Abstract
Glioblastoma multiforme is a highly migratory and invasive brain tumor in which hypoxia inducible factor-1α (HIF-1α) plays important roles. However, the underlying mechanisms regulating the action of HIF-1α in glioma cell migration and invasion ability remain unclear. We reported here that HIF-1α was regulated by geranylgeranyltransferase I (GGTI), a protein prenylation transferase, and then promoted glioma cell migration and invasion. The migratory and invasive ability of glioma cells were enhanced by hypoxia treatment but inhibited by down-regulation of HIF-1α. GGTI activity inhibition or GGTI specific β subunit (GGTI β) knocking-down decreased HIF-1α protein level. In addition, down-regulation of GGTI β inhibited migration and invasion of glioma cells under hypoxia, while GGTI β over-expression promoted it. Furthermore, the effect of GGTI β over-expression on cell migration and invasion was abolished by HIF-1α down-regulation. In summary, our study showed, for the first time, that HIF-1α was regulated by protein prenylation transferase GGTI and mediated the effect of GGTI on glioma cell migration and invasion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma multiforme (GBM) is the most common and malignant primary tumor developed in the central nervous system. Despite advances in surgery and adjuvant therapy, the median overall survival time does not exceed 15 months [1–3] due to the highly invasive capacity of tumor cells. Hypoxia and its master regulator, hypoxia inducible factor-1α (HIF-1α) play key roles in glioma migration and invasion [4–7].
Although HIF-1α maintains at a low level in normoxic cells due to proteasome mediated protein degradation [8], it plays a pivotal role in the cellular response to tumor hypoxia, which represents a major obstacle to the success of radiotherapy and chemotherapy [7]. It is reported that HIF-1α was upregulated in many common human cancers, such as prostate cancer, breast cancer and glioma [6, 9–11]. Suppression of HIF-1α expression via antisense oligonucleotides reduces the survival of glioblastoma cells and accelerates p53-independent apoptosis [12]. In addition, knocking-down of HIF-1α by RNA interference attenuates human glioma cell migration and invasion [7, 13, 14]. Although HIF-1α function is extensively studied under hypoxic stress, the mechanism regulating the action of HIF-1α is still unclear. Given its pivotal role in cancer biology, the underlying mechanisms involved in the activation of HIF-1α in glioma cells need to be further understood.
It is reported that members of the Rho small GTPases, which play essential roles in a variety of cellular events such as cell adhesion and invasion [15, 16], are activated in response to hypoxia and required for the induction of HIF-1α expression and transcriptional activity in hypoxic cells [17–19]. Rho GTPases recycle between GTP-bound active form and GDP-bound inactive states, and this process is regulated by GTPase activating proteins and guanine nucleotide exchange factors [20]. Importantly, before being activated by combining with GTP, Rho GTPases need to be translocated from the cytosol to the plasma membrane [21], which is achieved by prenylation, a lipid modification mainly catalyzed by geranylgeranyltransferase I (GGTI). GGTI acts to covalently couple a lipid moiety to the cystine of C-terminal “CAAX” box (Cys-aliphatic-aliphatic-X) of the GTPases [21, 22]. GGTI has two subunits: an α subunit (GGTI α) which is shared with farnesyltransferase (FT) and a distinct β subunit (GGTI β) [23]. As for FT, it has another distinct β subunit highly homologous with GGTI [23].
Since Rho small GTPases are demonstrated to be upregulated or highly mutated in many types of human tumors [15, 16], GGTI has been considered as a good cancer therapeutic target and great efforts have been made to develop GGTI inhibitors as anticancer drugs [23]. Many studies reported that GGTI specific inhibitors could induce apoptosis and inhibit cell proliferation in many tumors [23–25]. However, little is known about the migratory and invasive effect of GGTI on tumor cells.
In summary, because Rho GTPases could induce the expression of HIF-1α in hypoxic cells [17–19] and their activation need to be prenylated by GGTI first, we deduced that GGTI might regulate HIF-1α and play roles in tumor cell migration and invasion. In the present study, we found that HIF-1α promoted glioma cell migration and invasion, while down-regulation of HIF-1α inhibited it. GGTI regulated HIF-1α induction and GGTI β down-regulation inhibited glioma cell migration and invasion, similar to that of HIF-1α. Finally, over-expression of GGTI β induced migration and invasion were abrogated by HIF-1α down regulation. These results suggested that GGTI regulated HIF-1α induction, and then promoted glioma cell migration and invasion.
