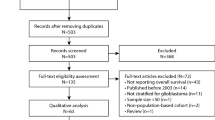
Abstract
With standard treatment for glioblastoma (GBM) consisting of surgery followed by radiotherapy (RT) with concurrent and adjuvant temozolomide (TMZ), median survival is ~14.6 months. This is not as informative to patients who have survived for some time. Conditional probability of survival may offer more relevant survival estimates. Outcomes/conditional probability of survival and post-progression survival (PPS) estimates were retrospectively reviewed in the TMZ treatment era of 882 consecutive patients with a diagnosis of GBM from January 2004 to August 2010. Median age of entire cohort was 62 years including 62 % males. Baseline performance status (PS) was 0–1 in 67, 23 % had frontal lobe tumors, 58 % received concurrent RT/TMZ ± adjuvant TMZ. Survival (OS) was similar for those with frontal lobe tumors versus other locations (P = 0.25). OS for patients receiving standard RT/TMZ ± TMZ was 14.2 months. Age, PS, extent of surgery, therapy post-surgery had significant effects on OS. OS for entire cohort at 1, 2, 3, 4, 5 years was 43.4, 17.9, 10.4, 8.4, 7.2 % respectively. Conditional probability of survival of an additional year given survival to 1, 2, 3, 4, 5 years was 41.4, 58, 80.7, 85.7, 81.5 % respectively. Conditional probability of survival for those patients receiving concurrent RT/TMZ ± adjuvant TMZ was similar. Patients who progress >18 months after their initial treatment for GBM had significantly greater 2 and 5 year PPS as well as OS. Conditional probabilities of survival may provide more meaningful life expectancy predictions for survivors of GBM than conventional survival outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary malignant central nervous system tumors represent ~2 % of all cancers but account for a disproportionate share of mortality [1]. Glioblastoma (GBM) is the most aggressive malignant primary brain tumor accounting for 54 % of all primary brain gliomas [2]. The mainstay of treatment is surgical removal without creating an unacceptable neurologic deficit followed by radiotherapy (RT). This is based on the early phase III clinical trials of the Brain Tumor study group which compared treatment with postoperative supportive care alone, carmustine alone, RT alone, and RT plus carmustine. The 1 year survival for each of these treatment arms was 3, 12, 24, and 25 % respectively [3].
Current standard of care for patients with newly diagnosed GBM includes maximal safe surgical resection, followed by concurrent temozolomide (TMZ)/RT and then adjuvant TMZ [4]. A median survival time of 14.6 months and an estimated 2 year survival rate of 27 % have been reported utilizing this treatment regimen [5].
However, patients enrolled in clinical trials are often highly selected and as a result may have better outcomes than patients treated in an unselected general population of GBM patients. In addition, advances in the therapeutic management of GBM patients have resulted in a larger proportion of patients surviving beyond 2 years after diagnosis. The disease outcome is typically described in terms of estimated survival from diagnosis but conditional probability offers more relevant information regarding survival for patients once they have survived for some time. Conditional probability of survival is defined as the probability of surviving to some Y years after diagnosis given survival to some X years (X < Y) [6]. For GBM, this has become more relevant in the TMZ treatment era as patients are now surviving for longer durations, and conditional probability may be a predictor of continued long-term survival.
Conditional probability of survival in GBM patients has previously been studied in patients enrolled in seven phase II clinical trials over a 22 year period up to 2007 [7] and in those who were treated with RT-containing regimens within surveillance, epidemiology, and end results (SEER) data over a 10 year period up to 2008 [8], but never specifically in patients presenting to a general neuro-oncology clinic setting in the era of TMZ treatment.
The aim of this study was to retrospectively review and report overall survival (OS) outcomes, the impact of previously reported prognostic clinical variables as well as conditional probability estimates in patients treated for GBM during the TMZ era at a large tertiary neuro-oncology clinic. A wide range of patients present to this institution with this diagnosis and therefore this population accurately reflects the full clinical spectrum of GBM. The gold-standard endpoint in clinical trials is OS. However, this endpoint takes years to observe and the availability of surrogate endpoints that would enable earlier assessments of treatment effects would be useful, therefore, the effects of progression status on survival and post-progression survival (PPS) were also investigated.
