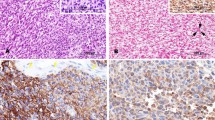
Abstract
Purely intracranial soft tissue sarcomas (ISTS) are very rare among children. A retrospective database analysis of the Cooperative Weichteilsarkom Studiengruppe (CWS) and brain tumor (HIT) registries was conducted to describe treatment and long-term outcome of children and adolescents with ISTS. Nineteen patients from Germany, Austria and Switzerland were reported between 1988 and 2009. Median age at diagnosis was 9.7 years (range, 0.5–17.8). Central pathological review was performed in 17 patients. Eleven patients underwent a total and five a subtotal tumor resection. A biopsy was done in one patient. In two patients no data concerning extent of initial resection was available. Radiotherapy was performed in 15 patients (first-line, n = 11; following progression, n = 4). All but one patient received chemotherapy (first-line, n = 7, following progression, n = 5; first-line and following progression, n = 6). With a median follow-up of 5.8 years (range, 0.6–19.8) ten patients were alive in either first or second complete remission. Seven patients died due to relapse or progression and two were alive with progressive disease. Estimated progression-free and overall survival at 5 years were 47 % (±12 %) and 74 % (±10 %), respectively. About 50 % of patients with ISTS remain relapse-free after 5 years. Multimodality treatment including complete tumor resection and radio-/chemotherapy is required to achieve sustained tumor control in patients with ISTS. Early initiation of postoperative non-surgical treatment seems to be important to prevent recurrence. Due to the intracranial localization local therapy should follow the recommendations used in brain tumors rather than in soft tissue sarcomas, whereas chemotherapy should be guided by histological subtype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soft tissue sarcomas (STS) account for approximately 6 % of all childhood malignancies [1]. In Germany the age-standardized incidence for all pediatric STS is 0.9 per 100.000/year, 0.7 per 100.000/year for rhabdomyosarcoma (RMS)-like, and 0.18 per 100.000/year for non-RMS-like STS [1]. In terms of histopathology, location and biological behaviour STS are extremely heterogenous [2]. Both RMS and non-RMS STS can occur in the head and neck region. However, except for parameningeal RMS with intracranial extension purely intracranial STS (ISTS) are exceptionally rare among children [2]. The true incidence of ISTS remains unknown, but estimates between 0.1 and 4 % of intracranial tumors have been reported [3–7]. Reports on pediatric ISTS are limited to single-institutional case reports or small, single-institutional case series [4, 7–16].
Children with STS or primary tumors of the brain are treated in Germany, Austria and Switzerland according to the prospective multicenter trials of the Cooperative Weichteilsarkom Studiengruppe (CWS) and brain tumor (HIT) study groups of the Pediatric Oncology and Hematology Society of the German Language [17–23]. However, neither prospective clinical trials nor established treatment guidelines are available for children with ISTS so far. Thus, these study groups encourage participating centres to report also patients with rare entities like ISTS serving as a national platform for data acquisition and analysis. Furthermore, they provide the best available management recommendations for such uncommon tumors and allow also performing central cytological [cerebrospinal fluid (CSF)], neuroradiological, and pathological review within an established network. The aim of the present retrospective study was to report the clinical characteristics, treatment and outcome of children and adolescents with ISTS registered in the databases of the CWS and HIT study groups.
Patients and methods
Between 1988 and 2009 nineteen patients (male, n = 12; female, n = 7) with non parameningeal RMS ISTS (ICD-O 8850/0-9364/3) from Germany, Austria and parts of Switzerland were reported as observational patients to the CWS (n = 8), HIT (n = 7) or both (n = 4) registries. The treating physicians decided which of the study centres (CWS, HIT or both) were contacted at the time of diagnosis. Institutional review board approval was obtained for all CWS and HIT trials. The HIT 2000 study was registered at the US National Cancer Institute (NCT00303810). All data were extracted without direct personal identification. Treatment centres were contacted by the CWS or HIT centres in order to obtain the most recent follow-up information. Patients’ data were carefully checked to avoid double reporting. Study records were reviewed at the study centres prior to inclusion to ensure that only patients with purely ISTS were reported.