Materials and methods
Materials
U251 and U87 cells were purchased from Shanghai Cell Bank, Type Culture Collection Committee, Chinese Academy of Sciences. pCAG–YFP–GGTI β was kindly gifted by professor ZG Luo at Shanghai Institute of Neuroscience of Chinese Academy of Sciences. Dulbecco’s modified Eagle’s medium, F-12 (DMEM/F-12) and Lipofectamine 2000 were purchased from Invitrogen (Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from Sijiqing Biological Engineering Materials Co., Ltd. Transwell invasion chambers were purchased from Corning Incorporated (Corning, USA). GGTI-298 was obtained from Calbiochem (Schwallbach, Germany). Following antibodies were used in this study: rabbit polyclonal anti-GGTI β (kindly gifted by professor ZG Luo); mouse monoclonal anti-HIF-1α (BD Transduction Laboratories, USA); mouse monoclonal anti-FTβ and rabbit polyclonal anti-GGTα/FTα (Santa Cruz Biotech, USA); mouse monoclonal anti-β-actin (Millipore Co., USA).
Cell culture
U251 and U87 cells were cultured in 5 % CO2 and 95 % humidified atmosphere air at 37 °C in Dulbecco’s modified Eagle’s medium, F-12 (DMEM/F-12) supplemented with 10 % FBS. For hypoxic exposure, cells were placed in a sealed Modular Incubator Chamber (150i, thermo Inc., USA) flushed with a gas mixture containing 1 % O2, 5 % CO2 and 94 % N2.
Small interfering RNAs and plasmids transfection
U251 and U87 cells were transiently transfected with siRNAs by using Lipofectamine 2000 according to the manufacturer’s instructions. siRNA duplexes purchased from Shanghai GenePharma Co. were listed below:
-
HIF-1α si1217: (5′-GCCGCUCAAUUUAUGAAUATT-3′);
-
HIF-1α si2791: (5′-CCAGUUAUGAUUGUGAAGUUATT-3′);
-
GGTI β si217: (5′-GGUGAACAAAGAUGAUAUATT-3′);
-
GGTI β si1015: (5′-GGAGGAAAGUGGAAUUUGUTT-3′).
Wound healing assay
A scratch was made in the middle of the well with a pipette tip 24 h after transfection and the monolayer was washed twice with phosphate-buffered saline (PBS, pH 7.4). Thereafter, the cells were maintained in serum-free media and incubated in hypoxic condition (1 % O2) at 37 °C for 24 h. Then five randomly selected fields at the lesion border were photographed using an inverted microscope (Olympus IX71). The number of cells across the wound was normalized to that of hypoxia group.
Invasion assay
Cell invasion was assessed by matrigel precoated transwell inserts (8.0-μm pore size with polyethylene tetraphthalate membrane) according to the manufacturer’s protocol. To assess invasion, filters were precoated with 10 μg of matrigel (BD Biosciences). A pretreated cell suspension (1 × 105) in serum-free culture media was added into the inserts, and each insert was placed in the lower chamber filled with culture media containing 10 % FBS as a chemoattractant. The invasion chambers were incubated in hypoxic condition (1 % O2) at 37 °C for 24 h. And then the invasion assay was performed as described [26].
Gelatin zymography assay
Cells (5 × 105) were seeded in 6-well plates in DMEM/F-12 media containing 10 % FBS for 24 h and transfected with GGTI β siRNA or HIF-1α siRNA. Thereafter, cells were incubated in serum-free DMEM/F-12 for an additional 24 h in hypoxic condition (1 % O2) at 37 °C. Then preformed as described previously [27].
Western blot analysis
At the designated time, the cells were removed quickly from the hypoxic chamber, washed twice with PBS, and lysed using ice-cold sodium dodecyl sulphate (SDS) buffer (60 mM Tris–HCl pH 6.8, 70 mM SDS, 10 % Glycerol) supplemented with protease inhibitors, and western blot was performed as described [28]. Band densities were quantified by ImageJ software (NIH; version 1.45), and the densitometric results were shown. The relative amount of proteins was determined by normalizing the densitometry value of interest to that of the internal loading control.
