Abstract
Children with high-grade glioma (HGG) have a poor prognosis compared to those with low-grade glioma (LGG). Adjuvant chemotherapy may be beneficial, but its optimal use remains undetermined. Histology and extent of resection are important prognostic factors. We tested the hypothesis that patients with midline HGG treated on Children’s Cancer Group Study (CCG) CCG-945 have a worse prognosis compared to the entire group. Of 172 children eligible for analysis, 60 had midline tumors primarily localized to the thalamus, hypothalamus and basal ganglia. Time-to-progression and death were determined from the date of initial diagnosis, and survival curves were calculated. Univariate analyses were undertaken for extent of resection, chemotherapy regimen, anatomic location, histology, proliferation index, MGMT status and p53 over-expression. For the entire midline tumor group, 5-year PFS and OS were 18.3 ± 4.8 and 25 ± 5.4 %, respectively. Many patients only had a biopsy (43.3 %). The sub-groups with near/total resection and hypothalamic location appeared to have better PFS and OS. However, the effect of tumor histology on OS was significant for children with discordant diagnoses on central pathology review of LGG compared to HGG. Proliferative index (MIB-1 > 36 %), MGMT and p53 over-expression correlated with poor outcomes. Children treated on CCG-945 with midline HGG have a worse prognosis when compared to the entire group. The midline location may directly influence the extent of resection. Central pathology review and entry of patients on clinical trials continue to be priorities to improve outcomes for children with HGG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Children’s Cancer Group (CCG) randomized clinical trial (RCT) CCG-943 compared irradiation followed by nitrosourea-based chemotherapy to irradiation alone. This was the first phase III trial demonstrating that adjuvant chemotherapy improved survival for children with HGG, with a 5-year event-free survival (EFS) and overall survival (OS) of 33 and 36 %, respectively [1].
Subsequently, the CCG-945 study compared a novel regimen known as “8-drugs-in-1-day” (“8-in-1”), given both pre- and post-irradiation, to the chemotherapy-containing arm in CCG-943. Although neither treatment arm was superior, important observations were made regarding central neuropathology review, tumor histology and the extent of resection (EOR) [2].
Although the original CCG-945 report assigned a poor prognosis to “midline” HGG patients, this group had the highest rate of pathological discordance and merited further study. Furthermore, the rarity and dismal outcome of HGG in the hypothalamic/optic chiasm region are new and important observations.
Materials and methods
Patient population
From 1985–1990, 172 eligible children with newly-diagnosed HGG outside of the brainstem or spinal cord were randomized to receive following surgery either eight cycles of the control regimen consisting of lomustine, vincristine and prednisone (Regimen A) following involved-field irradiation (54 Gy in 1.8 Gy fractions) with concomitant weekly vincristine ×8, or the experimental regimen known as “8-in-1” (Regimen B) which included lomustine, vincristine, hydroxyurea, procarbazine, cisplatin, cytarabinoside, dacarbazine and methylprednisolone. In Regimen B, two cycles of postoperative chemotherapy were administered prior to involved-field irradiation (54 Gy in 1.8 Gy fractions), the latter without weekly vincristine. Young patients (<2–3 years, depending upon enrollment year) were non-randomized to Regimen B (NB) to avoid or delay irradiation. Specimens were centrally reviewed by a panel of five neuropathologists, as were institutional radiology and surgical reports. EOR was defined as: biopsy only (<10 %), partial resection (10–50 %), subtotal (STR, 50–90 %), and near-total/total resection (NTR, >90 %). A pathologic diagnosis was termed discordant when central review determined the tumor to be other than HGG [2]. Surviving patients were followed up to 2004.
This report focused on 60 patients with “midline” HGG. In addition to progression-free survival (PFS) and OS, outcomes were determined according to EOR, chemotherapy regimen, tumor location and discordant diagnosis.
