Current status of immunotherapy and gene therapy for high-grade gliomas. Cancer Control 20:43–48
Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, Yong WH, Incardona F, Thompson RC, Riedinger MS, Zhang W, Prins RM, Black KL (2001) Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res 61:842–847
Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ (2004) Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res 64:4973–4979. doi:10.1158/0008-5472.CAN-03-3505
Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, Lin JW, Chute DJ, Mischel PS, Cloughesy TF, Roth MD (2005) Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res 11:5515–5525. doi:10.1158/1078-0432.CCR-05-0464
Jie X, Hua L, Jiang W, Feng F, Feng G, Hua Z (2012) Clinical application of a dendritic cell vaccine raised against heat-shocked glioblastoma. Cell Biochem Biophys 62:91–99. doi:10.1007/s12013-011-9265-6
Everson RG, Jin RM, Wang X, Safaee M, Scharnweber R, Lisiero DN, Soto H, Liau LM, Prins RM (2014) Cytokine responsiveness of CD8(+) T cells is a reproducible biomarker for the clinical efficacy of dendritic cell vaccination in glioblastoma patients. J Immunother Cancer 2:10. doi:10.1186/2051-1426-2-10
Fecci PE, Sweeney AE, Grossi PM, Nair SK, Learn CA, Mitchell DA, Cui X, Cummings TJ, Bigner DD, Gilboa E, Sampson JH (2006) Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res 12:4294–4305. doi:10.1158/1078-0432.CCR-06-0053
Poirier MD, Haban H, El Andaloussi A (2009) A combination of systemic and intracranial anti-CD25 immunotherapy elicits a long-time survival in murine model of glioma. J Oncol 2009:963037. doi:10.1155/2009/963037
Maes W, Rosas GG, Verbinnen B, Boon L, De Vleeschouwer S, Ceuppens JL, Van Gool SW (2009) DC vaccination with anti-CD25 treatment leads to long-term immunity against experimental glioma. Neuro Oncol 11:529–542. doi:10.1215/15228517-2009-004
Clark PA, Iida M, Treisman DM, Kalluri H, Ezhilan S, Zorniak M, Wheeler DL, Kuo JS (2012) Activation of multiple ERBB family receptors mediates glioblastoma cancer stem-like cell resistance to EGFR-targeted inhibition. Neoplasia 14(5):420–428
Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au H-J, Langer C, Moore MJ, Zalcberg JR (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359:1757–1765. doi:10.1056/NEJMoa0804385
Zhu X, Fujita M, Snyder LA, Okada H (2011) Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. J Neurooncol 104:83–92. doi:10.1007/s11060-010-0473-5
Vom Berg J, Vrohlings M, Haller S, Haimovici A, Kulig P, Sledzinska A, Weller M, Becher B (2013) Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J Exp Med 210:2803–2811. doi:10.1084/jem.20130678
Sampson JH, Schmittling RJ, Archer GE, Congdon KL, Nair SK, Reap EA, Desjardins A, Friedman AH, Friedman HS, Herndon JE 2nd, Coan A, McLendon RE, Reardon DA, Vredenburgh JJ, Bigner DD, Mitchell DA (2012) A pilot study of IL-2Ralpha blockade during lymphopenia depletes regulatory T-cells and correlates with enhanced immunity in patients with glioblastoma. PLoS ONE 7:e31046. doi:10.1371/journal.pone.0031046
Dunn GP, Dunn IF, Curry WT (2007) Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun 7:12
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723. doi:10.1056/NEJMoa1003466
Pedicord VA, Montalvo W, Leiner IM, Allison JP (2011) Single dose of anti-CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance. Proc Natl Acad Sci USA 108:266–271. doi:10.1073/pnas.1016791108
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369:134–144. doi:10.1056/NEJMoa1305133
Curran MA, Montalvo W, Yagita H, Allison JP (2010) PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA 107:4275–4280. doi:10.1073/pnas.0915174107
Zubairi S, Sanos SL, Hill S, Kaye PM (2004) Immunotherapy with OX40L-Fc or anti-CTLA-4 enhances local tissue responses and killing of Leishmania donovani. Eur J Immunol 34:1433–1440. doi:10.1002/eji.200324021
Korman AJ, Peggs KS, Allison JP (2006) Checkpoint blockade in cancer immunotherapy. Adv Immunol 90:297–339. doi:10.1016/S0065-2776(06)90008-X