Statistical analysis
The results are representative of experiments repeated at least three times and presented as the mean ± SEM. All statistical analyses were performed using SPSS software (SPSS; version 17.0). Statistical comparisons were performed using Student’s t test with two tails or one-way ANOVA for multiple comparisons. P values <0.05 were considered statistically significant (*P < 0.05).
Results
Hypoxia promotes the migration and invasion of glioma cells
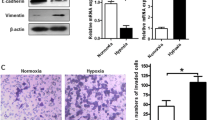
It is reported that HIF-1α is activated under hypoxia and plays a key role in glioma migration and invasion [6]. But the mechanism regulating the action of HIF-1α is still unclear. To further elucidate the role and regulating mechanism of HIF-1α in malignant gliomas, we first investigated the effects of HIF-1α on glioma U251 cell migration and invasion. As is shown in Fig. 1a, the protein level of HIF-1α increased at 6, 12, 24 and 48 h after hypoxia, and had a peak at 6 and 12 h, suggesting that hypoxia model has established successfully. The result of wound healing assay revealed that the wound healed obviously and had the tendency to fuse under hypoxia (Fig. 1b). Compared with the normoxic conditions, the migratory cell numbers increased by 46 % under hypoxic conditions (Fig. 1c). In addition, the result of matrigel precoated transwell assay showed that the number of invasive cells in hypoxia was higher than that under normoxic conditions by 67 % (Fig. 1d, e), suggesting that hypoxia also promoted cell invasion.
Hypoxia promotes the migration and invasion of glioma cells. a U251 glioma cells were grown under normoxia (20 % O2) or hypoxia (1 % O2) conditions for the indicated time. The protein level of HIF-1α was analyzed by Western blot. β-actin served as an internal control. b, c Hypoxia promotes glioma cell migration examined by wound healing assay. Representative digital pictures were taken at 0 and 24 h. d, e Hypoxia promotes glioma cell invasion examined by matrigel precoated transwell assay. f Hypoxia promotes MMP2 excretion examined by gelatin zymography. Results are presented as mean ± SEM from three separate experiments. Scale bar 100 μm. *P < 0.05
Many studies have pointed out a strong interaction between invasiveness of human gliomas and degradation of extracellular matrix (ECM) by matrix metalloproteases (MMPs), especially by MMP2 [29, 30]. Thus, the MMPs activity is considered as an index of tumor invasion ability. We therefore detected the effect of hypoxia treatment on the excretion of MMP-2 by gelatin zymography. As is shown in Fig. 1f, hypoxia treatment increased the excretion of MMP-2 significantly (Fig. 1f). Collectively, all these results indicated that hypoxia treatment promoted cell migration and invasion, consistent with the previous studies [6, 7, 12–14].
Down-regulation of HIF-1α inhibits hypoxia-induced cell migration and invasion
Next, we observed the migration and invasion behavior of cells by down-regulating the HIF-1α using RNA interference approach. After being transiently transfected with HIF-1α siRNAs (si-1217 and si-2791), the cells were harvested and the down-regulation efficacy was examined by western blotting. The results showed that both siRNAs knocked down HIF-1α remarkably (Fig. 2a).
Down-regulation of HIF-1α inhibits hypoxia-induced glioma cell migration and invasion. a Immunoblot was applied to detect the effect of HIF-1α si1217 and si2791 on HIF-1α expression. β-actin was used as a loading control. b–e 24 h after being transfected with siNC, HIF-1α si217 and si2791, cells were subjected to wounding assay and invasion assay under hypoxia condition. f 24 h after transfection, cells were exposed to hypoxia condition for further 24 h and the supernatant was harvested and subjected to gelatin zymography. Scale bar 100 μm. Results are presented as the mean ± SEM from three experiments. *P < 0.05
The result of wound healing assay showed that the number of migratory cells transfected with HIF-1α si1217 and si2791 was significantly reduced to 59 and 51 % respectively, compared with siNC group under hypoxia conditions (Fig. 2b, c). Furthermore, HIF-1α si1217 and si2791 decreased the number of invasive cells by about 47 and 45 % respectively (Fig. 2d, e), which were examined by matrigel precoated transwell assay. To further confirm the above result, we also used gelatin zymography to study the effect of HIF-1α down-regulation on the excretion of MMP-2. The intensity of the gelatinolytic bands clearly decreased to 59 and 60 % in U251 cells transfected with HIF-1α si1217 or si2791 respectively (Fig. 2f). These results indicated that down-regulation of HIF-1α reduced glioma cell migration and invasion under hypoxia conditions.
