Abstract
Surgical resection remains an important option for the treatment of brain metastases despite recent advancements in radiotherapy and systemic therapy. When selecting surgical candidates, it is important to exclude terminal cases who will receive neither a survival benefit nor an improvement in their quality of life. We reviewed a total of 264 surgical cases of brain metastases and analyzed the clinical characteristics of early death in order to clarify the indication for and the role of surgery. The median survival time (MST) after surgery in all cases was 12.4 months. Early death was defined as death within 6 months, and 23 % (62 cases) of this series were succumbed to this. A decrease in postoperative Karnofsky performance status (KPS) (<70) (P = 0.041), lack of systemic therapy after surgery (P < 0.0001), and uncontrolled extracranial malignancies (P = 0.0022) were significantly related to early death in multivariate analysis, while preoperative KPS (<70) and recursive partitioning analysis (RPA) class were related to early death only in univariate analysis (P < 0.05). When analyzing patients with uncontrolled extracranial malignancies and those with a postoperative KPS score of 70 or greater (who were generally candidates for systemic therapy), the MST was significantly longer in the systemic therapy (+) group compared with the systemic therapy (−) group (12.5 vs. 5.6 months; P = 0.0026). Our data indicate that the postoperative RPA class and treatment strategy were associated with early death. Deterioration of patients by surgery should be avoided in the treatment of brain metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastasis is a life-threatening event for cancer patients and indicates that cancer has reached the advanced stages. Surgical resection remains an important option for treatment despite recent advancements in radiotherapy and chemotherapy. The aims of surgical resection are mass reduction and rapid improvement of neurological status.
Knowledge regarding the prognosis of extracranial lesions is important when making decisions about surgery. Several studies have attempted to identify prognostic factors, and various classification systems including recursive partitioning analysis (RPA) classification and graded prognostic assessment (GPA) have been developed [1, 2]. These classification systems have mainly been validated in patient populations treated with radiotherapy; however, some reports have indicated that these systems are useful for predicting survival time after surgery [3–9]. Considering the risks associated with treatment, terminal cases who receive neither a survival benefit nor an improvement in their quality of life (QOL) should be excluded during the selection of surgical candidates.
Herein, we describe a retrospective analysis of the relationship between clinical characteristics and the outcome of surgery for brain metastases, and we discuss the indications for and the role of surgery.
Materials and methods
Patients
In total, we included 264 cases (156 men and 108 women) who underwent resection as their first surgery for brain metastases at the National Cancer Center Hospital in Japan between January 2000 and December 2011. The mean age of the included patients was 57.5 years (range 19–87), and their clinical characteristics were extracted from their medical records. Overall survival was calculated from the first resection surgery to death. The Karnofsky performance status (KPS) was determined as recorded or was retrospectively estimated from information obtained from the clinical chart by three neurosurgeons (Y.N., Y.M., and S.S.) who performed surgery on the patients. RPA classification of each patient was performed using published criteria [1]. Preoperative status, including performance status and RPA, was evaluated at the time of surgery, while postoperative status was evaluated approximately 1 month after surgery. The performance status and RPA class of patients who died within 1 month after surgery were recorded as 0 and III, respectively. Information regarding the RPA class and status of extracranial malignancy was not available for 1 case.
The cause of death was determined by clinical evaluation. Neurological deaths were defined as cases with neurological deterioration and stable extracranial disease as well as cases with apparent fatal progression of intracranial lesions or leptomeningeal metastases (LMM) regardless of systemic conditions.
The analysis in this study was approved by the local institutional review board (reference no. NCC16-066).
Treatment
Our basic surgical indications for brain metastases were described in a previous report [10]. Surgical candidates included patients with the following characteristics: (1) a post-surgery life expectancy of 6 months or more based on information from medical oncologists, (2) no clinical symptoms or apparent radiological findings indicating LMM, and (3) single metastases measuring ≧3 cm, or multiple or smaller tumors associated with severe neurological symptoms such as cerebellar metastases. In principle, adjuvant radiotherapy usually began 8 days after surgery. Adjuvant stereotactic radiosurgery (SRS) or stereotactic radiotherapy (SRT) was undergone only for the treatment of the surgical remnant or unresected lesion(s) in patients with multiple metastases. After brain metastases were controlled, patients received further systemic therapy or best supportive care (BSC) according to decisions made by medical oncologists.