Tumor biology
Study neuropathologists were blinded to patient status, EOR, tumor location or histology. The proliferation index (MIB-1 score) of formalin-fixed paraffin embedded (FFPE) sections used the percentage of Ki-67 antigen immunoreactivity in approx. 2,000 cells from 5 to 10 high power fields (hpf) [3]. O6-methylguanine-DNA methyltransferase (MGMT) expression was determined using immunohistochemistry (IHC) (mouse anti-MGMT antibody, Zymed) [4] with labeling score determined semi-quantitatively in 5–10 hpf: no/little expression (0/1), scattered positive cells (2), most/nearly all cells (3/4). A score of 3 or 4 was classified as “over-expression” whereas 0, 1 or 2 were considered “no over-expression” comparable to normal brain. p53 protein expression was detected by IHC using an antibody that recognized wild-type and/or mutant p53 (DO-7, Dako) [5], and assessed: little/no p53 expression (0/1), 25–50 % (2), 50–75 % (3) or >75 % of tumor cells (4).
Statistical considerations
The Kaplan–Meier method calculated time to recurrence and death from the date of diagnosis [6]. Follow-up stopped at death or the end of the study date for survival and at recurrence or the end of study (whichever happened first). Patients were censored for any other events when determining time to recurrence. The log rank test was used to compare time-to-event distributions. Data management and analyses were performed using SAS (v. 9.1.3).
Results
Patient characteristics
Of 172 enrolled patients, 60 with primarily midline tumor were eligible (Table 1). Eleven (18.3 %) are long-term survivors. There were 26 males (43.3 %) with 47 (78 %) Caucasians. The mean age at diagnosis was 7.61 years. Thirty-nine (65 %) were under 10 years with 21 (35 %) <5 years. Twenty-three patients received Regimen A (38.3 %); 1/23 was non-randomized (NA), whereas 37 received Regimen B (61.7 %), 16/37 being non-randomized. Tumors were primarily localized to the thalamus (37, 61.7 %), hypothalamus (10, 16.7 %), basal ganglia (6, 10.0 %), or mid-brain (4, 6.7 %). NTR was performed on only five patients (8.3 %); the remainder underwent a biopsy (26, 43.3 %), partial (15, 25.0 %) or subtotal (14, 23.3 %) resections. Institutional diagnoses were predominantly anaplastic astrocytoma (AA) in 39 patients (65 %) and glioblastoma (GBM) in 14 (23.3 %). Central review reclassified 19 patients as LGG, 26 patients (43.3 %) had AA (22, 36.7 %) or other anaplastic gliomas (4, 6.7 %) and 12 with GBM (20.0 %), representing 63.3 % of the cohort (Online resource Table 1).
Extent of resection
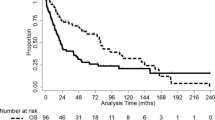
For the midline tumor group, the 3- and 5-year PFS was 23.3 ± 5.3 and 18.3 ± 4.8 %, respectively (Fig. 1). According to EOR the following 3- and 5-year PFS were: biopsy, 19.2 ± 7.1 %, 7.7 ± 4.3 %; partial resection, 33.3 ± 11.1 %, 33.3 ± 11.1 %; STR, 14.3 ± 7.6 %, 14.3 ± 7.6 %; NTR, 40.0 ± 17.9 %, 40.0 ± 17.9 % (Fig. 2a). NTR appeared to have better PFS but these results were not significant (NS, p = 0.26). The 3- and 5-year OS were 26.7 ± 5.5 and 25.0 ± 5.4 %, respectively (Fig. 1). Analysis of OS demonstrated the following 3- and 5-year survivals: biopsy, 23.1 ± 7.6 %, 19.2 ± 7.1 %; partial resection, 33.3 ± 11.1 %, 33.3 ± 11.1 %; STR, 21.4 ± 9.5 %, 21.4 ± 9.5 %; NTR, 40.0 ± 17.9 %, 40.0 ± 17.9 % (Fig. 2b). Similar to PFS, NTR appeared to have improved survival but the results were not significant (p = 0.55).
Chemotherapy regimen
There were no statistical differences between chemotherapy regimens (p = 0.48) with 3- and 5-year PFS for Regimen A/NA (26.1 ± 8.5 %, 26.1 ± 8.5 %) and Regimen B/NB (21.6 ± 6.4 %, 13.5 ± 5.1 %), respectively (Fig. 2c). Similarly there was no significant difference in OS between regimens (p = 0.49) with 3- and 5-year OS for Regimen A/NA (26.1 ± 8.5 %, 26.1 ± 8.5 %) and Regimen B/NB (27.0 ± 7.0 %, 24.3 ± 6.7 %), respectively (Fig. 2d).