GGTI regulates HIF-1α induction under hypoxic conditions
It is reported that Rho small GTPases are upregulated in hypoxia-driven angiogenesis and are involved in HIF-1α induction [17–19]. Turcotte et al. [31] even reported that the upregulated protein level of Cdc42, Rac1 and RhoA, the known substrates of GGT, was mainly located at membranes after hypoxia. Since Rho family members need to be prenylated by GGTI and then translocated to membranes before being activated [21], the above reports hinted us the possibility that GGTI may regulate HIF-1α under hypoxia. Thus, we detected the protein level of GGTI β upon hypoxia treatment first. Unexpectedly, we have not found any increase in GGTI β protein level (Fig. 3a). However, we found that the total and membrane protein level of RhoA increased gradually after hypoxia treatment, similar to the change of HIF-1α, suggesting that GGTI activity increased under hypoxia condition (Fig. 3a). Next, we used the siRNAs to downregulate GGTI β and examined the level of HIF-1α. As the Fig. 3b, c showed, GGTI β siRNAs (GGTIβ si217 and si1015) knocked down GGTI β significantly (Fig. 3b), but have not affect the level of GGTα/FTα or FTβ (Fig. 3c), suggesting the specificity of GGTI β siRNA. In addition, we found that the protein level of HIF-1α decreased after down-regulation of GGTI β under either normoxia or hypoxia conditions in both glioma U251 and U87 cells (Fig. 3d–f). Similarly, GGTI-298, a GGTI specific inhibitor, decreased the protein level of HIF-1α by 87 and 91 % under normoxia and hypoxic conditions, respectively (Fig. 3g). On the contrary, we tested the possibility that HIF-1α regulates GGTI. Since HIF-1α si2791 had a better down-regulating efficiency on HIF-1α than that of HIF-1α si-1217 (Fig. 2a), we selected HIF-1α si2791 to do this experiment. The result showed that in glioma U251 and U87 cells, down-regulation of HIF-1α has not change the protein level of GGTI β (Fig. 3h–j) under either normoxia or hypoxic conditions, suggesting that GGTI was located at the upstream of HIF-1α and could regulate HIF-1α in glioma cells.
GGTI regulates HIF-1α induction under normoxia and hypoxia conditions in glioma cells. a U251 glioma cells were grown under hypoxia (1 % O2) conditions for the indicated time. The protein level of GGTI β, Rho and the membrane level of Rho were analyzed by western blot. β-actin or Na+-K+-ATPase served as internal control respectively. b The effect of GGTI β si217, si1015 or siNC on GGTI β expression in glioma cells. c U251 cells were transfected with siNC and GGTI β si1015, and exposed to 1 % O2 for 24 h. The level of FTα and FTβ were analyzed by Western blot. d U251 and U87 cells were transfected with siNC and GGTI β si1015, and exposed to normoxia or hypoxia conditions for 24 h. GGTI β and HIF-1α level was analyzed by western blot. e, f Quantitative analysis of the relative protein levels of HIF-1α and GGTI β in glioma U251 and U87 cells. g U251 cells were treated with DMSO or GGTI-298 and exposed to normoxia or hypoxia conditions for 24 h. HIF-1α protein expression was analyzed by Western blot. h U251 and U87 cells were transfected with siNC and HIF1-α si2791, and exposed to normoxia or hypoxia conditions. GGTI β and HIF-1α level was analyzed by Western blot. i, j Quantitative analysis of the relative protein levels of HIF-1α and GGTIβ in glioma U251 and U87 cells. β-actin was used as the loading control. Results are presented as the mean ± SEM from three experiments. *P < 0.05
Depletion of GGTI β inhibits cell migration and invasion under hypoxia
Next, we wonder whether GGTI had a similar effect to that of HIF-1α on cell migration and invasion. As is shown in Fig. 4a, b, compared with siNC group, the number of migratory cells transiently transfected with GGTI β si217 or si1015 was reduced by 33 and 31 % in glioma U251 cells, 34.5 and 38 % in glioma U87 cells under hypoxic conditions respectively. Similarly, the invasive ability after down-regulation of GGTI β was reduced to 61 and 59 % (U251 cells), 57 and 48 % (U87 cells) of the level of siNC group respectively (Fig. 4c, d). In addition, down-regulation of GGTI β reduced the excretion of MMP2 significantly (Fig. 4e). These results indicated that, similar to the effect of HIF-1α, depletion of GGTI β reduced cell migration and invasion under hypoxia conditions.