Drake CG, Lipson EJ, Brahmer JR (2014) Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol 11:24–37. doi:10.1038/nrclinonc.2013.208
Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH (2011) T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. doi:10.1126/scitranslmed.3002842
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH (2013) Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368:1509–1518. doi:10.1056/NEJMoa1215134
Duraiswamy J, Freeman GJ, Coukos G (2014) Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors–response. Cancer Res 74:633–634. doi:10.1158/0008-5472.CAN-13-2752 (discussion 635)
Gregor PD, Wolchok JD, Ferrone CR, Buchinshky H, Guevara-Patino JA, Perales MA, Mortazavi F, Bacich D, Heston W, Latouche JB, Sadelain M, Allison JP, Scher HI, Houghton AN (2004) CTLA-4 blockade in combination with xenogeneic DNA vaccines enhances T-cell responses, tumor immunity and autoimmunity to self antigens in animal and cellular model systems. Vaccine 22:1700–1708. doi:10.1016/j.vaccine.2003.10.048
Fox BA, Schendel DJ, Butterfield LH, Aamdal S, Allison JP, Ascierto PA, Atkins MB, Bartunkova J, Bergmann L, Berinstein N, Bonorino CC, Borden E, Bramson JL, Britten CM, Cao X, Carson WE, Chang AE, Characiejus D, Choudhury AR, Coukos G, de Gruijl T, Dillman RO, Dolstra H, Dranoff G, Durrant LG, Finke JH, Galon J, Gollob JA, Gouttefangeas C, Grizzi F, Guida M, Hakansson L, Hege K, Herberman RB, Hodi FS, Hoos A, Huber C, Hwu P, Imai K, Jaffee EM, Janetzki S, June CH, Kalinski P, Kaufman HL, Kawakami K, Kawakami Y, Keilholtz U, Khleif SN, Kiessling R, Kotlan B, Kroemer G, Lapointe R, Levitsky HI, Lotze MT, Maccalli C, Maio M, Marschner JP, Mastrangelo MJ, Masucci G, Melero I, Melief C, Murphy WJ, Nelson B, Nicolini A, Nishimura MI, Odunsi K, Ohashi PS, O’Donnell-Tormey J, Old LJ, Ottensmeier C, Papamichail M, Parmiani G, Pawelec G, Proietti E, Qin S, Rees R, Ribas A, Ridolfi R, Ritter G, Rivoltini L, Romero PJ, Salem ML, Scheper RJ, Seliger B, Sharma P, Shiku H, Singh-Jasuja H, Song W, Straten PT, Tahara H, Tian Z, van Der Burg SH, von Hoegen P, Wang E, Welters MJ, Winter H, Withington T, Wolchok JD, Xiao W, Zitvogel L, Zwierzina H, Marincola FM, Gajewski TF, Wigginton JM, Disis ML (2011) Defining the critical hurdles in cancer immunotherapy. J Transl Med 9:214. doi:10.1186/1479-5876-9-214
Dancey JE, Dodd LE, Ford R, Kaplan R, Mooney M, Rubinstein L, Schwartz LH, Shankar L, Therasse P (2009) Recommendations for the assessment of progression in randomised cancer treatment trials. Eur J Cancer 45:281–289. doi:10.1016/j.ejca.2008.10.042
Keeren K, Friedrich M, Gebuhr I, Philipp S, Sabat R, Sterry W, Brandt C, Meisel C, Grutz G, Volk HD, Sawitzki B (2009) Expression of tolerance associated gene-1, a mitochondrial protein inhibiting T cell activation, can be used to predict response to immune modulating therapies. J Immunol 183:4077–4087. doi:10.4049/jimmunol.0804351
Anderson AC (2012) Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol 24:213–216. doi:10.1016/j.coi.2011.12.005
Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DA (2012) Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 72:917–927. doi:10.1158/0008-5472.CAN-11-1620
Hamid O, Carvajal RD (2013) Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Expert Opin Biol Ther 13:847–861. doi:10.1517/14712598.2013.770836
Ascierto PA, Kalos M, Schaer DA, Callahan MK, Wolchok JD (2013) Biomarkers for immunostimulatory monoclonal antibodies in combination strategies for melanoma and other tumor types. Clin Cancer Res 19:1009–1020. doi:10.1158/1078-0432.CCR-12-2982
Ng Tang D, Shen Y, Sun J, Wen S, Wolchok JD, Yuan J, Allison JP, Sharma P (2013) Increased frequency of ICOS+ CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res 1:229–234. doi:10.1158/2326-6066.CIR-13-0020
Li M, Bolduc AR, Hoda MN, Gamble DN, Dolisca SB, Bolduc AK, Hoang K, Ashley C, McCall D, Rojiani AM, Maria BL, Rixe O, MacDonald TJ, Heeger PS, Mellor AL, Munn DH, Johnson TS (2014) The indoleamine 2,3-dioxygenase pathway controls complement-dependent enhancement of chemo-radiation therapy against murine glioblastoma. J Immunother Cancer 2:21. doi:10.1186/2051-1426-2-21
