Abstract
Vemurafenib is indicated for the treatment of patients with BRAF V600-mutant metastatic melanoma. We studied for the first time the characteristics of brain metastases developed during treatment with vemurafenib in real-life conditions. We included all patients treated over 3 years with vemurafenib in our department for metastatic melanoma without initial brain involvement. Our primary endpoint was to assess the incidence of brain metastases in these patients. Our secondary endpoints were to identify the risk factors for metastases occurrence and their characteristics and course. In our retrospective cohort of 86 patients, 20 % had developed brain metastases on average 5.3 months after vemurafenib initiation. The median follow-up was 9 months (1–26 months). Radiological examinations revealed multiple brain metastases in 41 % of patients. The only risk factor for metastasis occurrence identified was a high number of metastatic sites when initiating vemurafenib (p = 0.045). Metastasis development was associated with a trend toward a decrease in overall survival from 12.8 to 8.5 months (p = 0.07) and a significant decrease in progression-free survival from 7 to 5 months (p = 0.04). Among the patients who developed brain metastases, 82 % died, of whom 64 % within 3 months, versus 58 % of patients without brain metastases over the same period. The extra-cerebral disease was well controlled in 59 % of patients during brain progression. In vemurafenib-treated melanoma patients, brain metastases are frequent and associated with a particularly poor prognosis. Because of their high frequency in patients with controlled extra-cerebral disease, brain explorations should be systematically performed during treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In adults, melanoma is the primary tumor associated with the highest propensity to metastasize to the brain. Brain metastases are diagnosed in 7–10 % of patients with melanoma regardless of the stage, including 40–50 % of patients with stage IV metastatic melanoma (Sixth AJCC classification), and autopsies have shown metastases in up to 75 % of patients died from melanoma [1–3]. Main risk factors for brain metastases include the male sex, mucosal malignant melanoma, location to the head or neck, and a high Breslow thickness. They are most often multiple at the time of diagnosis. However, performing more systematically radiological evaluations has allowed diagnosing more frequently asymptomatic metastases, in particular by MRI. They are associated with a poor prognosis: they are fatal in 95 % of patients, and the estimated median survival after occurrence is of 3–6 months [2, 4–6]. Recent therapeutic advances in vemurafenib, ipilimumab and more recently dabrafenib therapies have improved patient survival, leading to a higher risk of brain metastases. Some studies suggest that these new treatments could be effective on brain metastases with intracranial response rates of 16–50 % under vemurafenib, 31–39 % under dabrafenib and 5–25 % under ipilimumab [7]. Previously, the efficacy of chemotherapy has been shown in only less than 10 % of patients, without impacting the progression-free or overall survival [6, 7]. Whole brain radiotherapy, alone is not efficient. Conversely, the local control is improved at 1 year with stereotactic radiosurgery in up to 75 % of patients with only few small metastases, without any benefit outside of the radiation field. Management of brain metastases remains therefore a major therapeutic issue.
The BRAF oncogene encodes the protein kinase BRAF which belongs to the mitogen-activated protein kinase (MAPK) pathway and controls cell proliferation, differentiation, growth, survival and apoptosis [5]. An activating BRAF mutation is found in 60 % of cutaneous melanomas and corresponds to the V600E mutation in 75 % of cases. Vemurafenib, a BRAF-targeted therapy, is indicated for unresectable stage III or IV BRAF V600-mutant melanomas (Sixth AJCC classification). We report for the first time the incidence and progressive features of brain metastases in melanoma patients treated with vemurafenib.
Patients and methods
Patients
We included all patients treated with vemurafenib from November 2010 to November 2013 in the Dermato-Cancerology Department of Nantes University Hospital for BRAF V600-mutated metastatic melanoma. Exclusion criteria were: melanomas with brain involvement before treatment initiation and the absence of the first assessment scan in patients treated for less than 2 months. The initial dose of vemurafenib was 960 mg twice daily, with adaptation in case of toxicity according to the recommendations of the Summary of Product Characteristics.