Anatomic tumor location
PFS revealed 3- and 5- year PFS for thalamus (18.9 ± 6.0 %, 13.5 %), hypothalamus (30 ± 13.9 %, 30 ± 12.5 %), basal ganglia (16.7 ± 10.8 %, 16.7 ± 10.8 %) and midbrain (25.0 ± 15.3 %, 25.0 ± 15.3 %) (Fig. 3a). Survival analysis revealed 3- and 5 -year OS for thalamus (18.9 ± 6.0 %, 16.2 ± 5.6 %), hypothalamus (60 ± 14.3 %, 60 ± 14.3 %), basal ganglia (16.7 ± 10.8 %, 16.7 ± 10.8 %) and midbrain (25.0 ± 15.3 %, 25.0 ± 15.3 %) (Fig. 3c). There were no significant differences for PFS (p = 0.59) or OS (p = 0.39) among primary tumor locations. However, the patient group with hypothalamic tumors appeared to have better survival outcomes (p = 0.28).
For patients whose tumors were localized primarily to one site versus more than one site, PFS was significantly different (p = 0.0013) with 3- and 5-year PFS 36.4 ± 9.7 and 36.4 ± 9.7 % for one site versus 15.8 ± 5.5 and 7.9 ± 3.8 % for more than one site, respectively (Fig. 3b). OS was also significantly different (p = 0.0084) with 3- and 5-year OS 36.4 ± 9.7 and 36.4 ± 9.7 % for one site versus 21.1 ± 6.2 and 18.4 % for more than one site, respectively (Fig. 3d).
Histology
Central review showed 1- and 3-year PFS of 27.3 ± 8.8 and 0 % for AA and 33.3 ± 12.2 and 0 % for GBM, respectively. For those with a discordant diagnosis resulting in reclassification as low grade glioma (LGG), 1-, 3- and 5-year PFS were 73.1 ± 8.5, 53.8 ± 9.4 and 42.3 ± 9.3 % (Fig. 4a). One- and 3-year OS were 58.3 and 0 % for all HGG whose diagnoses remained unchanged. For AA, 1- and 3-year OS were 63.6 ± 9.9 and 0 %, and 41.7 ± 13.0 and 0 % for GBM (Fig. 4b). For patients revised to a LGG, 1-, 3- and 5-year OS were 80.8 ± 7.6, 61.5 ± 9.3 and 57.7 ± 9.4 % (Fig. 4b). There were significant differences for PFS (p < 0.0001) and OS (p < 0.0001) when comparing HGG (AA, GBM) to a discordant diagnosis.
Tumor histology and survival curves for a PFS or b OS. Solid line anaplastic astrocytoma (AA, WHO III); dashed line glioblastoma multiforme (GBM, WHO IV); dotted line diagnoses discordant for pediatric HGG. c, d Comparing changed diagnoses following central review to those patients with an unchanged diagnosis (p < 0.01). Diagnosis changed, dotted line; diagnosis unchanged, solid line
Patients were also classified into two groups: “No Change” and “Change” from Institutional Diagnosis. One-, 3- and 5-year PFS were 40.0 ± 9.3, 40.0 ± 2.8, and 0 % for no change and 54.3 ± 8.2, 37.1 ± 7.9, and 31.4 ± 7.5 % for changed diagnosis; these findings were significant (p = 0.0078, Fig. 4c), as were 1-, 3- and 5-year OS of 60.0 ± 9.5, 4 ± 2.8 and 4 ± 2.8 % for no change and 71.4 ± 7.5, 42.9 ± 8.1, and 40.0 ± 8.0 % for changed diagnosis (p = 0.0009, Fig. 4d).
Tumor biology
Analysis was also performed on patient subsets: (i) MIB-1 score (n = 32), (ii) MGMT expression score (n = 23) and (iii) p53 expression score (n = 27). The MIB-1 score separated into three groups: group 1 (“0”: <18 %; n = 21), group 2 (“1”: 18–36 %; n = 6) and group 3 (“2”: >36 %; n = 5) [3]. Three- and 5-year PFS were 28.6 ± 9.1 and 23.8 ± 8.5 % (group 1), 16.7 ± 10.8 and 16.7 ± 10.8 % (group 2) and 0 and 0 % (group 3), respectively (Online resource Fig. 1A). Similarly, 3- and 5-year OS were 33.3 ± 9.6 and 33.3 ± 9.6 % (group 1), 16.7 ± 10.8 and 16.7 ± 10.8 % (group 2) and 0 and 0 % (group 3), respectively (Online resource Fig. 1B). There were significant differences among the MIB-1 score groups for PFS (p = 0.03) and 0OS (p = 0.01).