Sippel TR, White J, Nag K, Tsvankin V, Klaassen M, Kleinschmidt-DeMasters BK, Waziri A (2011) Neutrophil degranulation and immunosuppression in patients with GBM: restoration of cellular immune function by targeting arginase I. Clin Cancer Res 17:6992–7002. doi:10.1158/1078-0432.CCR-11-1107
Huang JR, Tsai YC, Chang YJ, Wu JC, Hung JT, Lin KH, Wong CH, Yu AL (2014) alpha-Galactosylceramide but not phenyl-glycolipids induced NKT cell anergy and IL-33-mediated myeloid-derived suppressor cell accumulation via upregulation of egr2/3. J Immunol 192:1972–1981. doi:10.4049/jimmunol.1302623
Liang X, De Vera ME, Buchser WJ, de Vivar Romo, Chavez A, Loughran P, Beer Stolz D, Basse P, Wang T, Van Houten B, Zeh HJ 3rd, Lotze MT (2012) Inhibiting systemic autophagy during interleukin 2 immunotherapy promotes long-term tumor regression. Cancer Res 72:2791–2801. doi:10.1158/0008-5472.CAN-12-0320
Lotfi R, Lee JJ, Lotze MT (2007) Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J Immunother 30:16–28. doi:10.1097/01.cji.0000211324.53396.f6
Gustafson MP, Lin Y, LaPlant B, Liwski CJ, Maas ML, League SC, Bauer PR, Abraham RS, Tollefson MK, Kwon ED, Gastineau DA, Dietz AB (2013) Immune monitoring using the predictive power of immune profiles. J Immunother Cancer 1:7. doi:10.1186/2051-1426-1-7
Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, Nagtegaal ID, Palmqvist R, Masucci GV, Botti G, Tatangelo F, Delrio P, Maio M, Laghi L, Grizzi F, Asslaber M, D’Arrigo C, Vidal-Vanaclocha F, Zavadova E, Chouchane L, Ohashi PS, Hafezi-Bakhtiari S, Wouters BG, Roehrl M, Nguyen L, Kawakami Y, Hazama S, Okuno K, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Wang Y, Kopetz S, Sinicrope FA, Scripcariu V, Ascierto PA, Marincola FM, Fox BA, Pages F (2014) Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol 232:199–209. doi:10.1002/path.4287
Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K (2008) Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26:317–325. doi:10.1038/nbt1385
Nielsen T, Wallden B, Schaper C, Ferree S, Liu S, Gao D, Barry G, Dowidar N, Maysuria M, Storhoff J (2014) Analytical validation of the PAM50-based Prosigna Breast Cancer Prognostic Gene Signature Assay and nCounter Analysis System using formalin-fixed paraffin-embedded breast tumor specimens. BMC Cancer 14:177. doi:10.1186/1471-2407-14-177
Stricker TP, Morales La Madrid A, Chlenski A, Guerrero L, Salwen HR, Gosiengfiao Y, Perlman EJ, Furman W, Bahrami A, Shohet JM, Zage PE, Hicks MJ, Shimada H, Suganuma R, Park JR, So S, London WB, Pytel P, Maclean KH, Cohn SL (2014) Validation of a prognostic multi-gene signature in high-risk neuroblastoma using the high throughput digital NanoString nCounter system. Mol Oncol 8:669–678. doi:10.1016/j.molonc.2014.01.010
Veldman-Jones M, Lai Z, Wappett M, Harbron C, Barrett JC, Harrington EA, Thress KS (2014) Reproducible, quantitative and flexible molecular sub-typing of clinical DLBCL samples using the NanoString nCounter system. Clin Cancer Res. doi:10.1158/1078-0432.CCR-14-0357
Andorfer CA, Necela BM, Thompson EA, Perez EA (2011) MicroRNA signatures: clinical biomarkers for the diagnosis and treatment of breast cancer. Trends Mol Med 17:313–319. doi:10.1016/j.molmed.2011.01.006
Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N (2015) Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160:48–61. doi:10.1016/j.cell.2014.12.033
Spranger S, Gajewski T (2013) Rational combinations of immunotherapeutics that target discrete pathways. J Immunother Cancer 1:16. doi:10.1186/2051-1426-1-16
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331:1565–1570. doi:10.1126/science.1203486
Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM (2012) Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity 36:142–152. doi:10.1016/j.immuni.2012.01.002