Local magnetic resonance imaging (MRI) of the brain was done preoperatively in all patients. None of the patients had documented metastatic disease at the time of diagnosis. However, complete CNS staging including spinal MRI and cytological analysis of cerebrospinal fluid (CSF) was performed in nine patients (47 %) only. In six patients neither spinal MRI nor CSF analysis was done. In the remaining patients exclusion of CNS dissemination was based on CSF analysis (n = 2) only. In two patients data on CNS staging was missing. Central pathological review was done at the time of diagnosis in 17 patients at the Neuropathology Reference Centre of the HIT network at the Institute of Neuropathology, University of Bonn and/or at the Kiel Pediatric Tumor Registry according to the current World Health Organization (WHO) classifications of tumors of the central nervous system (CNS) and tumors of soft tissue and bone [24, 25]. Additional institutions provided pathological reviews for five patients. Survival probability was estimated by the Kaplan–Meier method. Overall survival (OS) was defined as date of diagnosis to death of any cause or to the date of last visit; progression-free survival (PFS) was defined as date of diagnosis to date of death, first progression, relapse or occurrence of secondary malignancy. Statistical analysis was done using SPSS software (IBM SPSS Statistics 19).
Results
Basic clinical characteristics
The clinical characteristics of study patients are shown in Table 1. Median age at diagnosis was 9.7 years (range, 0.5–17.8). Duration of symptoms was documented in 16 patients ranging from 1 day to 1 year (Table 1). Median duration of symptoms was 2 weeks. Histopathological diagnoses included sarcoma NOS (not otherwise specified) (n = 8), embryonal RMS (n = 2), chondrosarcoma (n = 2), malignant mesenchymal tumor (n = 2), alveolar RMS (n = 1), myxoid liposarcoma (n = 1), fibrosarcoma (n = 1), anaplastic hemangiopericytoma (n = 1) and small/spindle cell sarcoma (n = 1). In two cases (alveolar RMS, myxoid liposarcoma) diagnosis was confirmed by molecular genetics. In ten out of 17 patients local and central neuropathologies were concordant. Supratentorial tumor location was predominant. Two children had infratentorial tumors. In nine patients more than one lobe was involved (Table 1).
Surgery and non-surgical treatment
Treatment-related and outcome data are summarized in Tables 2 and 3. Eleven patients underwent a complete and five a subtotal tumor resection. A biopsy was done in one patient. In the remaining two patients no data concerning extent of resection was available. Radiotherapy was performed in 15 patients (first line, n = 11). Median tumor dose was 54 Gy (range, 44.8-68). Four patients were irradiated following progression. All but one patient received chemotherapy (first-line, n = 7, following progression, n = 5; first-line and following progression, n = 6) according to STS, brain tumor or mixed/individualized protocols (Table 2).
Clinical course and outcome
With a median follow-up of 5.8 years (range, 0.6–19.8) 10 patients were alive in either first (n = 7) or second (n = 3) complete remission (Table 3). Seven patients died due to relapse or progression and two were alive with progressive disease. The disease status according to the extent of resection and the use of postoperative non-surgical treatment is shown in Fig. 1. Estimated PFS and OS at 5 years were 47 % (±12 %) and 74 % (±10 %) (Fig. 2a, b). Progression-free survival at 5 years was 64 % (±15 %) for patients following gross total resection (GTR) compared with 33 % (± 19 %) for patients in whom a less than GTR was achieved (log-rank test, p = 0.072; Fig. 2c). No difference in OS at 5 years between the two groups was observed [73 % (±13 %) vs. 66 % (±19 %); log-rank test, p = 0.38].