MGMT scores were grouped as “0: no over-expression” (score = 0, 1 or 2; n = 19) or “1: over-expression” (score = 3 or 4; n = 4) [4]. Three- and 5-year PFS were 26.3 ± 9.2 and 21.1 ± 8.4 % (group 1) and 0 and 0 % (group 2), respectively (Online resource Fig. 2A). Similarly, 3- and 5-year OS were 26.3 ± 9.2 and 26.3 ± 9.2 % (group 1) and 0 and 0 % (group 2), respectively (Online resource Fig. 2B). Differences between MGMT score groups were not significant for PFS (p = 0.19) or OS (p = 0.13).
Finally, the p53 score was grouped as “0: normal” (score = 0 or 1; n = 17) or “1: over-expression” (score = 2, 3 or 4; n = 10) [5]. Three- and 5-year PFS were 29.4 ± 10.1 and 23.5 ± 9.2 % (group 1) and 10 ± 6.7 and 10 ± 6.7 % (group 2), respectively (Online resource Fig. 3A). Similarly, 3- and 5-year OS were 35.3 ± 10.7 and 35.3 ± 10.7 % (group 1) and 10 ± 6.7 and 10 ± 6.7 % (group 2), respectively (Online resource Fig. 3B). Differences between p53 score groups were not significant for PFS (p = 0.27) or OS (p = 0.099). Although MGMT and/or p53 over-expression correlated with poor PFS and OS, these results were not statistically significant (NS), unlike those observed for MIB-1 scores >36 %.
Discussion
In the current analysis of 60 children with midline HGG, the 5-year PFS and OS of 18.3 and 25.0 % were reduced when compared to the overall group of 172 patients with 5-year EFS and OS of 33 and 36 % [2]. EOR correlated with both PFS and OS in the midline group but did not reach significance; only 5 patients (8.3 %) underwent extensive resections. Posterior fossa (PF) and cerebral hemispheric locations were more amenable to aggressive resections [2]. CCG-945 patients were stratified according to the EOR combining operative reports with postoperative imaging (CT) [7]. A radical resection (>90 %) was performed in only 37 %, but 49 % with tumors in the cerebral hemispheres and 45 % in the PF compared with 8 % of midline tumors underwent NTR (p < 0.0001). Only radical resection was associated with significantly improved PFS [7]. A single institution retrospective study of 39 children with pre- and post-operative MRI studies reported similar outcomes with a median OS of 122.2 months in patients with GTR compared with 14.1 in those with residual disease [8]. HIT-GBM-C reported a 63 % 5-year OS for patients with complete resection [9]. Although the EOR on CCG-945 represented the spectrum of neurosurgery offered in the late 1980s, this was prior to the wider availability and adoption of MRI scanning, stereotactic navigation, advanced neurophysiologic intraoperative monitoring and dedicated pediatric neurosurgeons, so it is likely that more aggressive neurosurgical management will have a positive impact on pediatric HGG in midline locations.
In this midline HGG cohort, neither chemotherapy regimen was superior, similar to the overall group [2]. Concurrent temozolomide during involved-field irradiation followed by adjuvant temozolomide has become the standard of care for adult GBM [10]. However, a recent COG trial for newly-diagnosed HGG using a similar treatment strategy (ACNS0126) did not improve outcomes compared to CCG-945 (p = 0.98) [11]. MGMT over-expression was adversely associated with survival [12]. A follow-up Phase I/II COG study using chemo-irradiation and an adjuvant chemotherapy regimen combining temozolomide with BCNU also did not improve outcomes [13]. Bevacizumab-containing regimens are under evaluation for both children with newly diagnosed and recurrent HGG [14].
