Abstract
To evaluate the effect of stereotactic radiosurgery (SRS) on intracranial metastases with diffusion-weighted imaging/apparent diffusion coefficient maps. A total of 107 patients with 144 metastases larger than 1 cm in diameter were retrospectively reviewed. We calculated the DWITumor/white matter ratios (DWIT/WM ratio) between the metastases and the normal, contralateral frontal white matter at each time point. We also recorded the ADC values for metastases (ADCT values). The DWIT/WM ratio and ADCT values were assessed for correlation with the patients’ tumor response, brain edema, and survival. A decrease in DWIT/WM ratios was seen in the controlled metastases, and an increase in the DWIT/WM ratio were seen in the metastases with poor tumor control. On the other hand, an increase in ADCT values was seen in the controlled metastases, and a decrease in ADCT values was seen in the metastases with poor control. The differences were significant (p value: 0.001 and 0.002, respectively). Sensitivity of a decrease in the DWIT/WM ratio to make an early prediction of tumor control was 83.9 %, and specificity was 88.5 %. When using the initial ADCT values of metastases to predict tumor response, sensitivity and specificity were 85.5 and 72.7 %, respectively. DWI/ADC is a practical method for studying the efficacy of SRS and predicting early metastases response progression. A decrease signal on DWI and increased ADC values are indicators of good tumor control, and reflect the beneficial effect of SRS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intracranial metastases are the most common mass lesions in the brain [1], and 6 % of patients with newly diagnosed invasive cancer are expected to develop subsequent brain metastases as a progression of their original cancer diagnosis [2]. Magnetic resonance imaging (MRI) is the most important imaging modality to evaluate intracranial metastases. Most metastatic tumors can be diagnosed on conventional series of anatomical imaging including T1WI, T2WI, FLAIR, and T1WI plus gadolinium contrast medium. MR diffusion-weighted imaging (DWI), which correlates with tumor cellularity and nucleus-to-cytoplasm ratio of tumors, is also being used for the diagnosis of malignant brain tumors, such as malignant lymphomas, malignant gliomas, and metastases [3–5]. The apparent diffusion coefficients (ADCs) derived from DWI were found to be inversely correlated with cell density and certain proliferation indices [6]. Due to the rapid cell cycling of malignant metastatic lesions, diffusion-weighted MR images seem to provide valuable information beyond just anatomy and structure. Therefore, MR DWI at present is used almost routinely for imaging of metastases.
The goal of the current study was to evaluate the role of DWI/ADCs in assessing the efficacy and evaluating the response of metastases treated using gamma-knife stereotactic radiosurgery (SRS). More specifically, we wanted to determine whether the changes between the pre-SRS and post-SRS DWI/ADC values of metastases were predictive of tumor response, brain edema evolution, and patient survival.
Methods
Patient population
A retrospective review of 523 patients with 1,673 metastases who underwent SRS between November 2005 and January 2013 was conducted. One hundred and seven of these patients had at least 2 repeated MR imaging studies, with DWI series and ADC maps available. The study protocol was approved by the institutional review board of the University of Virginia. A total of 144 lesions larger than 1 cm were analyzed. Lesions <1 cm were excluded in this study because of the concern for measurements errors regions of interest (ROIs) smaller than 1 cm in diameter (Table 1).
Gamma knife surgery
Stereotactic radiosurgery was performed using the Leksell Gamma Unit Model C before 2007 and the Perfexion (Elekta Instrument, Inc) after 2007. GammaPlan software (Elekta Instrument, Inc) was used for treatment planning. A margin dose was prescribed at an isodose level of 40–86 %, median at 50 %. In this series, all patients with metastases had a higher margin dose (median 19.0 Gy, range 14.0–24.0 Gy) than those with benign tumors, in order to achieve better tumor control. Median radiation volume was 4.4 mL (range, 0.8–73.5 mL).