A total of 37 patients received RT prior to surgery. In patients who experienced tumor recurrence after radiotherapy, surgical indication was judged via discussion with senior radiologists.
Early death
Early death was defined as death within 6 months after the first surgery for brain metastases, and the clinical profiles between the early death group and the non-early death group were compared. This definition is based on a comparison between the outcome of whole brain radiation therapy (WBRT) and surgery. The median survival time (MST) after WBRT alone is approximately 6 months [11–13]; therefore, if surgery confers a survival benefit, it should extend this time period.
Statistical analysis
Statistical analysis was performed using JMP version 10 (SAS Institute, Cary, NC, USA). The data for survival time were analyzed using the Kaplan–Meier method. A P value below 0.05 was considered statistically significant.
Results
Analysis for all cases
When all cases were analyzed, the median follow-up, MST, 1-year overall survival rate, and 5-year overall survival rate were 11.2, 12.4 months, 52, and 12 %, respectively. The 3 and 6-month overall survival rates were 89 and 75 %, respectively. When patients were divided according to preoperative RPA class, we determined that MST was 21.8 months for class I (59 cases, 22 %), 12.4 months for class II (148 cases, 56 %), and 6.5 months for class III (56 cases, 21 %) (Fig. 1a). When we reevaluated the data using postoperative RPA classification, MST was 20.8 months for class I (66 cases, 25 %), 11.2 months for class II (176 cases, 67 %), and 4.3 months for class III (21 cases, 8 %) (Fig. 1b). Both of pre- and postoperative RPA class were significantly related with survival (P < 0.0001, log-rank test). The relationships between preoperative and postoperative RPA class are shown in Supplementary Table 1.
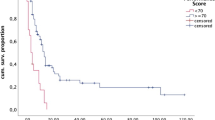
Survival analysis. a Survival curves according to preoperative RPA class. MST was 21.8 months for class I, 12.4 months for class II, and 6.5 months for class III. b Survival curves according to postoperative RPA class. MST was 20.8 months for class I, 11.2 months for class II, and 4.3 months for class III. c Survival curves according to type of adjuvant therapy in patients with high KPS (70 or more) and uncontrolled extracranial malignancies. MST was 12.5 months for the systemic therapy (+) group and 5.6 months for the systemic therapy (−) group. d Survival curves according to postoperative systemic therapy. Group 1 consisted of patients without systemic disease, group 2 consisted of patients undergoing systemic therapy for uncontrolled extracranial disease, and group 3 consisted of patients who had extracranial disease but did not receive systemic therapy. MST was 20.8 months for group 1, 12.4 months for group 2, and 5.1 months for group 3
KPS improved in 53 %, was unchanged in 40 %, and worsened in 7 % of all cases after surgery. Surgical complications were observed in 20 cases (7.6 %) including 8 instances of neurological deterioration due to surgical manipulation, 3 cerebral infarctions, 2 cases requiring evacuation of intraparenchymal hemorrhage, 1 case requiring evacuation of epidural hematoma, 1 case treated conservatively for intraparenchymal hemorrhage, 1 case requiring ventricular drainage for obstructive hydrocephalus, 1 instance of pulmonary embolism, 1 instance of surgical site infection, 1 sudden cardiopulmonary arrest, and 1 instance of vocal paralysis related to intubation. A permanent neurological deficit occurred in 11 (4.2 %) patients, but did not lead to early death in any case. Four patients (1.5 %) succumbed to surgery-related death (i.e., death within 30 days after surgery). Of these, two died of advanced systemic diseases 22 and 30 days after surgery, respectively. The other patients experienced neurological death: 1 died of LMM 23 days after surgery, while the other died of brainstem infarction 17 days after surgery for frontal lobe metastases.
Clinical characteristics of the early death group
A total of 62 patients (23 %) were included in the early death group. The early death rates were 10, 22, and 41 % in preoperative RPA class I, II, and III patients. When patients were divided according to postoperative RPA class, the early death rates were 11, 24, and 57 % in class I, II, and III patients, respectively.