Assessments
Patients underwent systematic tumor assessment through brain, chest, abdominal and pelvic scan before vemurafenib initiation, at month 2, and every 3 months thereafter. Brain imaging was also performed at the onset of neurological symptoms. Diagnosis of brain metastases was based on scan findings, sometimes completed with a MRI in case of doubt or stereotactic radiotherapy indication. Tumor responses were determined according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.
BRAF mutations were detected using an allele specific amplification completed in all cases by conventional Sanger DNA sequencing.
Statistics
Our primary endpoint was to assess the incidence of brain metastases developed during treatment with vemurafenib. Our secondary endpoints were to assess the median overall survival after development of brain metastases, the time between their development and the death, the median overall and progression-free survival in patients with and without brain metastases, the predictive factors for brain metastasis development, the time to develop following vemurafenib initiation, the diagnostic modalities, the number of metastases at the time of diagnosis seen on brain imaging, the type of extra-cerebral response at the time of brain progression, the efficacy of treatments initiated after brain progression and their incidence according to the type of V600 mutation. Progression-free survival was measured until development of relapse regardless of the site. Patients were included in the group “without brain metastases” when they developed brain metastases after vemurafenib cessation for extra-cerebral progression.
The statistical tests used were a χ2 or Fisher’s exact test depending on the sample size for qualitative data, a Wilcoxon–Mann–Whitney test for quantitative data and a log-rank test for survival analyzes. Differences were considered statistically significant if the p value was less than 0.05. All analyzes were performed using MedCalc software version 12.7.7.
Results
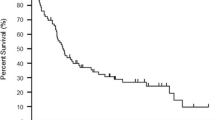
From November 2010 to November 2013, 86 patients were included with a 9-month median follow-up (1–26 months). Seventeen (19.8 %) patients developed brain metastases during treatment of whom 14 (82 %) died after a median time of 2.7 months (0.2–7.2 months), including four within 1 month following the diagnosis and 9 within 3 months (Fig. 1). The three patients still alive have a follow-up of 2, 7, and 10 months since the diagnosis of brain metastases. By comparison, over the same period, among the 69 patients treated with vemurafenib who did not develop brain metastases, only 58 % died. There was a trend toward a decrease in overall survival in patients with brain metastases compared to patients without brain involvement (p = 0.07) with respectively a median survival of 8.5 months (95 % CI: 4.5–11.5) and 12.8 months (95 % CI: 9.2–16.7) (Fig. 2a). The progression-free survival was significantly shorter in patients with brain metastases than in patients without brain involvement (p = 0.04) with respectively a median progression-free survival of 5 months (95 % CI: 3–8) and 7 months (95 % CI: 5–8) (Fig. 2b).
There was no significant difference in terms of characteristics between vemurafenib-treated patients with and without brain metastases regarding the gender, age, histological subtype of primary melanoma, its Breslow thickness, its ulceration status, not knowing the primary site or the number of previous therapeutic lines received at the metastatic stage (Table 1). Only the number of metastatic sites at the time of vemurafenib initiation was significantly associated with the development of brain metastases under vemurafenib (p = 0.045). Four patients without brain metastases experienced ipilimumab failure before vemurafenib initiation versus no patient with brain metastases.
Brain metastases developed after mean treatment duration of 5.3 months (±4.3) (1–15 months) (Fig. 3). The diagnosis was based on a systematic follow-up scan in 15 patients and on imaging requested because of the onset of neurological symptoms in two patients. Brain scan revealed no metastases in one case, one in nine cases, two to three in five cases and four or more in two cases. MRI revealed one metastases in two cases, two to three in four cases and four or more in six cases. In one patient, the scan performed because of neurological symptoms was normal while the MRI revealed one brain metastases and carcinomatous meningitis. No other carcinomatous meningitis was diagnosed. On imaging, multiple, mostly small brain metastases were diagnosed in 41 % of patients, based on scan and MRI findings in three and four patients, respectively. Among the 12 patients who performed both tests, the MRI was more sensitive in 11 patients. The number of brain metastases at the time of diagnosis was not significantly associated with the overall survival (p = 0.25) (Fig. 4a).