Magnetic resonance imaging (MRI)
MRI was performed on 1.5 tesla units using a standard head coil. All patients underwent a series of brain scanning, included precontrast axial T2-weighted, fluid attenuated inversion-recovery, and T1-weighted images with 3-mm slice thickness. After administrating gadolinium, and axial T1-weighted and coronal magnetization-prepared rapid acquisition gradient echo images were performed. DWI was obtained in transverse plane by using a spin echo–echo planar imaging sequence with diffusion gradient encoding in there orthogonal direction and with b = 0, 500, and 1,000 s/mm2. The ADC map were computed from the raw DWI data in the usual fashion on scanner console software.
Characteristics of MR images before and after radiosurgery
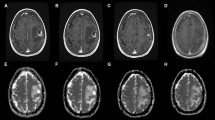
Pre- and post-SRS MR images were reviewed for number of metastases, tumor volume, and volume of peri-tumoral edema. The MR image analysis was designed to compare these parameters, as well as the DWI/ADC values from the first follow-up images to those from the last follow-up images (Fig. 1).
(Upper) The time table presents the way we collected DWI/ADC parameters, tumor volume, brain edema volume, and survival period. (Lower left) Illustration of the method for measuring signal intensities on DWI. MRI of the brain showed a metastasis located in the parietal lobe with peri-focal edema. In order to obtain DWI values, 2 ROIs were placed, one on tumor (T), and another on the normal white matter of the contralateral frontal lobe (WM). The DWIT/WM ratio is calculated as 0.78 before GKS and 0.37 after GKS. The negative change is correlated with a good tumoral response to radiation and edema regression. (Lower right) Illustration of the method for measuring signal intensities on ADC. MRI of the brain showed a metastasis located in the frontal lobe with peri-focal edema. The ROI was placed on the solid part of the tumor (T). The ADCT value was 1,364 before GKS and 2,772 after GKS. This ADC value increase was correlated with a good tumoral response to radiation and edema regression
Standardized ROIs were positioned over the solid component of each metastasis (round-shaped, around 5 mm in diameter), as well as in the contralateral frontal white matter, in a region devoid of metastasis. If the tumor is large enough, we usually put the ROI in the brightest part of tumor on DWI, and the darkest region of the ADC map. DWI intensity values were recorded in these ROIs. The tumor/white matter DWI ratio (referred to as “DWIT/WM ratio” below) was calculated as the mean DWI intensity value in the tumoral ROI divided by the mean DWI intensity value in the reference ROI (Fig. 1). The mean ADC value in the tumor ROI (referred to as “ADCT value” below) was also calculated (Fig. 1). Of note, while the 111 study patients had DWI at all time points, only 80 patients had ADC maps saved on our electronic archive (Table 2).
To evaluate the effect of GKS on the metastases, we quantified the changes in DWIT/WM ratios and ADCT values pre-GKS and post-GKS as below:
Changes in tumor volume were assessed on gadolinium-enhanced T1WI images. Changes in peritumoral edema volume was assessed on FLAIR images. Volumes were calculated applying the trapezoidal rule formula, which had an expected error rate of 10 % or less with accurate delineation on at least 5 slices.29 The tumor volume and brain edema responses to SRS were classified into 3 categories: (1) regressing (decreased volumes from baseline to follow-up); (2) stable; (3) progressive (increased volumes from baseline to follow-up).
Statistical analysis
Analysis was performed using bivariate tests: the one-way, and two-way analysis of variance to compare means. They were used to assess the tumor volume changes, edema volume changes, and survival rate in patients with increase/decrease in DWIT/WM ratios and ADCT values after SRS. The sensitivity, specificity, positive predictive value, and negative predictive value of these 2 prediction tools were evaluated. All statistical analyses in this study were performed using commercially available statistical software (version 20.0; SPSS, Inc., Chicago, IL).