Median time to first progression was 6 months (range 0.5–108). Only two patients had late relapses 65 and 108 months after diagnosis. In the majority of cases the first recurrence was either a local progression after initial incomplete resection or a locoregional relapse after complete resection. Relapses involved CNS except in one patient who developed multifocal bone metastases. Following diagnosis of recurrence, the disease was rapidly progressing with a median survival time of 9.5 (range, 5–132) months.
Seven patients (patients 1, 4, 5, 7, 9, 15, 18) were in first continuous complete remission (CCR) for a median of 10 years (range, 1.8–14.3). Histopathological diagnoses in these patients were sarcoma NOS (n = 3), embryonal RMS (n = 2), chondrosarcoma (n = 1), and myxoid liposarcoma (n = 1). A total resection was accomplished in all but one patient who underwent a subtotal resection. The patient with myxoid liposarcoma was treated by surgery alone. All other patients received postoperative chemo- and radiotherapy. These six patients were given chemotherapy after tumor resection, followed by radiotherapy (local, n = 5; craniospinal, n = 1). Chemotherapy was then continued in four patients after radiotherapy (Table 2).
Three patients [patient 12 (sarcoma NOS), patient 16 (malignant mesenchymal tumor), patient 17 (chondrosarcoma)] developed early local relapse (n = 1) or local progressive disease (n = 2) 3, 1, and 3 months following primary total or subtotal resection. Secondary surgery—information about secondary surgery was missing in one patient—was performed immediately after diagnosis of relapse followed by chemotherapy in two and radiotherapy in three patients. All patients are alive in second complete remission for 82, 89 and 108 months.
Seven patients (patients 6, 8, 10, 11, 13, 14, 19) died of disease and two (patients 2, 3) were alive with progressive disease. Diagnoses included sarcoma NOS (n = 4), malignant mesenchymal tumor (n = 1), alveolar RMS (n = 1), fibrosarcoma (n = 1), anaplastic hemangiopericytoma (n = 1) and small/spindle cell sarcoma (n = 1) in a patient with neurofibromatosis type 1. Four of these patients underwent a complete and two a subtotal resection. A biopsy was done in one patient. In the remaining two patients no data concerning extent of initial resection was available. Three of the nine patients received both radiotherapy and chemotherapy following initial surgery; two patients received chemotherapy and one patient had radiotherapy only. In the remaining three patients no adjuvant therapy was given.
Discussion
Available data on childhood intracranial soft tissue sarcomas
Due to their rarity our current knowledge of ISTS is based on case reports and few retrospective case series, most of them published before the year 2000 [7–16]. The largest series to date was presented by a Canadian group in 2003 and included 16 patients; 14 of them had ISTS and two spinal STS [7]. This series comprised patients with undifferentiated sarcoma (n = 7), meningeal sarcoma (n = 4), sarcoma NOS (n = 2), and RMS (n = 1). Fifty-three percent of patients achieved a total and 47 % a subtotal resection. Adjuvant radiotherapy and chemotherapy were used in 9 and 12 of patients with ISTS [7]. Thus our and the Canadian study compare very well with regard to treatment-related parameters. Data on outcome was provided for 12 children with ISTS in the Canadian study; six of them died after a median of 6.5 months (range, 1–41). The other six patients were alive 2–16 years after diagnosis [7]. An unfavourable outcome of children and adolescents with ISTS was also observed in smaller series and case reports [4, 10–12].
Diagnostic work-up and reference pathology
Despite the fact that a high incidence of CNS dissemination in ISTS was already emphasized in historical series [7–9], the number of patients who underwent complete CNS staging (including spinal MRI and cytological analysis of CSF) in our study was surprisingly low (47 %). Thus initial diagnostic evaluation should include complete CNS imaging as well as examination of CSF in all patients with ISTS. To our knowledge central pathological review was not performed in any of the previously published studies on childhood ISTS. Considering the relatively high rate of discordant results between local and central pathology (37 %), central pathological review by experienced neuropathologists and pediatric pathologists is of outstanding importance in these patients and clearly contributed to the quality of our study. Because of their clinical relevance the results of central pathological review should be obtained as soon as possible following tumor resection. Additional molecular genetic analysis of tumor specimens might be helpful in selected cases to confirm diagnosis [26].