A discrepancy was frequently observed between the cerebral and extra-cerebral response to vemurafenib. Indeed, the extra-cerebral disease was controlled in 59 % of patients at the time of brain progression with six patients in partial remission and four stabilized. Death was considered as directly related to the brain progression in 71 % of cases. The best response rates for extra-cerebral metastases were similar between both groups: 0 versus 9 % of complete remission, 47 versus 44 % of partial remission and 24 versus 19 % of stable disease respectively in patients with and without brain metastases. The importance of the extra-cerebral response was not significantly associated with the overall survival (p = 0.75) (Fig. 4b).
After development of brain metastases, six patients received palliative care, nine received chemotherapy with fotemustine and dacarbazine, and two received ipilimumab. One patient under fotemustine and dacarbazine and one under ipilimumab received their treatment in association with stereotactic brain radiotherapy. No patient responded to this first-line brain metastatic therapy. The patient treated with fotemustine and dacarbazine combined with stereotactic radiotherapy was the only one to receive a second-line therapy with vemurafenib resumption after a 3-month discontinuation, which is still ongoing. Vemurafenib resumption resulted in partial remission of brain, lung and axillary lymph node metastases at 3 months, which was maintained at 6 and 9 months.
In our cohort, 93 % of patients carried a BRAF V600E mutation: 20 % developed brain metastases, of whom 81 % were dead at the time of analysis. Among the five patients with V600K mutation, only one experienced a rapidly fatal brain progression at 3 months, associated with an extra-cerebral progressive disease. Another patient developed brain metastases 4 months after cessation of a 10 month-vemurafenib treatment, with a fatal outcome within 3 months following the diagnosis. The two remaining patients are still alive 5 and 9 months after vemurafenib initiation. The only patient carrying a V600R mutation is still alive 17 months after vemurafenib initiation, without development of brain metastases.
Discussion
Our study was the first to investigate the progressive features of brain metastases developed under vemurafenib in a cohort of patients whose treatment had been initiated for extra-cerebral metastases. Twenty percent of our patients developed brain metastases, which is consistent with the 29 % reported previously in the phase I study of vemurafenib [8]. Among the clinical criteria, only the high number of metastatic sites at the time of treatment initiation was a predictive factor for brain metastasis development (p = 0.045). Eighty-eight percent of diagnoses of brain involvement were based on systematic imaging in asymptomatic patients. Scan or MRI revealed particularly severe clinical forms in about half of patients with brain involvement: multiple, mostly small brain metastases in 41 % of patients and carcinomatous meningitis in 6 %. However, the small size of our subgroup of patients with brain metastases (17 patients) should be taken into account when interpreting these findings. The overall survival of these patients was not associated with the number of brain metastases at the time of diagnosis (p = 0.25). We cannot conclude if this finding is due to a too small size of our subgroup of patients or to a real absence of impact of the number of brain metastases. Our patients died soon after diagnosis: a quarter within 1 month and two thirds within 3 months. The development of brain metastases under vemurafenib seems therefore to be associated with a very poor prognosis, but it is not possible to conclude whether it is due to the presence of a BRAF mutation, to the usual poor prognosis of melanoma brain metastases or to the fact that they developed during vemurafenib treatment. By comparison, a median overall survival after diagnosis of brain metastases of 5.92 months was reported in 2011 in a large retrospective study in melanoma patients treated since 1996 with chemotherapy, radiotherapy or supportive care [4]. An estimated 3.7-month survival was found in patients with brain metastases developed under chemotherapy. To our knowledge, no study has been published about the prognosis of melanoma patients with brain metastases since the introduction of targeted therapies and BRAF mutational status analysis. Four published clinical cases reported the clinical course of BRAF inhibitor-treated patients who developed brain metastases: two cases with fatal outcome two weeks after diagnosis of brain metastases under vemurafenib [9, 10], one death a few days after diagnosis of carcinomatous meningitis under dabrafenib while the BRAF V600E mutation was still present in the cerebrospinal fluid [11] and one well-controlled case with leptomeningeal metastases with an 18-month follow-up after vemurafenib continuation and addition of whole-brain radiotherapy [12].