Results
The median age of patients included in our study was 58.4 years old, and there was no gender difference. The median tumor volume was 3.4 mL (range: 0.2–64.5 mL) based on MRI measurements. Most patients (67.6 %) had multiple intracranial lesions, and 18.7 % had metastasis to other organs. The locations of the metastases were the frontal lobe (34.7 %), parietal lobe (13.9 %), cerebellum (13.9 %), temporal lobe (12.5 %), occipital lobe (12.5 %), basal ganglia/thalamus (6.3 %), brainstem (1.4 %), and others (4.9 %). The primary cancers were mostly NSCLC (45.8 %), followed by melanoma (20.6 %), breast cancer (14.0 %), GI-origin cancer (6.5 %), SCLC (2.8 %), and others (7.5 %, including oral squamous cell carcinoma, prostate cancer, lacrimal gland carcinoma, Ewing sarcoma, and carcinoid). Prior microsurgical resection had been performed for 21 patients (19.6 %), and 35 patients (32.7 %) had whole brain radiotherapy before SRS. The median KPS in this group of patients was 90 (range, 30–100) (Table 1).
The median clinical follow-up was 8.4 months (range 2.1–83.5), and the median image follow-up was 6.4 months (range, 2.1–57.1 months). In the last follow-up of individual patients, 102 metastases had regressed, 16 were stable, and 26 had progressed (Table 2). The peri-tumoral edema had regressed for 107 metastases, was stable for 8, and had had progressed in 29. Overall, 81.9 % of the metastases were well-controlled (had regressed or remained stable), and 79.9 % of the metastases showed regression of and stable peri-tumoral brain edema (Table 2).
Changes in the DWIT/WM ratio in the initial MRI after SRS (n = 144)
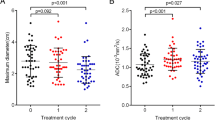
In the first 3 month follow-up, the mean DWIT/WM ratio declined from 1.3 to 0.9 (−0.4) in the 102 regressing metastases, and from 1.9 to 1.7 (−0.2) in the 16 stable metastases (Table 2). The mean DWIT/WM ratio increased from 1.2 to 1.7 (+0.5) in the 26 progressing metastases (Fig. 2). The differences in the changes between the controlled and uncontrolled metastases were significant (p value = 0.001).
Correlation between the DWIT/WM ratios/ADCT values and tumor and brain edema response. a Box plot showing a significant correlation between DWIT/WM ratios and tumor control (regression/stable vs. progression), p value was 0.001. b Box plot showing the correlation between DWIT/WM ratios and brain edema control, p value was also 0.001. c Box plot showing a significant correlation between ADCT values and edema control (regression/stable vs. progression), p value was 0.002. d Box plot showing the correlation between ADCT values and brain edema control (regression/stable vs. progression), p value was also 0.004
Decrease in DWIT/WM was observed in the metastases in which brain edema had regressed or was stable (−0.4 and −0.4 in the regressing and stable groups, respectively), and increase in DWIT/WM were seen in the metastases in which brain edema had progressed (+0.4 in the progressed group) (Fig. 2). The difference in the DWIT/WM changes was significant (p value = 0.001).
For those patients whose survival was longer in this series (>12 months), the mean change in the DWIT/WM ratio was −0.2. For the patients whose survival was shorter (<6 months), the mean change of DWIT/WM ratio was −0.1 (Table 2). There was no difference between these patient groups (p value = 0.238).
Changes in the ADCT value in the initial MRI after GKS (n = 80)
In the initial 3 months of follow-up, the mean ADCT value increased from 1,040.9 to 1,292.3 (+251.4) in the 60 regressing metastases, and from 521.4 to 615.9 (+94.5) in the 9 stable metastases. The mean ADCT value decreased from 1,156.9 to 941.8 (−215.1) in the 11 progressing metastases (Table 2 and Fig. 2). The difference in the ADCT changes between the controlled and uncontrolled metastases was significant (p value = 0.002).