It has been reported that the phenotypic features that distinguish isocitrate dehydrogenase 1R132MUT (IDH1R132MUT) GBM from other subtypes include better outcome and predominance of frontal lobe location [9], therefore effect of frontal lobe GBM tumor location on survival was also reviewed.
Materials and methods
Patient population
882 consecutive patients presenting to Princess Margaret Cancer Centre, Toronto with a new diagnosis of GBM were followed from January 2004 to August 2010. Complete demographics, Eastern Cooperative Oncology Group (ECOG) performance status (PS), GBM localization, extent of surgery (partial resection was defined as <90 % tumor removal, subtotal resection was defined as <100 % but >90 % tumor removal), percent receiving RT ± TMZ, time to first progression, PPS, OS and conditional probability of survival were analyzed. In patients with frontal lobe involvement as the primary tumor location, a distinction was not made for the degree of involvement of the corpus callosum. Thirty-four patients (4 %) had a radiological diagnosis without pathological confirmation due to tumor location and/or poor PS deeming biopsy unsafe. These patients were included in this analysis so as to represent the heterogenous GBM population that presents to a typical neuro-oncological clinic. O(6)-methylguanine DNA methyltransferase (MGMT) promoter methylation status or IDH1 mutation status were not available for patients as these were not routinely requested in the time period studied.
Institutional review board ethical approval was obtained for this study.
Statistical methods
Summary statistics were provided for patient demographics, disease, and treatment factors. The estimates of OS were calculated using the Kaplan–Meier method. Survival time was calculated from the date of diagnosis to the date of death; living patients were censored on the date of last follow-up. PPS was calculated from the date of first progression to the date of death; living patients were censored on the date of last follow-up. Group differences of OS and OS for survivors with time to first progression available were examined using the log-rank test. OS estimates from date of first progression were examined using the P value trend test. Conditional survival probability and confidence interval (CI) were calculated using the method described previously [6]. Conditional probability of surviving to 2 years, given survival to 1 year was calculated by dividing the 2 year survival rate by the 1 year survival rate. Conditional probabilities for other time intervals were calculated similarly. Variances of conditional survival were estimated using a variation of the usual “Greenwood’s formula” for unconditional survival [6].
Effects of clinical variables on survival were studied using parametric models. Parametric models used include exponential, Weibull, lognormal, and log-logistic. Because of the presence of non-constant hazard, the log-logistic model fitted the data better and was selected to do multivariable analysis. Multivariable analysis was performed by including clinical variables: age, ECOG PS, extent of surgery, frontal lobe localization and therapy received post surgery. All tests were two-sided with α = 0.05.
Results
Patients
The cohort included 882 consecutive patients with a median age of 62 years (18–93). The median follow up time of entire cohort of patients was 8.8 months and for censored patients (alive/status unknown) was 16.3 months. A summary of patient characteristics is detailed in Table 1.
For the 34 patients who did not have a biopsy, 17 (50 %) received supportive care only, 12 (35 %) received RT alone and 5 (15 %) received concurrent RT/TMZ followed by TMZ.
Of the 512 patients who received standard therapy with concurrent RT/TMZ followed by TMZ, 117 (23 %) received concurrent RT/TMZ only, 239 (47 %) received <6 cycles of adjuvant TMZ, and 156 (30 %) received at least 6 cycles adjuvant TMZ following concurrent treatment. Failure to complete standard treatment was either related to side effects of TMZ therapy or progressive disease. Two hundred and twenty-eight patients (26 %) received RT alone with 61 % of these having a PS ≥2, and 80 % aged >60 years. At the time of data analysis, 43 patients (5 %) were still alive, 718 (81 %) were deceased, and status was unknown/pending in 121 (14 %).
Overall survival
The median OS for the entire cohort was 10.0 months (95 % CI 8.8–11.0 months). Age, ECOG PS, extent of surgery and therapy received post-surgery had significant effects on OS utilizing multivariable analysis with the log-logistic model (Table 2).