When compared to the overall group, the midline location had poorer survival; however, tumors localized to the hypothalamus (10 of 60; 16.7 %) displayed a non-significant trend towards improved OS, whereas HGG originating in the thalamus (37 of 60; 61.7 %) or basal ganglia (6 of 60; 10 %) had worse outcomes (NS). Tumors that involved more than one contiguous site had significantly worse outcomes. The most likely explanation for the improved outcome for hypothalamic tumors is that 7 of 10 (70 %) were LGG after central review. Conversely, hypothalamic or optic chiasm patients with centrally reviewed HGG had no survivors; this observation has not been previously reported.
Central pathology review for children with HGG entered on treatment protocols is essential. Similar to the overall group, centrally reviewed AA histology correlated with improved PFS and OS when compared with GBM. Discordant diagnoses were evident in 21 of 60 patients (35 %), including revision of 19 of 39 AA and 1 of 14 GBM diagnoses to LGG. Of the other HGG, four AA were reclassified as GBM and five GBM were changed to AA. Patients with centrally reviewed midline LGG had significantly better outcomes on univariate analysis when compared with midline HGG. 70 of 250 patients entered on CCG-945 based upon institutional pathology were revised to LGG upon central review; 5-year PFS and OS were 63 and 79 % versus 19 and 22 %, respectively, in patients with confirmed HGG [15]. The most recent WHO guidelines post-dated our central re-review in 2003; a few additional HGG may have been reassigned to a LGG diagnosis [16]. Expedited central pathology review is therefore highly recommended prior to assignment of treatment on prospective HGG clinical trials [17, 18].
Pollack et al. retrospectively studied the proliferative indices of 98 eligible HGG from the overall CCG-945 cohort. The MIB-1 score was associated with 5-year EFS independent of tumor histology. MIB-1 labeling was a categorical variable and indices between 18 and 36 % had intermediate outcomes when compared to <18 or >36 % with better and worse prognoses, respectively [3]. In the midline HGG group, MIB-1 labeling >36 % had significantly worse PFS and OS when compared to patients with <36 % labeling; survival curves for tumors with indices from 18 to 36 % could not be distinguished from those with <18 % MIB-1 labeling.
Esteller et al. correlated MGMT inactivation with the response of adult HGG patients to alkylating agents [19]. Hegi et al. [20] retrospectively investigated adult GBM patients treated on an intergroup phase III RCT [10]. MGMT gene silencing significantly correlated with response to temozolomide and was an independent prognostic factor. Pollack et al. [4] studied 109 specimens from the entire CCG-945 group; all received alkylator therapy. Five-year PFS was 42.1 % in the 97 patients with low levels of MGMT expression versus 8.3 % in the 13 patients with MGMT over-expression (p < 0.02). Lee et al. assessed MGMT promoter methylation in a small cohort of 19 pediatric GBM and discovered low methylation rates (6–16 %) depending upon the laboratory assay used [21]. Buttarelli et al. found 7/24 pediatric HGG (30 %) had MGMT promoter methylation, whereas 19/28 (68 %) had MGMT protein over-expression [22]. However, using a methylation-specific polymerase chain reaction (PCR) assay, Srivastava et al. found that 10/20 pediatric GBM had MGMT promoter methylation [23].
MGMT expression was also an independent prognostic factor in this study. In the midline HGG cohort, the patients with MGMT over-expression had very poor outcomes; however, statistical significance could not be determined due to small patient numbers. Current clinical trials are prospectively assessing MGMT gene silencing and/or protein expression and future studies will stratify patients or assign treatments based on MGMT status. However, it may be necessary to assess both MGMT promoter methylation and protein expression in future pediatric HGG studies.
TP53 mutational status was assessed in 77 eligible tumor samples from the CCG-945 cohort; a higher frequency of TP53 mutations was found in 11.8 % of tumors from children under 3 years compared with 40 % in older children [24]. p53 expression (n = 115) as well as TP53 mutational status (n = 121) was then studied in a larger group of tumors from the initial CCG-945 cohort. p53 over-expression was significantly associated with adverse outcomes, independent of histology and other prognostic factors [5]. In the midline HGG cohort, only a few samples could be assessed for TP53 mutations; data was insufficient to generate statistical significance. However, in this group, 10/27 patients over-expressed p53 and had adverse PFS and OS when compared with patients without p53 over-expression. These results support assessing p53 expression status in pediatric HGG.