Depletion of GGTI β inhibits hypoxia-induced cell migration and invasion. a–d U251 and U87 cells were transfected with siNC, GGTI β si217 and si1015. After 24 h of transfection, cells were subjected to wounding healing assay or invasion assay under hypoxia condition. Representative microphotographs are shown. e 24 h after transfection, cells were exposed to hypoxia condition for further 24 h and the supernatant was harvested and subjected to gelatin zymography. Scale bar 100 μm. Results are presented as the mean ± SEM from three independent experiments. *P < 0.05
GGTI regulates cell migration and invasion via HIF-1α
The above data indicated that GGTI could not only regulate HIF-1α, but also affect the migratory and invasive behavior of glioma cells. Therefore, we examined whether HIF-1α could mediate the function of GGTI in cell migration and invasion by co-transfecting GGTI β and HIF-1α si2791. U251 cells were transfected with pCAG–YFP–GGTI β plasmid, which expressed YFP at the same time to indicate the positive cells, and the GGTI β overexpression was verified by the YFP fluorescence and western blot (Fig. 5a, b). In addition, western blot result showed that the GGTI β and HIF-1α si2791 were co-transfected into glioma U251 cells successfully (Fig. 5b) [32]. In line with the above result (Fig. 2b, c), the number of migratory cells decreased after down-regulation of HIF-1α in hypoxia and increased after GGTI β over-expression (Fig. 5c, d). Furthermore, the effect of GGTI β over-expression on cell migration and invasion was partially abolished by depletion of HIF-1α (Fig. 5c–f). In addition, GGTI β over-expression also increased the excretion of MMP2, which was blocked by down-regulation of HIF-1α at the same time. Above results suggested that GGTI regulates cell migration and invasion partially via HIF-1α in glioma cells under hypoxia.
GGTI β regulates cell migration and invasion via HIF-1α in glioma cells. a, b 24 h after being transfected with indicated plasmid and/or siRNA, cells were exposed to hypoxia conditions for further 24 h. YFP fluorescence and immunoblot was applied to detect the transient co-transfection efficiency. c–f Cell invasion and migration were examined by using cell invasion assay and wound healing assay. g 24 h after transfection, cells were exposed to hypoxia condition for further 24 h and the supernatant was harvested and subjected to gelatin zymography. Results are presented as the mean ± SEM from three independent experiments. Bar indicates 100 μm. *P < 0.05
Discussion
Hypoxia is one feature of glioblastoma, which is associated with poor prognosis, increased angiogenesis, tumor growth and resistance to radio- and chemotherapy [13]. HIF-1α, the key hypoxia regulatory factor, has been shown to be important in promoting both angiogenesis and invasion. Upregulation of HIF-1α induced by hypoxic stress is an essential event in the activation of glioma cell motility through alteration of invasion-related molecules, while knocking down of HIF-1α in glioma cells reduces migration and invasion [6, 7, 12–14]. Our data also showed that hypoxia promotes the migration and invasion of glioma cells, and down-regulation of HIF-1α inhibits them (Figs. 1, 2). These data supported that HIF-1α and hypoxia play important roles in glioma cell migration and invasion [4–6, 33] and that inhibition of HIF-1α is an attractive therapeutic strategy to target the tumor microenvironment.
Although the role of HIF-1α in glioma cell progression is well elucidated [6, 7, 12–14], the regulatory mechanism is not clear so far. Some studies demonstrated that mTOR and Myc could regulate HIF-1α induction [34–37]. Some reports suggested that the Rho small GTPases are upregulated under hypoxia and also involved in HIF-1α induction [17–19]. Rho small GTPases are often upregulated or mutated in tumors and play important roles in cell migration and invasion [16, 38–41]. The biological function of Rho GTPases depends on post-translational isoprenylation by GGTI, which in turn leads to their attachment to plasma membranes and then activation [21]. In our study, although the protein level of GGTI β was not increased under hypoxia, the total and membrane level of RhoA was up-regulated after hypoxia treatment, consistent with the previous studies [31, 42], suggesting that GGTI was activated under hypoxia. And we also found that specific down-regulation of GGTI β can reduce the expression of HIF-1α while the level of GGTI β has not changed after HIF-1α down-regulation, suggesting the regulation of GGTI on HIF-1α expression (Fig. 3).