Talmadge JE, Gabrilovich DI (2013) History of myeloid-derived suppressor cells. Nat Rev Cancer 13:739–752. doi:10.1038/nrc3581
Vahedi G, Takahashi H, Nakayamada S, Sun HW, Sartorelli V, Kanno Y, O’Shea JJ (2012) STATs shape the active enhancer landscape of T cell populations. Cell 151:981–993. doi:10.1016/j.cell.2012.09.044
Warren RL, Freeman JD, Zeng T, Choe G, Munro S, Moore R, Webb JR, Holt RA (2011) Exhaustive T-cell repertoire sequencing of human peripheral blood samples reveals signatures of antigen selection and a directly measured repertoire size of at least 1 million clonotypes. Genome Res 21:790–797. doi:10.1101/gr.115428.110
Wang C, Sanders CM, Yang Q, Schroeder HW Jr, Wang E, Babrzadeh F, Gharizadeh B, Myers RM, Hudson JR Jr, Davis RW, Han J (2010) High throughput sequencing reveals a complex pattern of dynamic interrelationships among human T cell subsets. Proc Natl Acad Sci USA 107:1518–1523. doi:10.1073/pnas.0913939107
Robins HS, Srivastava SK, Campregher PV, Turtle CJ, Andriesen J, Riddell SR, Carlson CS, Warren EH (2010) Overlap and effective size of the human CD8+ T cell receptor repertoire. Sci Transl Med. doi:10.1126/scitranslmed.3001442
Morris H, DeWolf S, Robins H, Sprangers B, LoCascio SA, Shonts BA, Kawai T, Wong W, Yang S, Zuber J, Shen Y, Sykes M (2015) Tracking donor-reactive T cells: evidence for clonal deletion in tolerant kidney transplant patients. Sci Transl Med. doi:10.1126/scitranslmed.3010760
Robert L, Harview C, Emerson R, Wang X, Mok S, Homet B, Comin-Anduix B, Koya RC, Robins H, Tumeh PC, Ribas A (2014) Distinct immunological mechanisms of CTLA-4 and PD-1 blockade revealed by analyzing TCR usage in blood lymphocytes. Oncoimmunology 3:e29244. doi:10.4161/onci.29244
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568–571. doi:10.1038/nature13954
Johnson PL, Kochin BF, McAfee MS, Stromnes IM, Regoes RR, Ahmed R, Blattman JN, Antia R (2011) Vaccination alters the balance between protective immunity, exhaustion, escape, and death in chronic infections. J Virol 85:5565–5570. doi:10.1128/JVI.00166-11
Blattman JN, Wherry EJ, Ha SJ, van der Most RG, Ahmed R (2009) Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J Virol 83:4386–4394. doi:10.1128/JVI.02524-08
Hess CF, Schaaf JC, Kortmann RD, Schabet M, Bamberg M (1994) Malignant glioma: patterns of failure following individually tailored limited volume irradiation. Radiother Oncol 30:146–149
Hochberg FH, Pruitt A (1980) Assumptions in the radiotherapy of glioblastoma. Neurology 30:907–911
Liang BC, Thornton AF Jr, Sandler HM, Greenberg HS (1991) Malignant astrocytomas: focal tumor recurrence after focal external beam radiation therapy. J Neurosurg 75:559–563. doi:10.3171/jns.1991.75.4.0559
Fujisawa HRR, Nakamura M, Colella S, Yonekawa Y, Kleihues P, Ohgaki H (2000) Loss of heterozygosity on chromosome 10 is more extensive in primary (de novo) than in secondary glioblastomas. Lab Invest 80:65–72
Tada K, Shiraishi S, Kamiryo T, Nakamura H, Hirano H, Kuratsu J, Kochi M, Saya H, Ushio Y (2001) Analysis of loss of heterozygosity on chromosome 10 in patients with malignant astrocytic tumors: correlation with patient age and survival. J Neurosurg 95:651–659. doi:10.3171/jns.2001.95.4.0651
Balesaria SBC, Bower M, Clark J, Nicholson SK, Lewis P, de Sanctis S, Evans H, Peterson D, Mendoza N, Glaser MG, Newlands ES, Fisher RA (1999) Loss of chromosome 10 is an independent prognostic factor in high-grade gliomas. Br J Cancer 81:1371–1377
Steck PAPM, Jasser SA, Tung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV (1997) Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 15:362–465
Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R (1997) PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275:1943–1947
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812. doi:10.1126/science.1164382
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN, Cancer Genome Atlas Research N (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110. doi:10.1016/j.ccr.2009.12.020