Table 1 shows the results of univariate analysis of data from the early death group and the non-early death group. The early death group contained a significantly higher ratio of patients with multiple brain metastases, KPS <70, uncontrolled primary cancers, and advanced RPA (II or III). The distribution of primary cancers did not differ significantly between these 2 groups. Fewer patients received systemic therapy after the resection of brain metastases in the early death group than in the non-early death group (26 vs. 55 %).
Multivariate logistic regression analysis was performed to identify which factors were most closely related with early death. Only clinical factors with P < 0.1 in univariate analysis (as described above) were used for this analysis. As shown in Table 2, uncontrolled primary tumors or extracranial metastases, lack of postoperative systemic therapy, and a postoperative decrease in KPS (<70) were significantly related to early death.
The impact of postoperative systemic therapy on the survival of patients with uncontrolled extracranial disease
The impact of treatment strategy on survival was further analyzed because postoperative systemic therapy was significantly related with early death in the univariate and multivariate analyses described above. Survival analysis using the Kaplan–Meier method did not reveal a difference in survival between patients in the systemic therapy (+) group (119 cases) and the (−) group (129 cases) (12.9 vs. 10.7 months; P = 0.68, log-rank test). Because systemic therapy is not usually administered to patients with poor performance status or without extra-cranial malignancies, we performed a further analysis including only patients with uncontrolled extracranial malignancies and those with a postoperative KPS of 70 or more. Based on this analysis, the MST was significantly longer in the systemic therapy (+) group (85 cases) than in the systemic therapy (−) group (54 cases) (12.5 vs. 5.6 months; P = 0.0026, log-rank test) (Fig. 1c).
The impact of postoperative treatment strategy on survival
All patients were divided into 3 groups according to treatment course after surgery for brain metastases: group 1 (102 cases) included patients without systemic disease, group 2 (89 cases) included patients who underwent systemic therapy for uncontrolled extracranial disease, and group 3 (65 cases) included patients who had extracranial disease but did not receive systemic therapy. Group 3 patients were treated with best supportive care. The MSTs of groups 1, 2, and 3 were 20.8, 12.4, and 5.1 months, respectively, and the difference among the groups was significant (P < 0.0001, log-rank test) (Fig. 1d). The early death rate was 12 % in group 1, 16 % in group 2 and 55 % in group 3, and the early death rate of group 3 was significantly higher than that of the other groups (P < 0.0001, Pearson’s Chi square test).
Cause of death
Data regarding cause of death was available for 55 of the early death cases. Twenty patients (32 %) died from neurological causes, while 35 patients (56 %) died from systemic diseases. Thirteen of the neurological deaths were attributed to LMM. The adjuvant radiation therapies used in LMM cases were WBRT in 5 and local brain radiation therapy in 3 cases. Five cases did not receive either therapy. Other neurological deaths were due to progression of brain metastases after RT (6 cases) and brain stem infarction (1 case).
Postoperative status and survival time in preoperative RPA class III patients
Patients assessed as preoperative RPA class III (n = 56) typically have shorter survival times; therefore, the clinical courses of these patients were further analyzed in order to evaluate the potential treatment benefit. Of these patients, 8 (14 %), 31 (55 %), and 17 (30 %) were postoperative RPA class I, II, and III, respectively. When patients were divided according to postoperative RPA class, MST was 13.6, 6.5, and 3.6 months in class I, II, and III patients, respectively. MST was significantly longer in patients who experienced an improvement in postoperative RPA class (n = 39) compared with patients who remained in class III (n = 17) (6.9 vs. 3.6 months; P = 0.019, log-rank test). KPS was improved in 43 (77 %), unchanged in 10 (18 %), and worsened in 3 (5.4 %) preoperative RPA class III cases after surgery.