It has been shown that a number of geranylgeranylated proteins play important roles in tumorigenesis and metastasis [23, 43, 44]. Many studies have reported that GGT inhibitors could induce apoptosis and inhibit cell proliferation in many tumors [43–46]. Similarly, we found that down-regulation of GGTI β also inhibited cell migration and invasion while GGTI β over-expression promoted them under hypoxia (Fig. 4, 5). Furthermore, the effect of GGTI on cell migration and invasion under hypoxia was partially abolished by knocking down of HIF-1α (Fig. 5). These results suggested HIF-1α mediated the effect of GGTI on cell migration and invasion.
Taken together, the results presented here indicated that GGTI regulated cell migration and invasion partially via HIF-1α. For the first time, our data provided the molecular mechanism of GGTI–HIF-1α signal pathway involved in glioma cell migration and invasion under hypoxia. As for the roles of Rho GTPases, the bridge between GGTI and HIF-1α, will be further studied in GGTI–HIF-1α signal pathway, which might play an important role in glioma progression.
Abbreviations
- GGTI:
-
Geranylgeranyltransferase I
- HIF-1α:
-
Hypoxia inducible factor-1 α
- ECM:
-
Extracellular matrix
- FT:
-
Farnesyltransferase
References
Clarke J, Butowski N, Chang S (2010) Recent advances in therapy for glioblastoma. Arch Neurol 67:279–283
Meyer MA (2008) Malignant gliomas in adults. N Engl J Med 359:1850 (author reply 1850)
Minniti G, De Sanctis V, Muni R, Filippone F, Bozzao A, Valeriani M, Osti MF, De Paula U, Lanzetta G, Tombolini V, Maurizi Enrici R (2008) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol 88:97–103
Wilson WR, Hay MP (2011) Targeting hypoxia in cancer therapy. Nat Rev Cancer 11:393–410
Vordermark D (2005) Significance of hypoxia in malignant glioma. Re: Evans et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res 2004;10:8177–8184; Clin Cancer Res 11:3966–3967 (author reply 3967–3968)
Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG (2005) Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol 7:134–153
Kessler J, Hahnel A, Wichmann H, Rot S, Kappler M, Bache M, Vordermark D (2010) HIF-1alpha inhibition by siRNA or chetomin in human malignant glioma cells: effects on hypoxic radioresistance and monitoring via CA9 expression. BMC Cancer 10:605
Tao BB, Zhang CC, Liu SY, Zhu YC (2012) Involvement of HIF-1 in the migration-promoting effects of hydrogen sulfide in vascular endothelial cells under normoxic conditions. Sheng Li Xue Bao 64:129–134
Jans J, van Dijk JH, van Schelven S, van der Groep P, Willems SH, Jonges TN, van Diest PJ, Bosch JL (2010) Expression and localization of hypoxia proteins in prostate cancer: prognostic implications after radical prostatectomy. Urology 75:786–792
Schwab LP, Peacock DL, Majumdar D, Ingels JF, Jensen LC, Smith KD, Cushing RC, Seagroves TN (2012) Hypoxia-inducible factor 1alpha promotes primary tumor growth and tumor-initiating cell activity in breast cancer. Breast Cancer Res 14:R6
Wong CC, Zhang H, Gilkes DM, Chen J, Wei H, Chaturvedi P, Hubbi ME, Semenza GL (2012) Inhibitors of hypoxia-inducible factor 1 block breast cancer metastatic niche formation and lung metastasis. J Mol Med (Berl) 90(7):803–815. doi:10.1007/s00109-011-0855-y
Dai S, Huang ML, Hsu CY, Chao KS (2003) Inhibition of hypoxia inducible factor 1alpha causes oxygen-independent cytotoxicity and induces p53 independent apoptosis in glioblastoma cells. Int J Radiat Oncol Biol Phys 55:1027–1036