Other markers of pediatric HGG are being discovered using advanced cytogenetic approaches [25]. Comparing pediatric GBM to either normal controls or adult GBM, two subsets of pediatric GBM have been identified, one associated with Ras/Akt pathway activation having a poor prognosis [26–28]. Insights of the genomic alterations of adult GBM from The Cancer Genome Atlas Research Network [29] and other consortia [30] are being applied to pediatric GBM, leading to identification of IDH1 mutations in adolescent HGG [31], and driver mutations in histone variant H3.3 and chromatin remodeling genes in pediatric and young adult GBM [32–34]. These findings will improve tumor classification and identify targets and pathways for novel therapeutic approaches for pediatric HGG.
In summary, children treated on CCG-945 with midline HGG fare poorly when compared to the entire group. Although EOR is limited by midline anatomic location, improvements in imaging, intraoperative monitoring and surgical adjuncts and techniques may contribute to improved outcomes. Our study reinforces the importance of central pathology review. Since optimal adjuvant chemotherapy in pediatric HGG is undetermined, patient enrolment on clinical trials remains a priority to improve outcomes.
References
Sposto R, Ertel IJ, Jenkin RD, Boesel CP, Venes JL, Ortega JA, Evans AE, Wara W, Hammond D (1989) The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: results of a randomized trial. A report from the Children’s Cancer Study Group. J Neurooncol 7:165–177
Finlay JL, Boyett JM, Yates AJ, Wisoff JH, Milstein JM, Geyer JR, Bertolone SJ, McGuire P, Cherlow JM, Tefft M et al (1995) Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. Children’s Cancer Group. J Clin Oncol 13:112–123
Pollack IF, Hamilton RL, Burnham J, Holmes EJ, Finkelstein SD, Sposto R, Yates AJ, Boyett JM, Finlay JL (2002) Impact of proliferation index on outcome in childhood malignant gliomas: results in a multi-institutional cohort. Neurosurgery 50:1238–1244 discussion 1244–1235
Pollack IF, Hamilton RL, Sobol RW, Burnham J, Yates AJ, Holmes EJ, Zhou T, Finlay JL (2006) O6-methylguanine-DNA methyltransferase expression strongly correlates with outcome in childhood malignant gliomas: results from the CCG-945 Cohort. J Clin Oncol 24:3431–3437. doi:10.1200/JCO.2006.05.7265
Pollack IF, Finkelstein SD, Woods J, Burnham J, Holmes EJ, Hamilton RL, Yates AJ, Boyett JM, Finlay JL, Sposto R (2002) Expression of p53 and prognosis in children with malignant gliomas. N Engl J Med 346:420–427. doi:10.1056/NEJMoa012224
Lee ET (1992) Non-parametric methods of estimating survival functions. Wiley, New York
Wisoff JH, Boyett JM, Berger MS, Brant C, Li H, Yates AJ, McGuire-Cullen P, Turski PA, Sutton LN, Allen JC, Packer RJ, Finlay JL (1998) Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: a report of the Children’s Cancer Group trial no. CCG-945. J Neurosurg 89:52–59
Bucci MK, Maity A, Janss AJ, Belasco JB, Fisher MJ, Tochner ZA, Rorke L, Sutton LN, Phillips PC, Shu HK (2004) Near complete surgical resection predicts a favorable outcome in pediatric patients with non brainstem, malignant gliomas: results from a single center in the magnetic resonance imaging era. Cancer 101:817–824. doi:10.1002/cncr.20422
Wolff JE, Driever PH, Erdlenbruch B, Kortmann RD, Rutkowski S, Pietsch T, Parker C, Metz MW, Gnekow A, Kramm CM (2010) Intensive chemotherapy improves survival in pediatric high-grade glioma after gross total resection: results of the HIT-GBM-C protocol. Cancer 116:705–712. doi:10.1002/cncr.24730
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi:10.1056/NEJMoa043330