We further analyzed the cases showing RPA class III preoperatively but better RPA class postoperatively (I, 8 cases; II 31 cases) in order to discuss the operative indication for preoperative RPA class III patients (Supplementary Table 1). Twelve cases (31 %) of this cohort (39 cases) succumbed to early death after surgery, and their postoperative RPA class was I in one and II in 11. The causes of their early death were mainly consisted of systemic death; systemic disease in 8 cases, leptomeningeal metastasis in 2 cases and unknown in 2 cases. To identify what factor contributed to the early death in this cohort (39 cases), the postoperative treatment strategy was compared between the early death cases (12 cases) and the non-early death cases (27 cases). Eight of the 12 early death cases received best supportive care while 7 of the 25 non-early death cases (2 cases lacked the data) did. Thus, lack of postoperative systemic therapy was also statistically related with the early death in this cohort despite improvement in RPA class (8/12 vs. 7/25; P = 0.025, Pearson’s Chi square test).
Discussion
In this study, we reviewed a surgical series from a single center and focused on the clinical characteristics of cases with poorer prognosis. Comparing with the recent studies presenting their surgical outcome, our series showed the comparable survival time [3, 6, 7, 9] according to RPA class and the comparable complication rate (7.6 vs. 4.5–14 %) despite the high ratio of RPA class III (21 vs. 5.7–6.8 %) [6, 14, 15]. We showed that postoperative treatment strategy and performance status were the significant factors for early death in multivariate analysis.
Systemic therapy after surgery was previously reported as being significantly related to survival time, but this was contradicted by the result in multivariate analysis [6]. This result simply seems to reflect the bias of the analysis: systemic therapy is usually avoided in patients with poorer performance status or patients without uncontrolled extracranial malignancy. We further analyzed only patients with favorable postoperative KPS scores and uncontrolled extracranial malignancies to ensure that we were only analyzing patients who truly needed further treatment for primary cancer. We showed that postoperative systemic therapy had a significant effect on survival in this population (Fig. 1c). Similarly, multivariate analysis showed that a lack of postoperative systemic therapy was a significant factor for early death, which was mainly analyzed in this study (Table 2). Thus, the treatment strategy for extracranial malignancies should be considered when determining operative indication, and this is supported by the results described in Fig. 1d. In other words, patients who cannot undergo chemotherapy (e.g., due to multidrug resistance to systemic therapy) are at high risk of early death after surgery. We also subjected our cohort to further analysis for survival by dividing three groups time according to the operative period (2000–2003, 2004–2007 and 2008–2011), but the difference in OS or early death rate was not apparent (data not shown). Despite the recent advances in systemic therapeutic agents, brain metastases may arise after acquiring drug resistance even for newly developed agents, and the survival after brain metastases might depend largely on whether further systemic therapy can be available or not.
One of the challenges in our study was evaluating both preoperative and postoperative status. The prognostic significance of pre- and postoperative RPA class was previously analyzed, and the multivariate analysis showed that only preoperative RPA was significant [9]. This observation was, however, based simply on the analysis of survival time. Our analysis differed from the previous study because we evaluated the factor related to early death and specifically analyzed the group with the poorest prognosis: preoperative RPA class III patients. In the present study, postoperative RPA class was related to survival and a higher early death rate, and the early death rate was extremely high in preoperative RPA class III patients without postoperative improvement. Because RPA class III simply indicates a poor KPS score (<70), improvement in performance status is a significant factor for survival in preoperative RPA class III patients. Therefore, when determining the indications for surgery in preoperative RPA class III patients, it is important to consider whether surgery is likely to improve KPS. Patients who are not likely to experience an improvement in performance status are also not likely to obtain a survival benefit. However, it is important to remember that the postoperative treatment strategy is also significant factor for survival as shown in our analysis for RPA class III patients.
Finally, we analyzed the cause of death. Previous studies have reported a neurological death rate of 15–37 % after surgery for brain metastases [5, 11, 13, 16–25] (Table 3). Our results were in line with this, although one limitation of our study was that the cause of death was available only for early death cases. Of note, 21 % (13/62) of early death cases were attributed to LMM in this study. Recent large studies reported a 5–16 % incidence of LMM after surgical removal [14, 17, 26, 27]. Considering these results, LMM appears to occur early after surgery and may be a significant cause of early death. An increased incidence of early death might be attributed to either (1) preoperative undiagnosed LMM without apparent radiological findings because of a lack of routine cerebrospinal fluid cytology [26] or (2) LMM caused by the surgery itself. In fact, several previous reports have shown an increased risk of LMM after surgery compared with SRS alone [14, 26–28]. In order to reduce early deaths due to LMM, adjuvant therapies will need to be developed. The protective effect of adjuvant radiation therapy for LMM remains controversial, and recent studies have failed to demonstrate this effect [14, 26]. Further studies are needed to clarify the efficacy of radiation therapy.