Lu H, Li Y, Shu M, Tang J, Huang Y, Zhou Y, Liang Y, Yan G (2009) Hypoxia-inducible factor-1alpha blocks differentiation of malignant gliomas. FEBS J 276:7291–7304
Gillespie DL, Flynn JR, Ragel BT, Arce-Larreta M, Kelly DA, Tripp SR, Jensen RL (2009) Silencing of HIF-1alpha by RNA interference in human glioma cells in vitro and in vivo. Methods Mol Biol 487:283–301
Reymond N, Riou P, Ridley AJ (2012) Rho GTPases and cancer cell transendothelial migration. Methods Mol Biol 827:123–142
Khalil BD, El-Sibai M (2012) Rho GTPases in primary brain tumor malignancy and invasion. J Neurooncol 108(3):333–339. doi:10.1007/s11060-012-0866-8
Zhou J, Li K, Gu Y, Feng B, Ren G, Zhang L, Wang Y, Nie Y, Fan D (2011) Transcriptional up-regulation of RhoE by hypoxia-inducible factor (HIF)-1 promotes epithelial to mesenchymal transition of gastric cancer cells during hypoxia. Biochem Biophys Res Commun 415:348–354
Xue Y, Li NL, Yang JY, Chen Y, Yang LL, Liu WC (2011) Phosphatidylinositol 3′-kinase signaling pathway is essential for Rac1-induced hypoxia-inducible factor-1(alpha) and vascular endothelial growth factor expression. Am J Physiol Heart Circ Physiol 300:H2169–H2176
Zhang P, Zhang X, Hao X, Wang Y, Hui Y, Wang H, Hu D, Zhou J (2009) Rac1 activates HIF-1 in retinal pigment epithelium cells under hypoxia. Graefes Arch Clin Exp Ophthalmol 247:633–639
Waheed F, Speight P, Dan Q, Garcia-Mata R, Szaszi K (2012) Affinity precipitation of active Rho-GEFs using a GST-tagged mutant Rho protein (GST-RhoA(G17A)) from epithelial cell lysates. J Vis Exp. doi:10.3791/3932
Zhou XP, Wu KY, Liang B, Fu XQ, Luo ZG (2008) TrkB-mediated activation of geranylgeranyltransferase I promotes dendritic morphogenesis. Proc Natl Acad Sci USA 105:17181–17186
Luo ZG, Je HS, Wang Q, Yang F, Dobbins GC, Yang ZH, Xiong WC, Lu B, Mei L (2003) Implication of geranylgeranyltransferase I in synapse formation. Neuron 40:703–717
Berndt N, Hamilton AD, Sebti SM (2011) Targeting protein prenylation for cancer therapy. Nat Rev Cancer 11:775–791
Ghavami S, Mutawe MM, Schaafsma D, Yeganeh B, Unruh H, Klonisch T, Halayko AJ (2012) Geranylgeranyl transferase 1 modulates autophagy and apoptosis in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 302:L420–L428
Lu J, Chan L, Fiji HD, Dahl R, Kwon O, Tamanoi F (2009) In vivo antitumor effect of a novel inhibitor of protein geranylgeranyltransferase-I. Mol Cancer Ther 8:1218–1226
Zhou X, Hua L, Zhang W, Zhu M, Shi Q, Li F, Zhang L, Song C, Yu R (2012) FRK controls migration and invasion of human glioma cells by regulating JNK/c-Jun signaling. J Neurooncol 110:9–19
Zhou X, Xu X, Meng Q, Hu J, Zhi T, Shi Q, Yu R (2012) Bex2 is critical for migration and invasion in malignant glioma cells. J Mol Neurosci. doi:10.1007/s12031-012-9864-8
Zhou X, Ma L, Li J, Gu J, Shi Q, Yu R (2012) Effects of SEMA3G on migration and invasion of glioma cells. Oncol Rep 28:269–275
Tremblay P, Beaudet MJ, Tremblay E, Rueda N, Thomas T, Vallieres L (2011) Matrix metalloproteinase 2 attenuates brain tumour growth, while promoting macrophage recruitment and vascular repair. J Pathol 224:222–233
Koul D, Parthasarathy R, Shen R, Davies MA, Jasser SA, Chintala SK, Rao JS, Sun Y, Benvenisite EN, Liu TJ, Yung WK (2001) Suppression of matrix metalloproteinase-2 gene expression and invasion in human glioma cells by MMAC/PTEN. Oncogene 20:6669–6678
Turcotte S, Desrosiers RR, Beliveau R (2003) HIF-1alpha mRNA and protein upregulation involves Rho GTPase expression during hypoxia in renal cell carcinoma. J Cell Sci 116:2247–2260