Cohen KJ, Pollack IF, Zhou T, Buxton A, Holmes EJ, Burger PC, Brat DJ, Rosenblum MK, Hamilton RL, Lavey RS, Heideman RL (2011) Temozolomide in the treatment of high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro Oncol 13:317–323. doi:10.1093/neuonc/noq191
Broniscer A, Chintagumpala M, Fouladi M, Krasin MJ, Kocak M, Bowers DC, Iacono LC, Merchant TE, Stewart CF, Houghton PJ, Kun LE, Ledet D, Gajjar A (2006) Temozolomide after radiotherapy for newly diagnosed high-grade glioma and unfavorable low-grade glioma in children. J Neurooncol 76:313–319. doi:10.1007/s11060-005-7409-5
Jakacki RI, Yates A, Blaney SM, Zhou T, Timmerman R, Ingle AM, Flom L, Prados MD, Adamson PC, Pollack IF (2008) A phase I trial of temozolomide and lomustine in newly diagnosed high-grade gliomas of childhood. Neuro Oncol 10:569–576. doi:10.1215/15228517-2008-019
Parekh C, Jubran R, Erdreich-Epstein A, Panigrahy A, Bluml S, Finlay J, Dhall G (2011) Treatment of children with recurrent high grade gliomas with a bevacizumab containing regimen. J Neurooncol 103:673–680. doi:10.1007/s11060-010-0444-x
Fouladi M, Hunt DL, Pollack IF, Dueckers G, Burger PC, Becker LE, Yates AJ, Gilles FH, Davis RL, Boyett JM, Finlay JL (2003) Outcome of children with centrally reviewed low-grade gliomas treated with chemotherapy with or without radiotherapy on Children’s Cancer Group high-grade glioma study CCG-945. Cancer 98:1243–1252. doi:10.1002/cncr.11637
Louis DNOH, Wiestler OD, Cavenee WK (eds) (2007) World Health Organization classification of tumours of the central nervous system. IARC Press, Lyon
Pollack IF, Boyett JM, Yates AJ, Burger PC, Gilles FH, Davis RL, Finlay JL (2003) The influence of central review on outcome associations in childhood malignant gliomas: results from the CCG-945 experience. Neuro Oncol 5:197–207. doi:10.1215/S1152-8517-03-00009-7
Gilles FH, Tavare CJ, Becker LE, Burger PC, Yates AJ, Pollack IF, Finlay JL (2008) Pathologist interobserver variability of histologic features in childhood brain tumors: results from the CCG-945 study. Pediatr Dev Pathol 11:108–117. doi:10.2350/07-06-0303.1
Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG (2000) Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med 343:1350–1354. doi:10.1056/NEJM200011093431901
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. doi:10.1056/NEJMoa043331
Lee JY, Park CK, Park SH, Wang KC, Cho BK, Kim SK (2011) MGMT promoter gene methylation in pediatric glioblastoma: analysis using MS-MLPA. Childs Nerv Syst 27:1877–1883. doi:10.1007/s00381-011-1525-7
Buttarelli FR, Massimino M, Antonelli M, Lauriola L, Nozza P, Donofrio V, Arcella A, Oliva MA, Di Rocco C, Giangaspero F (2010) Evaluation status and prognostic significance of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation in pediatric high grade gliomas. Childs Nerv Syst 26:1051–1056. doi:10.1007/s00381-010-1191-1
Srivastava A, Jain A, Jha P, Suri V, Sharma MC, Mallick S, Puri T, Gupta DK, Gupta A, Sarkar C (2010) MGMT gene promoter methylation in pediatric glioblastomas. Childs Nerv Syst 26:1613–1618. doi:10.1007/s00381-010-1214-y
Pollack IF, Finkelstein SD, Burnham J, Holmes EJ, Hamilton RL, Yates AJ, Finlay JL, Sposto R (2001) Age and TP53 mutation frequency in childhood malignant gliomas: results in a multi-institutional cohort. Cancer Res 61:7404–7407
Pollack IF, Hamilton RL, James CD, Finkelstein SD, Burnham J, Yates AJ, Holmes EJ, Zhou T, Finlay JL (2006) Rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: results from the Children’s Cancer Group 945 cohort. J Neurosurg 105:418–424. doi:10.3171/ped.2006.105.5.418
Faury D, Nantel A, Dunn SE, Guiot MC, Haque T, Hauser P, Garami M, Bognar L, Hanzely Z, Liberski PP, Lopez-Aguilar E, Valera ET, Tone LG, Carret AS, Del Maestro RF, Gleave M, Montes JL, Pietsch T, Albrecht S, Jabado N (2007) Molecular profiling identifies prognostic subgroups of pediatric glioblastoma and shows increased YB-1 expression in tumors. J Clin Oncol 25:1196–1208. doi:10.1200/JCO.2006.07.8626