The correlation between peri-focal brain edema evolution and changes in ADCT value of the metastases was similar to the that of tumor response: increases in ADCT were seen in the metastases in which brain edema had regressed or was stable (+252.7 and +32.2 in the regressing and stable groups, respectively), and decreases were seen in the metastases in which brain edema had progressed (−156.7 in the progressing group) (Fig. 2). The difference in the ADCT changes between the two groups was significant (p value = 0.004).
In terms of patient survival, ADCT values were not related to the survival period (p value = 0.831). Patients with higher ADCT values appear to be have longer survival, but this did not reach statistical significance. Increases in ADCT values after GKS were seen in all patients, but this did not correlate with overall survival (Table 2).
Concerning the overall relative time course of these changes, two 3-D plots to illustrate the changes of tumor volume at last follow, time of last follow up MRI, and DWI/ADC changes measured on the initial follow up MRI (Fig. 3).
Three-dimension plot to illustrate the changes of tumor volume at last MR follow-up, time period of last follow-up, and changes of DWIT/WM ratios (left)/ADCT values (right) changes derived from the first MRI follow-up within 3 months after SRS. (FU: follow-up, y-axis: 100 % tumor volume means no change; 200 % tumor volume means tumor growing doubled from the original size.)
Sensitivity, specificity, positive predictive value, and negative predictive value for evaluation of the effect of GKS on tumor and brain edema control by means of changes in the DWIT/WM ratio and ADCT value in the initial 3-month follow-up
The sensitivity of a decrease in the DWIT/WM ratios for early prediction of tumor control was 83.9, and 84.4 % for brain edema control. If the initial change in ADCT values was used to predict tumor and brain edema control, sensitivities were 85.5 and 85.1 %, respectively. The specificity of a decrease in the DWIT/WM ratio was 88.5 % to predict tumor control, and 82.8 % to predict brain edema control. If the initial change in ADCT values was used to predict tumor and brain edema control, specificities were 72.7 and 61.5 %, respectively (Table 3).
The positive predictive values for evaluation of the effect of radiosurgery on tumor and brain edema control by means of changes in the DWIT/WM ratio would be 97.1, and 95.1, and 95.2 and 91.9 % by means of changes in the ADCT value. The negative predictive values would be 54.8, 57.1, 44.4, and 44.4 %, respectively (Table 3).
Discussion
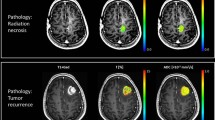
Stereotactic radiosurgery has been used for decades to treat metastases, either as a primary or adjunctive treatment after surgical resection and/or whole brain radiotherapy [7–9]. However, it usually takes months to assess the irradiated tumor for any morphological change or indirect evidence of tumor response or progression [10]. This delay may result in rescue treatment options being implemented later than they could. Furthermore, differentiating between tumor progression versus radiation necrosis after radiosurgery can be a challenge for clinicians. Therefore, in recent years, physicians have investigated new imaging modalities to early assess the effects of radiation.
Based on microscopic Brownian motion in tissue water, DWI/ADC quantitatively describes the effective mean diffusivity, which is determined by tissue cellularity, viscosity of the medium, and spacing of the diffusion barriers. High cellular brain tumors usually appear on DWI as hyperintense lesions, and have a low ADC. This characteristic is widely used in the diagnosis and grading of patients with gliomas and lymphomas [5, 6, 11, 12]. Most of these studies focused on correlating tumor grading and DWI ratio and ADC values. Wu’s and Guo’s reports showed a higher DWIT/WM ratio in high-grade gliomas, which correlate with proliferation indexes such as Ki-67 and MIB-1 [5, 12]. Murakami et al. [11] also demonstrated that the minimum ADC corresponded to the highest-grade glioma foci within heterogenous tumors, and helped distinguish grade I and grade IV tumor foci from other grade foci. This simple and useful imaging tool can be applied to guide tissue biopsy during a stereotactic intracranial tumor biopsy.