Wu KY, Zhou XP, Luo ZG (2010) Geranylgeranyltransferase I is essential for dendritic development of cerebellar Purkinje cells. Mol Brain 3:18
Chen JK, Hu LJ, Wang J, Lamborn KR, Kong EL, Deen DF (2005) Hypoxia-induced BAX overexpression and radiation killing of hypoxic glioblastoma cells. Radiat Res 163:644–653
Advani SH (2010) Targeting mTOR pathway: a new concept in cancer therapy. Indian J Med Paediatr Oncol 31:132–136
Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR (2008) Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol 217:674–685
Koh MY, Spivak-Kroizman TR, Powis G (2010) HIF-1alpha and cancer therapy. Recent Results Cancer Res 180:15–34
Kim JW, Gao P, Liu YC, Semenza GL, Dang CV (2007) Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol 27:7381–7393
Rathinam R, Berrier A, Alahari SK (2012) Role of Rho GTPases and their regulators in cancer progression. Front Biosci 17:2561–2571
Struckhoff AP, Rana MK, Worthylake RA (2011) RhoA can lead the way in tumor cell invasion and metastasis. Front Biosci 16:1915–1926
Kikuchi K, Li X, Zheng Y, Takano Y (2011) Invasion of breast cancer cells into collagen matrix requires TGF-alpha and Cdc42 signaling. FEBS Lett 585:286–290
Chen B, Gao Y, Jiang T, Ding J, Zeng Y, Xu R, Jiang X (2011) Inhibition of tumor cell migration and invasion through knockdown of Rac1 expression in medulloblastoma cells. Cell Mol Neurobiol 31:251–257
Gao Y, Xie M, He ZP, Zhang X, Zheng JQ (2010) The effect of hypoxia on the expression of RHO/RHO signaling pathway and CTGF of human embryonic fibroblasts. Sichuan Da Xue Xue Bao Yi Xue Ban 41:946–950
Lau CP, Huang L, Tsui SK, Ng PK, Leung PY, Kumta SM (2011) Pamidronate, farnesyl transferase, and geranylgeranyl transferase-I inhibitors affects cell proliferation, apoptosis, and OPG/RANKL mRNA expression in stromal cells of giant cell tumor of bone. J Orthop Res 29:403–413
van de Donk NW, Schotte D, Kamphuis MM, van Marion AM, van Kessel B, Bloem AC, Lokhorst HM (2003) Protein geranylgeranylation is critical for the regulation of survival and proliferation of lymphoma tumor cells. Clin Cancer Res 9:5735–5748
Sane KM, Mynderse M, Lalonde DT, Dean IS, Wojtkowiak JW, Fouad F, Borch RF, Reiners JJ Jr, Gibbs RA, Mattingly RR (2010) A novel geranylgeranyl transferase inhibitor in combination with lovastatin inhibits proliferation and induces autophagy in STS-26T MPNST cells. J Pharmacol Exp Ther 333:23–33
Unlu S, Mason CD, Schachter M, Hughes AD (2000) Perillyl alcohol, an inhibitor of geranylgeranyl transferase, induces apoptosis of immortalized human vascular smooth muscle cells in vitro. J Cardiovasc Pharmacol 35:341–344
Acknowledgments
The project was supported by National Natural Science Foundation of China (No. 81072072; No. 31070933); Natural Science Foundation of Jiangsu province (No. BK2011195); the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD); and Program for New Century Excellent Talents in University (NCET-10-0181).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiuping Zhou, Zhi Liu and Qiong Shi contributed equally to this study.
Rights and permissions
About this article
Cite this article
Zhou, X., Liu, Z., Shi, Q. et al. Geranylgeranyltransferase I regulates HIF-1α promoting glioblastoma cell migration and invasion. J Neurooncol 112, 365–374 (2013). https://doi.org/10.1007/s11060-013-1081-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1081-y