Overall survival of the entire cohort based on gender, ECOG PS, GBM localization, extent of surgery and therapy received post surgery is summarized in Table 3. There was no gender-related difference in OS between males and females (10.1 vs. 9.7 months, log-rank test P = 0.96). Similarly, there was no difference in OS for those with frontal lobe tumors versus other intracranial tumor location (10.3 vs. 9.7 months, log-rank P = 0.25).
Conditional probability of survival
The OS and conditional probability of survival for the entire cohort of 882 patients is tabulated (Table 4). The conditional probability of surviving an additional year at various time points for the entire cohort is graphically represented in Fig. 1. Survival probability decreases most rapidly in the first 2 years after initial diagnosis. The conditional probability of surviving one other year after surviving 2 years post diagnosis for the entire cohort was 58.0 % and higher than the observed 2 year survival rate. The OS and conditional probability of survival for the 228 patients who received RT alone and for the 512 patients who received concurrent RT/TMZ followed or not by adjuvant TMZ is also documented (Table 4). As there were only 10 patients who received TMZ alone, OS and conditional probability of survival for this group were not reported.
Impact of time to first progression on overall survival and post-progression survival
In patients with time to first progression available (N = 505), the median OS from diagnosis for patients with time to first progression (TTP) at ≤6 months (group 1) (N = 214), 6–18 months (group 2) (N = 233), and >18 months (group 3) (N = 58) was 7.3 months (95 % CI 6.3–8.8), 15.2 months (95 % CI 14.5–16.9), and 50.6 months (95 % CI 36.6–82.4) respectively (log-rank test P < 0.001).
The Kaplan–Meier plot of OS after progression stratified for each of these three TTP groups, as described above, is graphically represented in Fig. 2. The median OS from first progression for each of these three TTP groups; group 1: ≤6 months, group 2: 6–18 months, and group 3: >18 months was 3.5 months (95 % CI 2.7–4.5), 5.3 months (95 % CI 4.4–6.3) and 15.9 months (12.4–26.4) respectively.
For each of these three TTP groups, the 2 year PPS was 9.3 % (95 % CI 5.4–14.6), 9.9 % (95 % CI 5.9–15.0) and 38.6 % (95 % CI 21.6–55.3) respectively (trend test P < 0.001) and the 5 year PPS was 3.3 % (95 % CI 0.9–8.5), non-evaluable and 25.7 % (95 % CI 21.6–55.3) (trend test P < 0.001) respectively.
There was significant association of TTP groups with age [analysis of variance (ANOVA) P < 0.001], PS (Fisher’s exact test P < 0.001), extent of surgery (Fisher’s exact test P < 0.05), and treatment received (χ2 test P < 0.001).
Discussion
Despite a higher median age (62 years) and lower rate of debulking surgery (69 %) compared with data from Stupp et al. [4] (84 %), the median survival and survival rate at 2 years were similar in this study for the subset of patients who received the standard regimen of RT with concurrent and adjuvant TMZ. The 2-year survival rate for the entire cohort in this study was 17.9 % and this lower survival rate reflects the greater heterogeneity in the patients seen with newly diagnosed GBM in our neuro-oncology centre including patients with greater age, worse PS and less extensive surgery compared with patients who participate in clinical trials.
Advances in the therapeutic management of GBM have resulted in a larger proportion of patients surviving beyond 2 years after diagnosis. As the observed and previous reported survival rates for GBM are largely limited beyond 2 years, conditional probability allows for more relevant survival estimates that are adjusted in ‘real-time’, based on their survival to-date. The probability of surviving an additional year for the entire cohort of 882 patients given survival to 1, 2, 3, 4, and 5 years was 41.4, 58, 80.7, 85.7 and 81.5 % respectively and for the 512 patients who received concurrent RT/TMZ followed by adjuvant TMZ was 43.1, 59.6, 80.2, 88.6 and 88.9 % respectively. For those patients who received RT alone, who survived beyond 2 years (4.7 %), their conditional probability of survival also increased. There were fewer long term survivors following RT alone but in those that did, their conditional probability of survival was similar to those following standard concurrent RT/TMZ followed by TMZ, indicating that perhaps the treatment modality choice may only play a minor role once patients have responded to treatment and survived for a certain amount of time.