Histological subtypes and outcome
Due to the variety of different tumor entities included in the present series it is difficult to assess the impact of a particular histopathology on prognosis. Histopathological diagnoses of the ten survivors in our series were sarcoma NOS (n = 4), embryonal RMS (n = 2), chondrosarcoma (n = 2), myxoid liposarcoma (n = 1), and malignant mesenchymal tumor (n = 1). Accordingly successful treatment is reported anecdotally in individual patients with intracranial fibrosarcoma [8, 13], chondrosarcoma [12, 14, 15], and embryonal RMS [7, 9, 16] following intensive multimodality treatment. In the Canadian study 4 out of 8 patients with undifferentiated or unclassifiable sarcoma and two out of three with meningeal sarcoma died [7]. In our series four out of eight patients with sarcoma NOS relapsed; two of them died and two were alive with progressive disease. Thus entities such as undifferentiated, unclassifiable or sarcoma NOS seem to have an unfavourable prognosis.
Extent of resection, time to progression and outcome
In the study by Al-Gahtany et al. [7] mean survival following total resection was 6.2 years compared to 3.4 years in patients who underwent a subtotal resection, but no further statistical analysis to compare these figures was provided. In our series there was a trend toward improved PFS among patients who underwent a GTR compared with patients in whom a less than GTR was obtained (PFS 64 % vs. 33 %, p = 0.072). Thus a complete tumor resection seems to be a prerequisite for longer survival in patients with ISTS. Of note, very few histological subtypes such as liposarcoma, might be cured by complete surgical resection alone.
An aggressive clinical behaviour with early progression and death within months is reported in many series [4, 7–10, 13]. In our study the median time to progression was 6 months and only two patients had late relapses. In three patients who developed early local relapse or local progressive disease between 1 and 3 months following primary resection a second complete remission could be achieved. Thus, the short PFS seems to require rapid initiation of non-surgical treatment after tumor resection to improve local tumor control.
Postoperative non-surgical treatment
Although postoperative local radiotherapy with adequate doses (44.8–54 Gy) seems to be essential to achieve local tumor control [7–10, 12–16], all our patients in first CCR started with postoperative chemotherapy before radiotherapy; four of them continued chemotherapy after radiotherapy. Thus administration of (one or two courses) of chemotherapy before the start of radiotherapy is one treatment option until completion of radiotherapy planning.
Since six out of seven patients in first CCR received first-line radiotherapy in contrast to five out of 12 patients who subsequently relapsed, radiotherapy is a prerequisite to achieve long-term disease control. Craniospinal irradiation (CSI) was done in three of our patients (patient 4, 6, 16), who all had localized disease at the time of diagnosis confirmed by complete CNS staging. Five patients received CSI in the study by Al-Gahtany et al. [7]; two of them had initial CNS spread. Following CSI two patients were alive, two died and in the remaining fifth patient follow-up data was missing. Thus far, there are no data to suggest that CSI is able to prevent disease recurrence and/or dissemination in patients with non metastatic disease at diagnosis. Craniospinal irradiation, however, seems to be the most appropriate therapeutic option in case of initial CNS dissemination. The potential benefits and risks of CSI, particularly in younger children have to be carefully weighted and discussed with parents.
Chemotherapy is used in a considerable number of reported patients with ISTS [4, 7, 9, 16], even for chemoresistant histological subtypes [10, 13, 15], but its impact on outcome is still unknown. However, almost the vast majority of long-term survivors reported in the literature [7, 9, 13, 15, 16] has received multimodality treatment including chemotherapy. Although the optimal treatment schedule, duration and combination of drugs have not yet been established, a “sandwich” regimen that uses chemotherapy before and after radiotherapy might be appropriate. Six of seven patients were treated by such a “sandwich” approach. The early administration of chemotherapy when the blood brain barrier is disrupted postoperatively might have contributed to the more favourable outcome of patients treated by a “sandwich strategy”. It is quite obvious to use cytotoxic drugs with known efficacy in STS such as vincristine, ifosfamide, etoposide, anthracyclines or dactinomycin. The type of chemotherapy in patients with ISTS should be guided by histology/histological subtype. Some of our patients continued to receive chemotherapy after radiotherapy, but the benefit of this approach is not defined for non-RMS ISTS.