Kim YW, Koul D, Kim SH, Lucio-Eterovic AK, Freire PR, Yao J, Wang J, Almeida JS, Aldape K, Yung WK (2013) Identification of prognostic gene signatures of glioblastoma: a study based on TCGA data analysis. Neuro-oncology 15:829–839. doi:10.1093/neuonc/not024
Bhat KP, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L, James JD, Goodman LD, Conroy S, Long L, Lelic N, Wang S, Gumin J, Raj D, Kodama Y, Raghunathan A, Olar A, Joshi K, Pelloski CE, Heimberger A, Kim SH, Cahill DP, Rao G, Den Dunnen WF, Boddeke HW, Phillips HS, Nakano I, Lang FF, Colman H, Sulman EP, Aldape K (2013) Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell 24:331–346. doi:10.1016/j.ccr.2013.08.001
Mischel PS, Shai R, Shi T, Horvath S, Lu KV, Choe G, Seligson D, Kremen TJ, Palotie A, Liau LM, Cloughesy TF, Nelson SF (2003) Identification of molecular subtypes of glioblastoma by gene expression profiling. Oncogene 22:2361–2373. doi:10.1038/sj.onc.1206344
Gan HK, Kaye AH, Luwor RB (2009) The EGFRvIII variant in glioblastoma multiforme. J Clin Neurosci 16:748–754. doi:10.1016/j.jocn.2008.12.005
Schlegel J, Merdes A, Stumm G, Albert FK, Forsting M, Hynes N, Kiessling M (1994) Amplification of the epidermal-growth-factor-receptor gene correlates with different growth behaviour in human glioblastoma. Int J Cancer 56:72–77
Wong AJBS, Bigner DD, Kinzler Hamilton SR, Vogelstein B (1987) Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci USA 84:6899–6903
Wong AJ, Ruppert JM, Bigner SH, Grzeschik CH, Humphrey PA, Bigner DS, Vogelstein B (1992) Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci USA 89:2965–2969
Humphrey PAWA, Vogelstein B, Zalutsky MR, Fuller GN, Archer GE, Friedman HS, Kwatra MM, Bigner SH, Bidgner DD (1990) Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci USA 97:4207–4211
Shinojima NTK, Shiraishe S, Kamirya T, Kochi M, Nakamura H, Makio K, Saya H, Hirano H, Kuratsu J, Oka K, Ishimaru Y, Ushio Y (2003) Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res 63:6962–6970
Heimberger AB, Hlatky R, Suki D, Yang D, Weinberg J, Gilbert M, Sawaya R, Aldape K (2005) Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res 11:1462–1466. doi:10.1158/1078-0432.CCR-04-1737
Han SJ, Zygourakis C, Lim M, Parsa AT (2012) Immunotherapy for glioma: promises and challenges. Neurosurg Clin N Am 23:357–370. doi:10.1016/j.nec.2012.05.001
Stragliotto G, Vega F, Stasiecki P, Gropp P, Poisson M, Delattre JY (1996) Multiple infusions of anti-epidermal growth factor receptor (EGFR) monoclonal antibody (EMD 55,900) in patients with recurrent malignant gliomas. Eur J Cancer 32A:636–640
Snelling L, Miyamoto CT, Bender H, Brady LW, Steplewski Z, Class R, Emrich J, Rackover MA (1995) Epidermal growth factor receptor 425 monoclonal antibodies radiolabeled with iodine-125 in the adjuvant treatment of high-grade astrocytomas. Hybridoma 14:111–114
Katanasaka Y, Kodera Y, Kitamura Y, Morimoto T, Tamura T, Koizumi F (2013) Epidermal growth factor receptor variant type III markedly accelerates angiogenesis and tumor growth via inducing c-myc mediated angiopoietin-like 4 expression in malignant glioma. Mol Cancer 12:31. doi:10.1186/1476-4598-12-31
Li G, Wong AJ (2008) EGF receptor variant III as a target antigen for tumor immunotherapy. Expert Rev Vaccines 7:977–985. doi:10.1586/14760584.7.7.977
Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE 2nd, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling RJ, Shi W, Vredenburgh JJ, Bigner DD (2010) Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 28:4722–4729. doi:10.1200/JCO.2010.28.6963
Heimberger AB, Crotty LE, Archer GE, Hess KR, Wikstrand CJ, Friedman AH, Friedman HS, Bigner DD, Sampson JH (2003) Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res 9:4247–4254
Kaina B, Christmann M, Naumann S, Roos WP (2007) MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst) 6:1079–1099. doi:10.1016/j.dnarep.2007.03.008
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. doi:10.1056/NEJMoa043331
Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C, Pfaff E, Tonjes M, Sill M, Bender S, Kool M, Zapatka M, Becker N, Zucknick M, Hielscher T, Liu XY, Fontebasso AM, Ryzhova M, Albrecht S, Jacob K, Wolter M, Ebinger M, Schuhmann MU, van Meter T, Fruhwald MC, Hauch H, Pekrun A, Radlwimmer B, Niehues T, von Komorowski G, Durken M, Kulozik AE, Madden J, Donson A, Foreman NK, Drissi R, Fouladi M, Scheurlen W, von Deimling A, Monoranu C, Roggendorf W, Herold-Mende C, Unterberg A, Kramm CM, Felsberg J, Hartmann C, Wiestler B, Wick W, Milde T, Witt O, Lindroth AM, Schwartzentruber J, Faury D, Fleming A, Zakrzewska M, Liberski PP, Zakrzewski K, Hauser P, Garami M, Klekner A, Bognar L, Morrissy S, Cavalli F, Taylor MD, van Sluis P, Koster J, Versteeg R, Volckmann R, Mikkelsen T, Aldape K, Reifenberger G, Collins VP, Majewski J, Korshunov A, Lichter P, Plass C, Jabado N, Pfister SM (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22:425–437. doi:10.1016/j.ccr.2012.08.024
Stricker SH, Feber A, Engstrom PG, Caren H, Kurian KM, Takashima Y, Watts C, Way M, Dirks P, Bertone P, Smith A, Beck S, Pollard SM (2013) Widespread resetting of DNA methylation in glioblastoma-initiating cells suppresses malignant cellular behavior in a lineage-dependent manner. Genes Dev 27:654–669. doi:10.1101/gad.212662.112
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773. doi:10.1056/NEJMoa0808710
Minniti G, Scaringi C, Arcella A, Lanzetta G, Di Stefano D, Scarpino S, Bozzao A, Pace A, Villani V, Salvati M, Esposito V, Giangaspero F, Enrici RM (2014) IDH1 mutation and MGMT methylation status predict survival in patients with anaplastic astrocytoma treated with temozolomide-based chemoradiotherapy. J Neurooncol 118:377–383. doi:10.1007/s11060-014-1443-0
Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, Sulman EP, Anne SL, Doetsch F, Colman H, Lasorella A, Aldape K, Califano A, Iavarone A (2010) The transcriptional network for mesenchymal transformation of brain tumours. Nature 463:318–325. doi:10.1038/nature08712
Sonabend AM, Bansal M, Guarnieri P, Lei L, Amendolara B, Soderquist C, Leung R, Yun J, Kennedy B, Sisti J, Bruce S, Bruce R, Shakya R, Ludwig T, Rosenfeld S, Sims PA, Bruce JN, Califano A, Canoll P (2014) The transcriptional regulatory network of proneural glioma determines the genetic alterations selected during tumor progression. Cancer Res 74:1440–1451. doi:10.1158/0008-5472.CAN-13-2150
Allen BK, Stathias V, Maloof ME, Vidovic D, Winterbottom EF, Capobianco AJ, Clarke J, Schurer S, Robbins DJ, Ayad NG (2014) Epigenetic pathways and glioblastoma treatment: insights from signaling cascades. J Cell Biochem. doi:10.1002/jcb.24990
Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, Menn O, Osswald M, Oezen I, Ott M, Keil M, Balss J, Rauschenbach K, Grabowska AK, Vogler I, Diekmann J, Trautwein N, Eichmuller SB, Okun J, Stevanovic S, Riemer AB, Sahin U, Friese MA, Beckhove P, von Deimling A, Wick W, Platten M (2014) A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 512:324–327. doi:10.1038/nature13387
Shah N, Lankerovich M, Lee H, Yoon JG, Schroeder B, Foltz G (2013) Exploration of the gene fusion landscape of glioblastoma using transcriptome sequencing and copy number data. BMC Genom 14:818. doi:10.1186/1471-2164-14-818
Singh D, Chan JM, Zoppoli P, Niola F, Sullivan R, Castano A, Liu EM, Reichel J, Porrati P, Pellegatta S, Qiu K, Gao Z, Ceccarelli M, Riccardi R, Brat DJ, Guha A, Aldape K, Golfinos JG, Zagzag D, Mikkelsen T, Finocchiaro G, Lasorella A, Rabadan R, Iavarone A (2012) Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 337:1231–1235. doi:10.1126/science.1220834
Wikstrand CJ, Grahmann FC, McComb RD, Bigner DD (1985) Antigenic heterogeneity of human anaplastic gliomas and glioma-derived cell lines defined by monoclonal antibodies. J Neuropathol Exp Neurol 44:229–241
Dutoit V, Herold-Mende C, Hilf N, Schoor O, Beckhove P, Bucher J, Dorsch K, Flohr S, Fritsche J, Lewandrowski P, Lohr J, Rammensee HG, Stevanovic S, Trautwein C, Vass V, Walter S, Walker PR, Weinschenk T, Singh-Jasuja H, Dietrich PY (2012) Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain 135:1042–1054. doi:10.1093/brain/aws042