Other studies used DWI and ADC to evaluate the radiation effects on intracranial malignancies. The first investigative target were patients with high-grade gliomas [13, 14] and metastatic brain tumors [15–19] (Table 4). Although their study included only a few patients (n = 10), Mardor et al. [15] utilized DWI/ADC to detect the development of radiation necrosis, and found a significant correlation between early ADC changes (8 days after initiation of radiation) and later tumor response (7 weeks later by conventional MRI). In 2006, Tomura et al. reported the relationship between ADC changes and tumor response to radiation in 19 patients. The ADC of the tumors was significantly higher 2–4 weeks after stereotactic irradiation, and a significant difference in the ADC was observed between the responder and non-responder groups [17]. In 2008, Huang and colleagues reported a small series of metastases, in which they found that ADC values increased in most tumors after SRS, and significantly increased in those tumors in which central necrosis was present [18]. In this study, we hypothesized that the high dose radiation that SRS delivered can cause cellular injury, apoptosis, and finally decrease in intensity of cellularity in a malignant brain tumor. As result, we found that the ADCT value and DWIT/WM ratio were correlated with tumor and brain edema responses after irradiation. In brief, a decrease in DWI intensity and an increase in ADC values were found to be early markers of tumor response [19–23]. In addition, the ADC of brain tumors is relatively insensitive to steroid therapy, which facilitates the post-treatment follow-up of metastases [24].
Different from previous studies that have used DWI/ADC to predict survival in patients with glioma [25–27], CNS lymphoma [28], and single metastasis [29], changes in ADCT values and DWIT/WM ratios in our study were not related to patient survival. This is likely related to the fact that the natural course of patients with multiple metastasis is decided by multiple factors including the extent and control of systemic disease as well as local intracranial disease response.
With the advances in medical imaging, the use of serial functional and metabolic images during follow-up provides detailed information on radiosurgery-induced pathophysiological reactions within the intracranial malignancy [20]. These imaging modalities include conventional MRI, DWI, ADC, MRS, dynamic susceptibility contrast (DSC-enhanced) perfusion MRI (DSC-MRP), rCBV, FDG-PET, MET-PET, SPECT, CTP and so on. Several studies have demonstrated that SPECT imaging has the highest specificity (97.8 %) of these modalities, using either technetium-99 (Tc-99) or thallium-201 (Tl-201) [30, 31]. Other studies also suggested that DSC-MRP is helpful in differentiating radiation necrosis and tumor progression by making precise measurements of cerebral blood flow [32]. However, how and when to choose these imaging modalities are still controversial issues, and they are usually time-consuming, expensive, and occasionally denied by private payers. As a result, highly cost-effective method was attractive. Kano et al. in 2010 [33] utilized a correspondence between the contrast-enhanced volume on T1WI and a low signal-defined lesion margin on T2WI to differentiate tumor progression and radiation effects. The sensitivity of the T1/T2 mismatch in identifying radiation effects was 83.3 %, and the specificity was 91.1 %. Similarly, the present report provided another high cost-effective method using diffusion-weighted MR images, and demonstrated a sensitivity of 83.9 % and a specificity of 88.5 % in tumor control with a decreased DWIT/WM ratio and a sensitivity of 85.5 % and a specificity of 72.7 % in tumor control with an increased ADCT value. Compared to other advanced and expensive diagnostic tools, diffusion-weighted MR images provide acceptable specificities and an easier way to access.
There are a few limitations inherent to the DWI technique. The signal intensity of DWI/ADC is influenced by: (1) the presence of peritumoral vasogenic edema (so called T2 shine-through), (2) the degree of neuroarchitectural destruction, (3) the nucleus-to-cytoplasm ratio, (4) the presence of tumoral necrosis, (5) the porosity of extracellular space, (6) the cellular features of the tumor, (7) presence of hemoglobin and its degradation products, and (8) presence calcification within the metastases. Because of this, metastases with intra-tumoral hemorrhage, calcification, and cyst formation had to be excluded from this study. We also excluded metastases that underwent recent surgical resection (<1 month before GKS). Remote surgical resection (defined for the purposes of this study as >1 month before SRS and compromising 19.6 % in this series) and mixed cyst-solid metastases (12.5 % in this series) were included for evaluation (Table 1).