Conclusion
The present study is the first multi-institutional series on children and adolescents with centrally reviewed ISTS. The prospective collection of clinical, pathological, treatment-related and follow-up data of children and adolescents with ISTS within national or international registries is needed. This allows better defining favourable and unfavourable prognostic parameters and is a prerequisite for the development of risk-adapted therapeutic standards. The CWS and HIT study groups have, therefore, agreed on a common policy and close cooperation in patients with ISTS.
References
Weihkopf T, Blettner M, Dantonello T, Jung I, Klingebiel T, Koscielniak E, Lückel M, Spix C, Kaatsch P (2008) Incidence and time trends of soft tissue sarcomas in German children 1985–2004—a report from the population-based German Childhood Cancer Registry. Eur J Cancer 44:432–440
Meyer WH, Spunt SL (2004) Soft tissue sarcoma of childhood. Cancer Treat Rev 30:269–280
Paulus W, Slowik F, Jellinger K (1991) Primary intracranial sarcomas: histopathological features of 19 cases. Histopathology 18:395–402
Ellams ID, Hildebrandt G, Vuia O, Kaufmann U (1985) Intracranial sarcoma in childhood. Childs Nerv Syst 1:169–171
Ironside JW (1991) Classification of primary intracranial sarcomas and other central nervous system neoplasms. Histopathology 18:483–486
Mena H, Garcia JH (1978) Primary brain sarcomas: light and electron microscopic features. Cancer 42:1298–1307
Al-Gahtany M, Shroff M, Bouffet E, Dirks P, Drake J, Humphreys R, Laperriere N, Hawkins C, Rutka J (2003) Primary central nervous system sarcomas in children: clinical, radiological, and pathological features. Childs Nerv Syst 19:808–817
Tomita T, Gonzalez-Crussi F (1984) Intracranial primary nonlymphomatous sarcomas in children: experience with eight cases and review of the literature. Neurosurgery 14:529–540
Olson JJ, Menezes AH, Godersky JC, Lobosky JM, Hart M (1985) Primary intracranial rhabdomyosarcoma. Neurosurgery 17:25–34
Eckhardt BP, Brandner S, Zollikofer CL, Wentz KU (2004) Primary cerebral leiomyosarcoma in a child. Pediatr Radiol 34:495–498
Scheithauer BW, Rubinstein LJ (1978) Meningeal mesenchymal chondrosarcoma: report of 8 cases with review of the literature. Cancer 42:2744–2752
Rushing EJ, Armonda RA, Ansari Q, Mena H (1996) Mesenchymal chondrosarcoma: a clinicopathologic and flow cytometric study of 13 cases presenting in the central nervous system. Cancer 77:1884–1891
Bisogno G, Roganovic J, Carli M, Scarzello G, Calderone M, Faggin R, Perilongo G (2002) Primary intracranial fibrosarcoma. Childs Nerv Syst 18:648–651
Gerszten PC, Pollack IF, Hamilton RL (1998) Primary parafalcine chondrosarcoma in a child. Acta Neuropathol 95:111–114
Sardi I, Massimino M, Genitori L, Buccoliero AM, Giangaspero F, Ferrari A (2011) Intracranial mesenchymal chondrosarcoma: report of two pediatric cases. Pediatr Blood Cancer 56:685–686
Guilcher GM, Hendson G, Goddard K, Steinbok P, Bond M (2008) Successful treatment of a child with a primary intracranial rhabdomyosarcoma with chemotherapy and radiation therapy. J Neurooncol 86:79–82
Dantonello TM, Int-Veen C, Harms D, Leuschner I, Schmidt BF, Herbst M, Juergens H, Scheel-Walter HG, Bielack SS, Klingebiel T, Dickerhoff R, Kirsch S, Brecht I, Schmelzle R, Greulich M, Gadner H, Greiner J, Marky I, Treuner J, Koscielniak E (2009) Cooperative trial CWS-91 for localized soft tissue sarcoma in children, adolescents, and young adults. J Clin Oncol 27:1446–1455