In summary, early death after resection of brain metastases can be attributed to neurologic factors and systemic factors. Of the neurological factors, LMM is a critical factor that is related to early death. Further studies exploring the prevention and treatment of LMM are necessary. Of the systemic factors, a poor performance status after surgery (rather than before surgery), uncontrolled extracranial malignancies, and a lack of systemic therapy after surgery are related to early death. The limitation of our retrospective study lies in the possibility of the bias derived from patient selection. Further analysis including non-surgically treated cases may confirm our observations. When making decisions regarding surgery for brain metastases, physicians should be aware of the importance of a systemic treatment strategy after surgery, while surgeons should recognize that a poor performance status deprives patients of QOL and a chance for systemic therapy. The role of surgery for brain metastases is not only to improve the QOL and prevent neurological death but also to give patients a chance for further systemic therapy.
References
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37:745–751
Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, Bhatt A, Jensen AW, Brown PD, Shih H, Kirkpatrick J, Schwer A, Gaspar LE, Fiveash JB, Chiang V, Knisely J, Sperduto CM, Mehta M (2010) Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 77:655–661. doi:10.1016/j.ijrobp.2009.08.025
Tendulkar RD, Liu SW, Barnett GH, Vogelbaum MA, Toms SA, Jin T, Suh JH (2006) RPA classification has prognostic significance for surgically resected single brain metastasis. Int J Radiat Oncol Biol Phys 66:810–817. doi:10.1016/j.ijrobp.2006.06.003
Golden DW, Lamborn KR, McDermott MW, Kunwar S, Wara WM, Nakamura JL, Sneed PK (2008) Prognostic factors and grading systems for overall survival in patients treated with radiosurgery for brain metastases: variation by primary site. J Neurosurg 109(Suppl):77–86. doi:10.3171/JNS/2008/109/12/S13
Agboola O, Benoit B, Cross P, Da Silva V, Esche B, Lesiuk H, Gonsalves C (1998) Prognostic factors derived from recursive partition analysis (RPA) of Radiation Therapy Oncology Group (RTOG) brain metastases trials applied to surgically resected and irradiated brain metastatic cases. Int J Radiat Oncol Biol Phys 42:155–159
Paek SH, Audu PB, Sperling MR, Cho J, Andrews DW (2005) Reevaluation of surgery for the treatment of brain metastases: review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery 56:1021–1034 (discussion 1021–1034)
Nieder C, Astner ST, Andratschke NH, Marienhagen K (2011) Postoperative treatment and prognosis of patients with resected single brain metastasis: how useful are established prognostic scores? Clin Neurol Neurosurg 113:98–103. doi:10.1016/j.clineuro.2010.09.009
Chidel MA, Suh JH, Reddy CA, Chao ST, Lundbeck MF, Barnett GH (2000) Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys 47:993–999. doi:S0360-3016(00)00527-7
Schackert G, Lindner C, Petschke S, Leimert M, Kirsch M (2013) Retrospective study of 127 surgically treated patients with multiple brain metastases: indication, prognostic factors, and outcome. Acta Neurochirurg. doi:10.1007/s00701-012-1606-8
Narita Y, Shibui S (2009) Strategy of surgery and radiation therapy for brain metastases. Int J Clin Oncol Jpn Soc Clin Oncol 14:275–280. doi:10.1007/s10147-009-0917-0
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ Jr (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672. doi:10.1016/S0140-6736(04)16250-8
Datta R, Jawahar A, Ampil FL, Shi R, Nanda A, D’Agostino H (2004) Survival in relation to radiotherapeutic modality for brain metastasis: whole brain irradiation versus gamma knife radiosurgery. Am J Clin Oncol 27:420–424
Mintz AH, Kestle J, Rathbone MP, Gaspar L, Hugenholtz H, Fisher B, Duncan G, Skingley P, Foster G, Levine M (1996) A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 78:1470–1476