We acknowledge several limitations to our study. Beside the retrospective nature of our study, we did not distinguish between metastases of different origin. Further studies are required to assess whether DWI/ADC changes are influenced by type of primary cancer. Except the tumor origin, several risk factors, including age, sex, KPS, initial tumor volume, primary tumor control, and remote tumor control, may also influence the predict rate of DWI/ADC changes. In addition, the placement of the ROI on the tumor portion with the brightest signal relies upon the skill as well as the subjectivity of the authors’, and partial volume effects might also affect the signal ratios. This study attempted to set a limitation that the metastases should be larger than 1 cm for better visualization, and further study of this technique would be required to test its applicability for tumor <1 cm in diameter since they were excluded from the study. Various factors can affect the response of tumors to radiosurgery. The utility of DWI and ADC changes for predicting tumor response will require study in a larger cohort of patients in which the effect of additional variables (e.g. tumor type, size, and location) can be controlled for. Last, although the advancement of image fusion technique, we mostly use T1-weighted, contrast-enhanced images for SRS planning in brain metastases cases. On occasion, fusing diffusion images for planning and follow-up can be performed. Inevitably, some enlarged tumors extend beyond the area of ROI that we initially measured. A future prospective study with a point to point comparison for targeting metastases may further validate the power of this method.
Conclusion
The application of DWI/ADC in studying the outcomes from SRS and specifically, the prediction of metastases progression is not only a novel but also a practical concept. The DWIT/WM ratio and ADCT value were correlated to tumor and brain edema responses after irradiation. A DWI signal decrease and an increased ADC intensity were indicative of good tumor control and brain edema control, and reflected a positive response to radiation. For metastasis control after SRS, a decreased DWIT/WM ratio had a sensitivity of 83.9 % and a specificity of 88.5 %. An increasing ADCT value had a sensitivity of 85.5 % and a specificity of 72.7 %.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- ARE:
-
Adverse radiation effect
- CTP:
-
Computed tomography perfusion
- DWI:
-
Diffusion-weighed images
- FDG-PET:
-
Positron emission tomography with 18Ffluoro-2-deoxy-d-glucose
- FLAIR:
-
Fluid attenuated inversion recovery
- GI:
-
Gastrointestinal
- GKS:
-
Gamma-knife surgery
- Gy:
-
Gray
- KPS:
-
Karnofsky Performance Scale
- MET-PET:
-
Positron emission tomography with 11Cmethyl-l-methionine
- MR:
-
Magnetic resonance
- MRP:
-
Magnetic resonance perfusion
- MRS:
-
Magnetic resonance spectroscopy
- NSCLC:
-
Non-small cell lung cancer
- rCBV:
-
Relative cerebral blood volume
- SCLC:
-
Small cell lung cancer
- SPECT:
-
Single photon emission computed tomography
- SRS:
-
Stereotactic radiosurgery
- T1WI:
-
T1-weighted images
- T2WI:
-
T2-weighted images
- WBRT:
-
Whole brain radiotherapy
- WM:
-
White mater
References
Walker AE, Robins M, Weinfeld FD (1985) Epidemiology of brain tumors: the national survey of intracranial neoplasms. Neurology 35:219–226
Davis FG, Dolecek TA, McCarthy BJ, Villano JL (2012) Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol 14:1171–1177