The poor prognostic value of brain metastases developed under vemurafenib was also confirmed in our cohort by the high mortality rate (82 %) after a median follow-up of 9 months versus 58 % for patients without brain metastases. The development of brain metastases was associated with a trend toward a decrease in overall survival from 12.8 to 8.5 months (p = 0.07) and a significant decrease in progression-free survival from 7 to 5 months (p = 0.04). In our cohort, vemurafenib seemed less effective in the brain: 59 % of our patients with brain metastases development had well-controlled extra-cerebral involvement, with 60 % of partial remission and 40 % of stable disease. Similar dissociated courses have been previously published [9, 10]. Death was directly related to brain metastases in 71 % of patients. The overall survival of these patients was not related to the type of extra-cranial response (p = 0.75). These data show that the prognosis of these patients depends primarily on their brain progression, regardless of their extra-cerebral melanoma severity. In our cohort, no patient with brain metastases responded to fotemustine and dacarbazine or ipilimumab, regardless of their association with stereotactic radiotherapy. No conclusion can be drawn on the efficacy of these different treatments after vemurafenib, given that only 11 patients could be treated after brain metastases development. Treatment for brain metastases was chosen in our patients according to patient general condition and the possibility to be included in clinical research protocols, because, in France, ipilimumab is only available in routine practice for patients without BRAF V600 mutations. Only one patient received a second-line therapy for his brain metastases and was stabilized from the third month following vemurafenib resumption, the latter having been discontinued 3 months before because of brain progression. Among the 7 % of patients who did not carry a V600E mutation, one in five patients with V600K mutation experienced a rapidly fatal brain evolution at 3 months and the patient with V600R mutation had no brain progression.
In our cohort, brain metastases developed after mean treatment duration of 5.3 months and 59 % were associated with a well-controlled extra-cerebral disease, raising the question of brain-specific and unspecific resistance mechanisms. Under vemurafenib, secondary resistance is most often associated with a reactivation of the MAPK pathway, mediated by specific BRAF aberrations [13, 14], switches between RAF isoforms [15], neomutations in NRAS and MEK [16] and the increased expression of a “partner” kinase, COT [5, 17]. In rarer cases, BRAF-independent signaling pathways such as insulin growth factor 1 receptor (IGF1R)—phosphatidylinositol-3 kinase (PI3K) [18, 19] or platelet-derived growth factor β (PDGFRβ) pathways are activated [11, 20]. Brain-specific phenomena have also been shown from tumor samples of melanoma patients with over-activation of the AKT survival pathway and loss of PTEN expression, induced by the brain environment, contributing to melanoma cell survival in the brain parenchyma [21].
Furthermore, vemurafenib physico-chemical properties suggest that this molecule could hardly cross the blood–brain barrier because of its high molecular weight (489.9 g/mol) and poor liposolubility [10]. No pharmacokinetic study has been conducted in humans in the cerebrospinal fluid or extracellular fluid of the brain parenchyma [2]. However, the discrepancy between the efficacy of BRAF inhibitors for treating brain metastases present before treatment initiation and those which developed during treatment [22, 23] could be explained by an altered blood–brain barrier permeability [10], although the consequences on the intra-cerebral drug diffusion remain poorly understood [24]. The encouraging results of the combination of vemurafenib and radiotherapy could also be due to a transient radiation-induced impairment of the blood–brain barrier, facilitating vemurafenib intra-cerebral diffusion [25].
Finally, it has been shown in vivo in mice that vemurafenib was actively excreted by the P-glycoprotein and breast cancer resistance protein efflux pumps, reducing significantly its passage through the blood–brain barrier [26]. The inhibition of both pumps in vitro restored vemurafenib intracellular accumulation. Vemurafenib intra-cerebral level was 30–225 times higher in mice carrying mutations in both pumps than in wild-type mice or mice carrying mutations in only one pump, both after a single intravenous or oral and a continuous intraperitoneal vemurafenib administration [26]. A second study confirmed in vitro and in vivo in mice the role of these two pumps in vemurafenib transport, with a 21.4-fold increase in the brain/plasma level ratio in mice carrying mutations in both pumps while the levels were barely increased in mice carrying mutations in only one pump [27]. In addition, the plasma concentrations were increased by 6.6 after a single vemurafenib oral administration in mice carrying mutations in both pumps, also suggesting their role in vemurafenib intestinal absorption. The oral coadministration of vemurafenib and lacridar, which inhibits both efflux pumps, also allowed achieving similar plasma and brain concentrations than in mice carrying mutations in both pumps. Radiotherapy could also increase vemurafenib intracellular concentration by inhibiting these two pumps [6].