Haque T, Faury D, Albrecht S, Lopez-Aguilar E, Hauser P, Garami M, Hanzely Z, Bognar L, Del Maestro RF, Atkinson J, Nantel A, Jabado N (2007) Gene expression profiling from formalin-fixed paraffin-embedded tumors of pediatric glioblastoma. Clin Cancer Res 13:6284–6292. doi:10.1158/1078-0432.CCR-07-0525
Pollack IF, Hamilton RL, Burger PC, Brat DJ, Rosenblum MK, Murdoch GH, Nikiforova MN, Holmes EJ, Zhou T, Cohen KJ, Jakacki RI (2010) Akt activation is a common event in pediatric malignant gliomas and a potential adverse prognostic marker: a report from the Children’s Oncology Group. J Neurooncol 99:155–163. doi:10.1007/s11060-010-0297-3
McLendon R et al (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068. doi:10.1038/nature07385
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812. doi:10.1126/science.1164382
Pollack IF, Hamilton RL, Sobol RW, Nikiforova MN, Lyons-Weiler MA, LaFramboise WA, Burger PC, Brat DJ, Rosenblum MK, Holmes EJ, Zhou T, Jakacki RI (2011) IDH1 mutations are common in malignant gliomas arising in adolescents: a report from the Children’s Oncology Group. Childs Nerv Syst 27:87–94. doi:10.1007/s00381-010-1264-1
Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tonjes M, Hovestadt V, Albrecht S, Kool M, Nantel A, Konermann C, Lindroth A, Jager N, Rausch T, Ryzhova M, Korbel JO, Hielscher T, Hauser P, Garami M, Klekner A, Bognar L, Ebinger M, Schuhmann MU, Scheurlen W, Pekrun A, Fruhwald MC, Roggendorf W, Kramm C, Durken M, Atkinson J, Lepage P, Montpetit A, Zakrzewska M, Zakrzewski K, Liberski PP, Dong Z, Siegel P, Kulozik AE, Zapatka M, Guha A, Malkin D, Felsberg J, Reifenberger G, von Deimling A, Ichimura K, Collins VP, Witt H, Milde T, Witt O, Zhang C, Castelo-Branco P, Lichter P, Faury D, Tabori U, Plass C, Majewski J, Pfister SM, Jabado N (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482:226–231. doi:10.1038/nature10833
Sturm D, Bender S, Jones DT, Lichter P, Grill J, Becher O, Hawkins C, Majewski J, Jones C, Costello JF, Iavarone A, Aldape K, Brennan CW, Jabado N, Pfister SM (2014) Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat Rev Cancer 14:92–107. doi:10.1038/nrc3655
Bender S, Tang Y, Lindroth AM, Hovestadt V, Jones DT, Kool M, Zapatka M, Northcott PA, Sturm D, Wang W, Radlwimmer B, Hojfeldt JW, Truffaux N, Castel D, Schubert S, Ryzhova M, Seker-Cin H, Gronych J, Johann PD, Stark S, Meyer J, Milde T, Schuhmann M, Ebinger M, Monoranu CM, Ponnuswami A, Chen S, Jones C, Witt O, Collins VP, von Deimling A, Jabado N, Puget S, Grill J, Helin K, Korshunov A, Lichter P, Monje M, Plass C, Cho YJ, Pfister SM (2013) Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell 24:660–672. doi:10.1016/j.ccr.2013.10.006
Acknowledgments
Biological analyses were supported by RO1NS37704 (IFP).
Conflict of interest
None of the study authors declares any conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Sapp M, Boyett JM, Yates AJ, Burger P, Becker L, Davies R, Gilles F and Finlay JL. Outcome for children with midline primary site high grade astrocytoma (HGA) treated on the Childrens Cancer Group Trial (CCG-945). Oral presentation: Seventh International Symposium on Pediatric Neuro-oncology, Washington DC, J Neuro-oncol 1996; 33:269.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Eisenstat, D.D., Pollack, I.F., Demers, A. et al. Impact of tumor location and pathological discordance on survival of children with midline high-grade gliomas treated on Children’s Cancer Group high-grade glioma study CCG-945. J Neurooncol 121, 573–581 (2015). https://doi.org/10.1007/s11060-014-1669-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1669-x