Sugahara T, Korogi Y, Kochi M, Ikushima I, Shigematu Y, Hirai T, Okuda T, Liang L, Ge Y, Komohara Y, Ushio Y, Takahashi M (1999) Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 9:53–60
Kono K, Inoue Y, Nakayama K, Shakudo M, Morino M, Ohata K, Wakasa K, Yamada R (2001) The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 22:1081–1088
Guo AC, Cummings TJ, Dash RC, Provenzale JM (2002) Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology 224:177–183
Higano S, Yun X, Kumabe T, Watanabe M, Mugikura S, Umetsu A, Sato A, Yamada T, Takahashi S (2006) Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology 241:839–846
Da Silva AN, Nagayama K, Schlesinger D, Sheehan JP (2009) Early brain tumor metastasis reduction following Gamma Knife surgery. J Neurosurg 110:547–552
Jagannathan J, Yen CP, Ray DK, Schlesinger D, Oskouian RJ, Pouratian N, Shaffrey ME, Larner J, Sheehan JP (2009) Gamma knife radiosurgery to the surgical cavity following resection of brain metastases. J Neurosurg 111:431–438
Basina BR, Olson C, Roy DK, Yen CP, Schlesinger D, Nagayama K, Sheehan JP (2010) Radiation dose and incidence of new metastasis in the anterior temporal lobe structures of radiosurgically treated patients. J Neurosurg 112:122–129
Stinauer MA, Diot Q, Westerly DC, Schefter TE, Kavanagh BD (2012) Fluorodeoxyglucose positron emission tomography response and normal tissue regeneration after stereotactic body radiotherapy to liver metastases. Int J Radiat Oncol Biol Phys 83:e613–e618
Murakami R, Hirai T, Sugahara T, Fukuoka H, Toya R, Nishimura S, Kitajima M, Okuda T, Nakamura H, Oya N, Kuratsu J, Yamashita Y (2009) Grading astrocytic tumors by using apparent diffusion coefficient parameters: superiority of a one- versus two-parameter pilot method. Radiology 251:838–845
Wu CC, Guo WY, Chen MH, Ho DM, Hung AS, Chung HW (2012) Direct measurement of the signal intensity of diffusion-weighted magnetic resonance imaging for preoperative grading and treatment guidance for brain gliomas. J Chin Med Assoc 75:581–588
Hein PA, Eskey CJ, Dunn JF, Hug EB (2004) Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol 25:201–209
Asao C, Korogi Y, Kitajima M, Hirai T, Baba Y, Makino K, Kochi M, Morishita S, Yamashita Y (2005) Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am J Neuroradiol 26:1455–1460
Mardor Y, Pfeffer R, Spiegelmann R, Roth Y, Maier SE, Nissim O, Berger R, Glicksman A, Baram J, Orenstein A, Cohen JS, Tichler T (2003) Early detection of response to radiation therapy in patients with brain malignancies using conventional and high b-value diffusion-weighted magnetic resonance imaging. J Clin Oncol 21:1094–1100
Mardor Y, Roth Y, Ochershvilli A, Spiegelmann R, Tichler T, Daniels D, Maier SE, Nissim O, Ram Z, Baram J, Orenstein A, Pfeffer R (2004) Pretreatment prediction of brain tumors’ response to radiation therapy using high b-value diffusion-weighted MRI. Neoplasia 6:136–142
Tomura N, Narita K, Izumi J, Suzuki A, Anbai A, Otani T, Sakuma I, Takahashi S, Mizoi K, Watarai J (2006) Diffusion changes in a tumor and peritumoral tissue after stereotactic irradiation for brain tumors: possible prediction of treatment response. J Comput Assist Tomogr 30:496–500
Huang CF, Chou HH, Tu HT, Yang, Lee JK, Lin LY (2008) Diffusion magnetic resonance imaging as an evaluation of the response of brain metastases treated by stereotactic radiosurgery. Surg Neurol 69:62–68 discussion 68
Goldman M, Boxerman JL, Rogg JM, Noren G (2006) Utility of apparent diffusion coefficient in predicting the outcome of Gamma knife-treated brain metastases prior to changes in tumor volume: a preliminary study. J Neurosurg 105(Suppl):175–182