Gill BJ, Pisapia DJ, Malone HR, Goldstein H, Lei L, Sonabend A, Yun J, Samanamud J, Sims JS, Banu M, Dovas A, Teich AF, Sheth SA, McKhann GM, Sisti MB, Bruce JN, Sims PA, Canoll P (2014) MRI-localized biopsies reveal subtype-specific differences in molecular and cellular composition at the margins of glioblastoma. Proc Natl Acad Sci USA 111:12550–12555. doi:10.1073/pnas.1405839111
Joshi BH, Plautz GE, Puri RK (2000) Interleukin-13 receptor alpha chain: a novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res 60:1168–1172
Kawakami KKM, Puri RK (2001) Overexpressed cell surface interleukin-4 receptor molecules can be successfully targeted for antitumor cytotoxin therapy. Crit Rev Immunol 21:299–310
Kunwar S, Prados MD, Chang SM, Berger MS, Lang FF, Piepmeier JM, Sampson JH, Ram Z, Gutin PH, Gibbons RD, Aldape KD, Croteau DJ, Sherman JW, Puri RK, Cintredekin Besudotox Intraparenchymal Study G (2007) Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol 25:837–844. doi:10.1200/JCO.2006.08.1117
Weber F, Asher A, Bucholz R, Berger M, Prados M, Chang S, Bruce J, Hall W, Rainov NG, Westphal M, Warnick RE, Rand RW, Floeth F, Rommel F, Pan H, Hingorani VN, Puri RK (2003) Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J Neurooncol 64:125–137
Sampson JH, Archer G, Pedain C, Wembacher-Schroder E, Westphal M, Kunwar S, Vogelbaum MA, Coan A, Herndon JE, Raghavan R, Brady ML, Reardon DA, Friedman AH, Friedman HS, Rodriguez-Ponce MI, Chang SM, Mittermeyer S, Croteau D, Puri RK, Investigators PT (2010) Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg 113:301–309. doi:10.3171/2009.11.JNS091052
Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, Shaffrey M, Ram Z, Piepmeier J, Prados M, Croteau D, Pedain C, Leland P, Husain SR, Joshi BH, Puri RK, Group PS (2010) Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro-oncology 12:871–881. doi:10.1093/neuonc/nop054
Han J, Yang L, Puri RK (2005) Analysis of target genes induced by IL-13 cytotoxin in human glioblastoma cells. J Neurooncol 72:35–46. doi:10.1007/s11060-004-3119-7
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB, 3rd, Eastern Cooperative Oncology Group Study E (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25:1539–1544. doi:10.1200/JCO.2006.09.6305
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285:1182–1186
If T (1968) The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer 22:259–273
Tannock IF (1970) Population kinetics of carcinoma cells, capillary endothelial cells, and fibroblasts in a transplanted mouse mammary tumor. Cancer Res 30:2470–2476
Maxwell MNS, Wolfe HJ, Hedley-Whyte ET, Galanopoulos T, Neville-Golden J, Antoniades HN (1991) Expression of angiogenic growth factor genes in primary human astrocytomas may contribute to their growth and progression. Cencer Res 51:1345–1351
Stefanik DF, Fellows WK, Rizkalla LR, Rizkalla WM, Stefanik PP, Deleo AB, Welch WC (2001) Monoclonal antibodies to vascular endothelial growth factor (VEGF) and the VEGF receptor, FLT-1, inhibit the growth of C6 glioma in a mouse xenograft. J Neurooncol 55:91–100
Thompson EM, Frenkel EP, Neuwelt EA (2011) The paradoxical effect of bevacizumab in the therapy of malignant gliomas. Neurology 76:87–93. doi:10.1212/WNL.0b013e318204a3af
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745. doi:10.1200/JCO.2008.16.3055
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740. doi:10.1200/JCO.2008.19.8721
Norden AD, Drappatz J, Muzikansky A, David K, Gerard M, McNamara MB, Phan P, Ross A, Kesari S, Wen PY (2009) An exploratory survival analysis of anti-angiogenic therapy for recurrent malignant glioma. J Neurooncol 92:149–155. doi:10.1007/s11060-008-9745-8
Omuro A, Chan TA, Abrey LE, Khasraw M, Reiner AS, Kaley TJ, Deangelis LM, Lassman AB, Nolan CP, Gavrilovic IT, Hormigo A, Salvant C, Heguy A, Kaufman A, Huse JT, Panageas KS, Hottinger AF, Mellinghoff I (2013) Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro-oncology 15:242–250. doi:10.1093/neuonc/nos295