In our study, the patients who received RT alone had similar 3, 4 and 5 year OS, therefore as the 3 year conditional survival probability is calculated by dividing the 4 year OS rate by the 3 year OS rate [6], the 3 year conditional survival probability of one additional year is 100 %. However, in the clinical setting, guarantees of this nature can never be made to patients with a diagnosis of GBM, and thus longer follow up data is required, as it is anticipated that most patients with a diagnosis of GBM will eventually progress.
A previous report by other authors on the conditional probability of survival in patients with newly diagnosed GBM demonstrated the probabilities of surviving an additional year given survival to 1, 2, 3, and 4 years as 35, 49, 69, and 93 % respectively [7]. However, this study included patients enrolled onto seven phase two clinical trials between 1975 and 2007, who received broad variations in therapeutic management and are often highly selected. Another study in 2,743 patients with high-grade glioma, diagnosed in Los Angeles County during the years 1990–2000 reported that the conditional probabilities of surviving one additional year increases as the post-diagnosis survival time increases (from 43 ± 2 % conditional on surviving 1 year after diagnosis to 91 ± 2 % conditional on surviving 5 years after diagnosis) [10]. In that study, patients diagnosed with World Health Organisation (WHO) grade III gliomas had higher conditional survival probabilities than those diagnosed with WHO grade IV gliomas. An additional study reported the conditional probability of survival in patients diagnosed with GBM from 1998 to 2008 who were treated with radiation-containing regimens identified within SEER data. Conditional probability of surviving an additional 2 years in this manuscript ranged from 19.8 % at diagnosis to 65.9 % at 5 years after diagnosis [8].
In our study, the conditional probability of surviving an additional year after survival to 2 years post diagnosis exceeds the 1 year survival rate, indicating that the future prognosis of a patient who has survived for 2 years may be as good as those recently diagnosed. Therefore, the conditional probability of survival increases with duration of life. In addition, the conditional probability of survival for the entire cohort of patients is similar to that for those who received standard concurrent RT/TMZ followed by TMZ, indicating that those receiving standard treatment are more likely to be those patients who survive for longer. However, those patients receiving either RT or TMZ alone may have already been pre-selected by the treating physicians as patients unable to tolerate standard therapy with poorer functional activity.
Indeed, on analysis of the 5 year summary data from the landmark phase III study by Stupp et al. [5], 2, 3 and 4 year conditional survival probabilities were 58.8, 75.6 and 81 % respectively, calculated using the method described by Davis et al. [6], for those patients receiving RT with concurrent and adjuvant TMZ, and are not dissimilar to our reported conditional survival probability data.
In studies previously published on conditional probability of survival in patients with a diagnosis of GBM [6–8, 10], patients included were those treated on clinical trials, treated with RT-containing regimens, and patients treated mainly prior to the TMZ therapy era. Our series differs through inclusion of patients treated in the post TMZ therapy era and is reflective of the entire spectrum of GBM patients presenting to neuro-oncology clinics. In all of the studies discussed [5–8, 10], including the data reported in this manuscript, it is clear that there is a gain in conditional survival probability over time in patients with a diagnosis of GBM who receive either RT or systemic therapy. Despite TMZ-based therapy [4] being accepted as standard of care in upfront treatment of patients with GBM, conditional probability of survival values have not changed drastically in the years 1975–2010 (Table 5) [5–8, 10]. Therefore, regardless of therapy, long survival is not unprecedented and highlights the importance of determining whether molecular heterogeneity in these tumors or lack thereof, in this specific longer surviving population of patients, is contributory and also highlights the need for continued and improved drug development in this disease.
In this study, GBM survivors who have experienced progression have a much different prognosis depending on when they progress, and patients who progress >18 months from diagnosis have greater 2 and 5 year PPS than those progressing before that time point. This information is likely to be useful for this population of patients as their options for further treatment may expand as novel agents continue to be developed. Although prior studies have also reported that tumors located in the frontal lobe were associated with better survival [9], we did not find this association and given the lack of availability of IDH1 mutation status in our population of patients, firm conclusions cannot be made.