Chernov M, Hayashi M, Izawa M, Ochiai T, Usukura M, Abe K, Ono Y, Muragaki Y, Kubo O, Hori T, Takakura K (2005) Differentiation of the radiation-induced necrosis and tumor recurrence after gamma knife radiosurgery for brain metastases: importance of multi-voxel proton MRS. Minim Invasive Neurosurg 48:228–234
Kang TW, Kim ST, Byun HS, Jeon P, Kim K, Kim H, Lee JI (2009) Morphological and functional MRI, MRS, perfusion and diffusion changes after radiosurgery of brain metastasis. Eur J Radiol 72:370–380
Nagel FJ, Van As H, Tramper J, Rinzema A (2002) Water and glucose gradients in the substrate measured with NMR imaging during solid-state fermentation with Aspergillus oryzae. Biotechnol Bioeng 79:653–663
Gao X, Zhang XN, Zhang YT, Yu CS, Xu DS (2011) Magnetic resonance imaging in assessment of treatment response of gamma knife for brain tumors. Chin Med J (Engl) 124:1906–1910
Young GS (2007) Advanced MRI of adult brain tumors. Neurol Clin 25:947–973 viii
Hamstra DA, Chenevert TL, Moffat BA, Johnson TD, Meyer CR, Mukherji SK, Quint DJ, Gebarski SS, Fan X, Tsien CI, Lawrence TS, Junck L, Rehemtulla A, Ross BD (2005) Evaluation of the functional diffusion map as an early biomarker of time-to-progression and overall survival in high-grade glioma. Proc Natl Acad Sci U S A 102:16759–16764
Hamstra DA, Galban CJ, Meyer CR, Johnson TD, Sundgren PC, Tsien C, Lawrence TS, Junck L, Ross DJ, Rehemtulla A, Ross BD, Chenevert TL (2008) Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol 26:3387–3394
Murakami R, Sugahara T, Nakamura H, Hirai T, Kitajima M, Hayashida Y, Baba Y, Oya N, Kuratsu J, Yamashita Y (2007) Malignant supratentorial astrocytoma treated with postoperative radiation therapy: prognostic value of pretreatment quantitative diffusion-weighted MR imaging. Radiology 243:493–499
Barajas RF Jr, Rubenstein JL, Chang JS, Hwang J, Cha S (2010) Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol 31:60–66
Berghoff AS, Spanberger T, Ilhan-Mutlu A, Magerle M, Hutterer M, Woehrer A, Hackl M, Widhalm G, Dieckmann K, Marosi C, Birner P, Prayer D, Preusser M (2013) Preoperative diffusion-weighted imaging of single brain metastases correlates with patient survival times. PLoS ONE 8:e55464
Yoshii Y, Satou M, Yamamoto T, Yamada Y, Hyodo A, Nose T, Ishikawa H, Hatakeyama R (1993) The role of thallium-201 single photon emission tomography in the investigation and characterisation of brain tumours in man and their response to treatment. Eur J Nucl Med 20:39–45
Shah AH, Snelling B, Bregy A, Patel PR, Tememe D, Bhatia R, Sklar E, Komotar RJ (2012) Discriminating radiation necrosis from tumor progression in gliomas: a systematic review what is the best imaging modality? J Neurooncol 112:141–152
Fatterpekar GM, Galheigo D, Narayana A, Johnson G, Knopp E (2012) Treatment-related change versus tumor recurrence in high-grade gliomas: a diagnostic conundrum–use of dynamic susceptibility contrast-enhanced (DSC) perfusion MRI. AJR Am J Roentgenol 198:19–26
Kano H, Kondziolka D, Lobato-Polo J, Zorro O, Flickinger JC, Lunsford LD (2010) T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery 66:486–491 discussion 491-482
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, CC., Wintermark, M., Xu, Z. et al. Application of diffusion-weighted magnetic resonance imaging to predict the intracranial metastatic tumor response to gamma knife radiosurgery. J Neurooncol 118, 351–361 (2014). https://doi.org/10.1007/s11060-014-1439-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1439-9