Paradoxically, the potential benefit of BRAF inhibitors on brain metastases is currently being assessed in six clinical trials identified on December 16, 2013 on the website clinicaltrials.gov (vemurafenib: NTC01378975, NCT01781026, NCT01983124; dabrafenib: NCT01721603, NCT01978236, NCT01677741). While data of the first studies did not allow drawing conclusions on vemurafenib intra-cerebral efficacy, various studies have shown in patients with V600E mutation an intracranial response rate of 16–50 %, with a progression-free survival of 3.9–4.6 months and an overall survival of 5–8 months, increased up to 13.7 months in a series of 12 patients in association with radiotherapy. The 6-month local control rate was of 75 % in this series [7, 25, 28–30]. Patients with V600K mutation seem to be less responsive with an estimated progression-free survival of 2–4 months and overall survival of 4–5 months. Vemurafenib and radiotherapy seem to have a synergistic effect for both efficacy [31] and toxicity [32], raising the question of the therapeutic interest of their combined use. Data on vemurafenib efficacy in patients with pre-existing brain metastases cannot be compared with those of our cohort of patients who developed brain metastases under vemurafenib: the presence of the metastases themselves before treatment initiation modifies the blood–brain barrier permeability. Dabrafenib, another BRAF inhibitor, is also effective to treat melanoma brain metastases with an intracranial response rate of 31–39 % depending on the treatment line, a progression-free survival of 4 months and an overall survival of 7–8 months for patients with V600E mutation [33]. As under vemurafenib, response rates are lower for patients carrying the V600K mutation, estimated between 7 and 22 %, with a progression-free survival of 2–4 months and an overall survival of 4–5 months. No study has compared both molecules, so it is not possible to determine which drug is the most effective on brain metastases [33]. In recent studies, BRAF inhibitors seem to be more efficient than chemotherapy, whole brain radiotherapy or best supportive care for patients with brain metastases, which is consistent with the superiority of these drugs on extra-cerebral metastases [6, 7]. Stereotactic radiosurgery is another effective treatment, but intended for few small metastases, which can be a limit in melanoma. Therefore, the best treatment sequence and the interest of combining treatments still need to be clarified.
Conclusion
In our cohort of vemurafenib-treated patients, one in five patients developed brain metastases, mostly asymptomatic at the time of diagnosis. Their development was associated with a very poor prognosis and was fatal in more than 80 % of cases, mostly within the 3 months following the diagnosis. Because of brain metastases frequency and severity, brain evaluations should be systematically performed in patients treated with vemurafenib. Brain MRI should be preferred to a scan because about half of patients had a particularly severe clinical form, with multiple, mostly small brain metastases, whose diagnosis could only be established by MRI in four out of seven patients. Interestingly, the extra-cerebral disease was controlled in most patients during brain progression, suggesting brain-specific resistance phenomena whose exploration could help opening new therapeutic strategies.