Recent developments in glioma research include The Cancer Genome Atlas Network and the report that classical GBM tumors are characterized by abnormally high levels of EGFR and survive the longest of the subgroups in response to aggressive treatment [11, 12]. Furthermore, MGMT promoter methylation, and chromosome 1p19q co-deletion have been associated with prolonged progression-free survival in secondary GBM, and patients with both IDH mutation [13] and MGMT promoter methylation have had the best reported response rate to TMZ [14]. In contrast, loss of heterozygosity 10q has been reported to be predictive of shorter survival in GBM patients [15].
The main limitations of this study include its retrospective nature, the lack of correlative molecular data and the smaller numbers of patients who survive beyond 2 years, therefore making any strong conclusions challenging. However, given the current median survival of this population of patients following standard treatment in the era studied, our study is relatively large and provides useful information for long term survivors with a diagnosis of GBM in the general neuro-oncology clinic setting, and also provides information on the survival implication of later progression. Ideally, these concepts could be investigated in large randomized prospective trials with full molecular data availability.
In conclusion, conditional probabilities of survival and PPS data in this general disease population in the era of TMZ therapy may provide more meaningful life expectancy predictions than conventional survival outcomes for long term survivors. However, greater integration of clinical and molecular information in GBM patients is necessary, and stratified treatment plans are warranted considering the molecular heterogeneity and difference in prognosis among these patients.
References
Central Brain Tumor Registry of the United States (CBTRUS) (2009) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2005. www.cbtrus.org/reports/reports.html. Accessed 10 Dec 2013
Gurney JG, Kadan-Lottick N (2001) Brain and other central nervous system tumors: rates, trends, and epidemiology. Curr Opin Oncol 13:160–166
Walker MD, Alexander E Jr, Hunt WE et al (1978) Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg 49:333–343
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC–NCIC trial. Lancet Oncol 10:459–466
Davis FG, McCarthy BJ, Freels S, Kupelian V, Bondy ML (1999) The conditional probability of survival of patients with primary malignant brain tumors: surveillance, epidemiology, and end results (SEER) data. Cancer 85:485–491
Polley MY, Lamborn KR, Chang SM, Butowski N, Clarke JL, Prados M (2011) Conditional probability of survival in patients with newly diagnosed glioblastoma. J Clin Oncol 29:4175–4180
Johnson DR, Ma DJ, Buckner JC, Hammack JE (2012) Conditional probability of long-term survival in glioblastoma: a population-based analysis. Cancer 118:5608–5613
Lai A, Kharbanda S, Pope WB et al (2011) Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol 29:4482–4490
Tsao-Wei DD, Hu J, Groshen SG, Chamberlain MC (2012) Conditional survival of high-grade glioma in Los Angeles County during the year 1990–2000. J Neurooncol 110:145–152
Verhaak RG, Hoadley KA, Purdom E et al (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110
Malkoun N, Chargari C, Forest F et al (2012) Prolonged temozolomide for treatment of glioblastoma: preliminary clinical results and prognostic value of p53 overexpression. J Neurooncol 106:127–133
Krell D, Assoku M, Galloway M, Mullholland P, Tomlinson I, Bardella C (2011) Screen for IDH1, IDH2, IDH3, D2HGDH and L2HGDH mutations in glioblastoma. PLoS ONE 6:e19868
SongTao Q, Lei Y, Si G et al (2012) IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci 103:269–273
Ohgaki H, Dessen P, Jourde B et al (2004) Genetic pathways to glioblastoma: a population-based study. Cancer Res 64:6892–6899
Acknowledgments
We thank Princess Margaret Cancer Centre Cancer Registry. Dr. Mairéad G. McNamara is funded by the Princess Cancer Centre clinical fellowship fund. All other authors have no funding to declare.
Conflict of interest
The authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McNamara, M.G., Lwin, Z., Jiang, H. et al. Conditional probability of survival and post-progression survival in patients with glioblastoma in the temozolomide treatment era. J Neurooncol 117, 153–160 (2014). https://doi.org/10.1007/s11060-014-1368-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1368-7