Koscielniak E, Harms D, Henze G, Jürgens H, Gadner H, Herbst M, Klingebiel T, Schmidt BF, Morgan M, Knietig R, Treuner J (1999) Results of treatment for soft tissue sarcoma in childhood and adolescence: a final report of the German Cooperative Soft Tissue Sarcoma Study CWS-86. J Clin Oncol 17:3706–3719
Koscielniak E, Jürgens H, Winkler K, Bürger D, Herbst M, Keim M, Bernhard G, Treuner J (1992) Treatment of soft tissue sarcoma in childhood and adolescence. A report of the German Cooperative Soft Tissue Sarcoma Study. Cancer 70:2557–2567
von Hoff K, Hinkes B, Gerber NU, Deinlein F, Mittler U, Urban C, Benesch M, Warmuth-Metz M, Soerensen N, Zwiener I, Goette H, Schlegel PG, Pietsch T, Kortmann RD, Kuehl J, Rutkowski S (2009) Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT’91. Eur J Cancer 45:1209–1217
von Bueren AO, von Hoff K, Pietsch T, Gerber NU, Warmuth-Metz M, Deinlein F, Zwiener I, Faldum A, Fleischhack G, Benesch M, Krauss J, Kuehl J, Kortmann RD, Rutkowski S (2011) Treatment of young children with localized medulloblastoma by chemotherapy alone: results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro Oncol 13:669–679
Wolff JE, Driever PH, Erdlenbruch B, Kortmann RD, Rutkowski S, Pietsch T, Parker C, Metz MW, Gnekow A, Kramm CM (2010) Intensive chemotherapy improves survival in pediatric high-grade glioma after gross total resection: results of the HIT-GBM-C protocol. Cancer 116:705–712
Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Soerensen N, Graf N, Emser A, Pietsch T, Wolff JE, Kortmann RD, Kuehl J (2005) Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med 352:978–986
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (2007) World Health Organization Classification of Tumours of the Central Nervous System. International Agency for Research on Cancer (IARC), Lyon
Fletcher CDM, Unni KK, Mertens F (2002) World Health Organization of Tumors. Pathology and genetics of tumours of soft tissue and bone. International Agency for Research on Cancer (IARC) Press, Lyon
Romeo S, Dei Tos AP (2011) Clinical application of molecular pathology in sarcomas. Curr Opin Oncol 23:379–384
Acknowledgments
The authors thank Wibke Treulieb and Christine Lindow (HIT study centre Hamburg, Germany), Gabriele Pammer (HIT study centre Graz, Austria), Erika Hallmen, Iris Veit-Friedrich and Simone Feuchtgruber (CWS study centre Stuttgart, Germany) for excellent data management, Claudia Bremensdorfer for administrative support and Franz Quehenberger (Medical University of Graz) for statistical support. The HIT and CWS studies and the Reference Centre for Neuropathology at the University of Bonn are supported by Grants from the Deutsche Kinderkrebsstiftung (German Childhood Cancer Foundation). The Austrian HIT study centre is supported by the Steirische Kinderkrebshilfe.
Conflict of interest
All authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benesch, M., von Bueren, A.O., Dantonello, T. et al. Primary intracranial soft tissue sarcoma in children and adolescents: a cooperative analysis of the European CWS and HIT study groups. J Neurooncol 111, 337–345 (2013). https://doi.org/10.1007/s11060-012-1020-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-012-1020-3