Zuniga RM, Torcuator R, Jain R, Anderson J, Doyle T, Schultz L, Mikkelsen T (2010) Rebound tumour progression after the cessation of bevacizumab therapy in patients with recurrent high-grade glioma. J Neurooncol 99:237–242. doi:10.1007/s11060-010-0121-0
Kennedy BC, Showers CR, Anderson DE, Anderson L, Canoll P, Bruce JN, Anderson RC (2013) Tumor-associated macrophages in glioma: friend or foe? J Oncol 2013:486912. doi:10.1155/2013/486912
Pello OM, De Pizzol M, Mirolo M, Soucek L, Zammataro L, Amabile A, Doni A, Nebuloni M, Swigart LB, Evan GI, Mantovani A, Locati M (2012) Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood 119:411–421. doi:10.1182/blood-2011-02-339911
Moutsopoulos NM, Wen J, Wahl SM (2008) TGF-beta and tumors–an ill-fated alliance. Curr Opin Immunol 20:234–240. doi:10.1016/j.coi.2008.04.003
Lei L, Sonabend AM, Guarnieri P, Soderquist C, Ludwig T, Rosenfeld S, Bruce JN, Canoll P (2011) Glioblastoma models reveal the connection between adult glial progenitors and the proneural phenotype. PLoS ONE 6:e20041. doi:10.1371/journal.pone.0020041
Parker JJ, Dionne KR, Massarwa R, Klaassen M, Foreman NK, Niswander L, Canoll P, Kleinschmidt-Demasters BK, Waziri A (2013) Gefitinib selectively inhibits tumor cell migration in EGFR-amplified human glioblastoma. Neuro-oncology 15:1048–1057. doi:10.1093/neuonc/not053
Vauleon E, Tony A, Hamlat A, Etcheverry A, Chiforeanu DC, Menei P, Mosser J, Quillien V, Aubry M (2012) Immune genes are associated with human glioblastoma pathology and patient survival. BMC Med Genomics 5:41. doi:10.1186/1755-8794-5-41
Watanabe K, Tachibana O, Sata K, Yonekawa Y, Kleihues P, Ohgaki H (1996) Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol 6:217–223 (discussion 223–214)
Phuphanich S, Wheeler CJ, Rudnick JD, Mazer M, Wang H, Nuno MA, Richardson JE, Fan X, Ji J, Chu RM, Bender JG, Hawkins ES, Patil CG, Black KL, Yu JS (2013) Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother 62:125–135. doi:10.1007/s00262-012-1319-0
Mineo JF, Bordron A, Baroncini M, Maurage CA, Ramirez C, Siminski RM, Berthou C, Dam Hieu P (2007) Low HER2-expressing glioblastomas are more often secondary to anaplastic transformation of low-grade glioma. J Neurooncol 85:281–287. doi:10.1007/s11060-007-9424-1
Mastronardi L, Guiducci A, Puzzilli F, Ruggeri A (1999) Relationship between Ki-67 labeling index and survival in high-grade glioma patients treated after surgery with tamoxifen. J Neurosurg Sci 43:263–270
Liu G, Ying H, Zeng G, Wheeler CJ, Black KL, Yu JS (2004) HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer Res 64:4980–4986. doi:10.1158/0008-5472.CAN-03-3504
Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H, Whittle N, Waterfield MD, Ullrich A, Schlessinger J (1985) Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature 313:144–147
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285:1182–1186. doi:10.1056/NEJM197111182852108
Eng LF, Vanderhaeghen JJ, Bignami A, Gerstl B (1971) An acidic protein isolated from fibrous astrocytes. Brain Res 28:351–354
Duffy PE, Huang YY, Rapport MM (1982) The relationship of glial fibrillary acidic protein to the shape, motility, and differentiation of human astrocytoma cells. Exp Cell Res 139:145–157
Bigner SH, Mark J, Burger PC, Mahaley MS Jr, Bullard DE, Muhlbaier LH, Bigner DD (1988) Specific chromosomal abnormalities in malignant human gliomas. Cancer Res 48:405–411
Chandramohan V, Bao X, Keir ST, Pegram CN, Szafranski SE, Piao H, Wikstrand CJ, McLendon RE, Kuan CT, Pastan IH, Bigner DD (2013) Construction of an immunotoxin, D2C7-(scdsFv)-PE38KDEL, targeting EGFRwt and EGFRvIII for brain tumor therapy. Clin Cancer Res 19(17):4717–4727. doi:10.1158/1078-0432.CCR-12-3891
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sims, J.S., Ung, T.H., Neira, J.A. et al. Biomarkers for glioma immunotherapy: the next generation. J Neurooncol 123, 359–372 (2015). https://doi.org/10.1007/s11060-015-1746-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1746-9