Suki D, Hatiboglu MA, Patel AJ, Weinberg JS, Groves MD, Mahajan A, Sawaya R (2009) Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery 64:664–674. doi:10.1227/01.NEU.0000341535.53720.3E (discussion 674–666)
Lee CH, Kim DG, Kim JW, Han JH, Kim YH, Park CK, Kim CY, Paek SH, Jung HW (2013) The role of surgical resection in the management of brain metastasis: a 17-year longitudinal study. Acta Neurochirurg. doi:10.1007/s00701-013-1619-y
Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N, Metsaars JA, Wattendorff AR et al (1993) Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 33:583–590. doi:10.1002/ana.410330605
Hashimoto K, Narita Y, Miyakita Y, Ohno M, Sumi M, Mayahara H, Kayama T, Shibui S (2011) Comparison of clinical outcomes of surgery followed by local brain radiotherapy and surgery followed by whole brain radiotherapy in patients with single brain metastasis: single-center retrospective analysis. Int J Radiat Oncol Biol Phys 81:e475–e480. doi:10.1016/j.ijrobp.2011.02.016
Muacevic A, Wowra B, Siefert A, Tonn JC, Steiger HJ, Kreth FW (2008) Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol 87:299–307. doi:10.1007/s11060-007-9510-4
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483–2491. doi:10.1001/jama.295.21.2483
Manon R, O’Neill A, Knisely J, Werner-Wasik M, Lazarus HM, Wagner H, Gilbert M, Mehta M (2005) Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, and sarcoma: an Eastern Cooperative Oncology Group study (E 6397). J Clin Oncol 23:8870–8876. doi:10.1200/JCO.2005.01.8747
Serizawa T, Saeki N, Higuchi Y, Ono J, Iuchi T, Nagano O, Yamaura A (2005) Gamma knife surgery for brain metastases: indications for and limitations of a local treatment protocol. Acta Neurochirurg 147:721–726. doi:10.1007/s00701-005-0540-4 (discussion 726)
Jawahar A, Matthew RE, Minagar A, Shukla D, Zhang JH, Willis BK, Ampil F, Nanda A (2004) Gamma knife surgery in the management of brain metastases from lung carcinoma: a retrospective analysis of survival, local tumor control, and freedom from new brain metastasis. J Neurosurg 100:842–847. doi:10.3171/jns.2004.100.5.0842
Petrovich Z, Yu C, Giannotta SL, O’Day S, Apuzzo ML (2002) Survival and pattern of failure in brain metastasis treated with stereotactic gamma knife radiosurgery. J Neurosurg 97:499–506. doi:10.3171/jns.2002.97.supplement5.0499
Wronski M, Arbit E, Burt M, Galicich JH (1995) Survival after surgical treatment of brain metastases from lung cancer: a follow-up study of 231 patients treated between 1976 and 1991. J Neurosurg 83:605–616. doi:10.3171/jns.1995.83.4.0605
Bindal RK, Sawaya R, Leavens ME, Lee JJ (1993) Surgical treatment of multiple brain metastases. J Neurosurg 79:210–216. doi:10.3171/jns.1993.79.2.0210
Ahn JH, Lee SH, Kim S, Joo J, Yoo H, Shin SH, Gwak HS (2012) Risk for leptomeningeal seeding after resection for brain metastases: implication of tumor location with mode of resection. J Neurosurg 116:984–993. doi:10.3171/2012.1.JNS111560
Suki D, Abouassi H, Patel AJ, Sawaya R, Weinberg JS, Groves MD (2008) Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg 108:248–257. doi:10.3171/JNS/2008/108/2/0248
van der Ree TC, Dippel DW, Avezaat CJ, Sillevis Smitt PA, Vecht CJ, van den Bent MJ (1999) Leptomeningeal metastasis after surgical resection of brain metastases. J Neurol Neurosurg Psychiatry 66:225–227
Acknowledgments
This work was supported in part by Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan [No. 24592180 (YN) and 24659650 (HA)].
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arita, H., Narita, Y., Miyakita, Y. et al. Risk factors for early death after surgery in patients with brain metastases: reevaluation of the indications for and role of surgery. J Neurooncol 116, 145–152 (2014). https://doi.org/10.1007/s11060-013-1273-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1273-5