References
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22(14):2865–2872
Fonkem E, Uhlmann EJ, Floyd SR, Mahadevan A, Kasper E, Eton O, Wong ET (2012) Melanoma brain metastases: overview of current management and emerging targeted therapies. Expert Rev Neurother 12(10):1207–1215
Preusser M, Capper D, Ilhan-Mutlu A, Berghoff AS, Birner P, Bartsch R, Marosi C, Zielinski C, Mehta MP, Winkler F, Wick W, von Deimling A (2012) Brain metastases: pathobiology and emerging targeted therapies. Acta Neuropathol 123(2):205–222
Davies MA, Liu P, McIntyre S, Kim KB, Papadopoulos N, Hwu WJ, Hwu P, Bedikian A (2011) Prognostic factors for survival in melanoma patients with brain metastases. Cancer 117(8):1687–1696
Menzies AM, Long GV, Murali R (2012) Dabrafenib and its potential for the treatment of metastatic melanoma. Drug Des Devel Ther 6:391–405
Murrell J, Board R (2013) The use of systemic therapies for the treatment of brain metastases in metastatic melanoma: Opportunities and unanswered questions. Cancer Treat Rev 39(8):833–838
Long GV, Margolin KA (2013) Multidisciplinary approach to brain metastases from melanoma. Am Soc Clin Oncol Educ Book 2013:393–398
Kim KB, Flaherty KT, Chapman PB, Sosman JA, Ribas A, McArthur GA, Amaravadi RK, Lee RJ, Nolop KB, Puzanov I (2011) Pattern and outcome of disease progression in phase I study of vemurafenib in patients with metastatic melanoma (MM). J Clin Oncol 29(15):1628–1634
Maleka A, Enblad G, Sjors G, Lindqvist A, Ullenhag GJ (2013) Treatment of metastatic malignant melanoma with vemurafenib during pregnancy. J Clin Oncol 31(11):e192–e193
Rochet NM, Dronca RS, Kottschade LA, Chavan RN, Gorman B, Gilbertson JR, Markovic SN (2012) Melanoma brain metastases and vemurafenib: need for further investigation. Mayo Clin Proc 87(10):976–981
Simeone E, De Maio E, Sandomenico F, Fulciniti F, Lastoria S, Aprea P, Staibano S, Montesarchio V, Palmieri G, Mozzillo N, Ascierto PA (2012) Neoplastic leptomeningitis presenting in a melanoma patient treated with dabrafenib (a V600EBRAF inhibitor): a case report. J Med Case Rep 6(1):131
Lee JM, Mehta UN, Dsouza LH, Guadagnolo BA, Sanders DL, Kim KB (2013) Long-term stabilization of leptomeningeal disease with whole-brain radiation therapy in a patient with metastatic melanoma treated with vemurafenib: a case report. Melanoma Res 23(2):175–178
Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, Shi H, Atefi M, Titz B, Gabay MT, Salton M, Dahlman KB, Tadi M, Wargo JA, Flaherty KT, Kelley MC, Misteli T, Chapman PB, Sosman JA, Graeber TG, Ribas A, Lo RS, Rosen N, Solit DB (2011) RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 480(7377):387–390
Shi H, Moriceau G, Kong X, Lee MK, Lee H, Koya RC, Ng C, Chodon T, Scolyer RA, Dahlman KB, Sosman JA, Kefford RF, Long GV, Nelson SF, Ribas A, Lo RS (2012) Melanoma whole-exome sequencing identifies (V600E)B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat Commun 3:724
Montagut C, Sharma SV, Shioda T, McDermott U, Ulman M, Ulkus LE, Dias-Santagata D, Stubbs H, Lee DY, Singh A, Drew L, Haber DA, Settleman J (2008) Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res 68(12):4853–4861
Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, Kehoe SM, Johannessen CM, Macconaill LE, Hahn WC, Meyerson M, Garraway LA (2011) Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol 29(22):3085–3096
Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J, Caponigro G, Hieronymus H, Murray RR, Salehi-Ashtiani K, Hill DE, Vidal M, Zhao JJ, Yang X, Alkan O, Kim S, Harris JL, Wilson CJ, Myer VE, Finan PM, Root DE, Roberts TM, Golub T, Flaherty KT, Dummer R, Weber BL, Sellers WR, Schlegel R, Wargo JA, Hahn WC, Garraway LA (2010) COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 468(7326):968–972
Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, Wubbenhorst B, Xu X, Gimotty PA, Kee D, Santiago-Walker AE, Letrero R, D’Andrea K, Pushparajan A, Hayden JE, Brown KD, Laquerre S, McArthur GA, Sosman JA, Nathanson KL, Herlyn M (2010) Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3 K. Cancer Cell 18(6):683–695
Smalley KS, Sondak VK (2010) Melanoma–an unlikely poster child for personalized cancer therapy. Engl J Med 363(9):876–878
Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, Chodon T, Nelson SF, McArthur G, Sosman JA, Ribas A, Lo RS (2010) Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 468(7326):973–977
Meier F, Forschner A, Klumpp B, Flaherty KT, Honegger JB, Witte M, Bornemann A, Dummer R, Bauer J, Tabatabai G, Weide B, Eigentler TK, Schadendorf D, Quintanilla-Fend L, Niessner H, Garbe C (2013) Targeting hyperactivation of the AKT survival pathway to overcome therapy resistance of melanoma brain metastases Cancer Med 2(1):76–85
Mittapalli RK, Vaidhyanathan S, Dudek AZ, Elmquist WF (2013) Mechanisms limiting distribution of the threonine-protein kinase B-RaF(V600E) inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J Pharmacol Exp Ther 344(3):655–664
Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, Hamid O, Infante JR, Millward M, Pavlick AC, O’Day SJ, Blackman SC, Curtis CM, Lebowitz P, Ma B, Ouellet D, Kefford RF (2012) Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 379(9829):1893–1901
Fortin D (2012) The blood-brain barrier: its influence in the treatment of brain tumors metastases. Curr Cancer Drug Targets 12(3):247–259
Narayana A, Mathew M, Tam M, Kannan R, Madden KM, Golfinos JG, Parker EC, Ott PA, Pavlick AC (2013) Vemurafenib and radiation therapy in melanoma brain metastases. J Neurooncol 113(3):411–416
Mittapalli RK, Vaidhyanathan S, Sane R, Elmquist WF (2012) Impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on the brain distribution of a novel BRAF inhibitor: vemurafenib (PLX4032). J Pharmacol Exp Ther 342(1):33–40
Durmus S, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH (2012) Oral availability and brain penetration of the B-RAFV600E inhibitor vemurafenib can be enhanced by the P-GLYCOprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Mol Pharm 9(11):3236–3245
Harding JJ, Catalanotti F, Yaqubie A, McDermott GC, Kersellius R, Merghoub T (2013) Vemurafenib (VEM) in patients (pts) with BRAF-mutant melanoma and brain metastases (mets). J Clin Oncol 31:9060
Dzienis MR, Atkinson VG (2014).Response rate to vemurafenib in patients with B-RAF-positive melanoma brain metastases. Melanoma Res 24(4):349–353
Dummer R, Goldinger SM, Turtschi CP, Eggmann NB, Michielin O, Mitchell L, Veronese L, Hilfiker PR, Felderer L, Rinderknecht JD (2014) Vemurafenib in patients with BRAF mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur J Cancer 50(3):611–621
Baroudjian B, Boussemart L, Routier E, Dreno B, Tao Y, Deutsch E, Blanchard P, Dhermain F, Vilcot L, Vagner S, Eggermont A, Mateus C, Robert C (2014) Dramatic response to radiotherapy combined with vemurafenib. Is vemurafenib a radiosensitizer? Eur J Dermatol 24(2):265–267
Peuvrel L, Ruellan AL, Thillays F, Quereux G, Brocard A, Saint-Jean M, Aumont M, Drouet F, Dreno B (2013) Severe radiotherapy-induced extracutaneous toxicity under vemurafenib. Eur J Dermatol 23(6):879–881
Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, Puzanov I, Hauschild A, Robert C, Algazi A, Mortier L, Tawbi H, Wilhelm T, Zimmer L, Switzky J, Swann S, Martin AM, Guckert M, Goodman V, Streit M, Kirkwood JM, Schadendorf D (2012) Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 13(11):1087–1095
Conflict of interest
The authors declare that they have no conflict of interest and no funding source.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peuvrel, L., Saint-Jean, M., Quéreux, G. et al. Incidence and characteristics of melanoma brain metastases developing during treatment with vemurafenib. J Neurooncol 120, 147–154 (2014). https://doi.org/10.1007/s11060-014-1533-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1533-z