Abstract
We tested the validity of two prognostic indices for stereotactic radiosurgically (SRS)-treated patients with brain metastases (BMs) from five major original cancer categories. The two indices are Diagnosis-Specific Graded Prognostic Assessment (DS-GPA) and our Modified Recursive Partitioning Analysis (RPA). Forty-six hundred and eight BM patients underwent gamma knife SRS during the 1998–2011 period. Primary cancer categories were non-small cell lung cancer (NSCLC, 2827 patients), small cell lung cancer (SCLC, 460), gastro-intestinal cancer (GIC, 582), breast cancer (BC, 547) and renal cell cancer (RCC, 192). There were statistically significant survival differences among patients stratified into four groups based on the DS-GPA systems (p < 0.001) in all five original cancer categories. In the NSCLC category, there were statistically significant mean survival time (MST) differences (p < 0.001) among the four groups without overlapping of 95 % confidence intervals (CIs) between any two pairs of groups with the DS-GPA system. However, among the SCLC, GIC, BC and RCC categories, MST differences between some pairs of groups failed to reach statistical significance with this system. There were, however, statistically significant MST differences (p < 0.001) among the three groups without overlapping of 95 % CIs between any two pairs of groups with the Modified RPA system in all five categories. The DS-GPA system is applicable to our set of patients with NSCLC only. However, the Modified RPA system was shown to be applicable to patients with five primary cancer categories. This index should be considered when designing future clinical trials involving BM patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases (BMs) are a common and life-threatening neurological problem for cancer patients, in the absence of effective treatment. Controversy in the field of clinical oncology, regarding the optimal treatment of BM patients, persists due mainly to patient heterogeneity. Numerous factors in BM patients impact outcomes. Furthermore, data allowing the roles and benefits of various treatment modalities to be defined, i.e., whole brain radiotherapy (WBRT), surgery, stereotactic radiosurgery (SRS) or radiotherapy (SRT), anti-cancer agent administration, or combinations of these modalities, are lacking. Thus, clinicians are often uncertain as to the optimal treatment selection. An improved prognostic index might resolve some of the uncertainty in making treatment decisions as well as guiding future research efforts.
Gaspar et al. [1] proposed the now well-established Recursive Partitioning Analysis (RPA) based on the Radiation Therapy Oncology Group (RTOG) databases of BM patients receiving WBRT. This index divides patients into three classes using four factors; age, Karnofsky Performance Status (KPS), primary tumor status and extracranial metastases. The RPA index is very simple and has long been widely used for predicting survival periods of BM patients and was shown to also be applicable to those treated with SRS alone, as we reported previously [2]. The RTOG study 9508 provided prospective evidence that a tumor number of one versus two or three impacted survival benefits [3]. According to this finding, Sperduto et al. [4] reevaluated and updated the RPA system, thereby devising the Graded Prognostic Assessment (GPA) index. This index divides patients into four classes based on age, KPS, extracranial metastasis status and tumor number.
However, it is widely known that there are considerable differences in oncological and clinical features as well as treatment responses among various primary tumor types. Therefore, in 2010, Sperduto et al. [5] modified their original GPA system and developed a new index, the Diagnosis-Specific GPA (DS-GPA); a user-friendly worksheet of this system was demonstrated very recently [6]. Their new system uses different scoring which takes into account different primary tumor types. Six common original tumor sites associated with BMs, i.e., non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), breast cancer (BC), melanoma, renal cell cancer (RCC) and gastro-intestinal cancer (GIC) were scored to allow comparisons among post-WBRT survivals (Table 1).
We recently published a sub-classification of RPA class II patients into IIa, IIb and IIc based on KPS, tumor number, original tumor status and extracranial metastases, as outlined in Table 1, as an index for cancer patients with SRS-treated BMs [2]. In our prior study, this index was confirmed to be applicable to class II patients with individual subsets of four major primary tumors, i.e., LC, GIC, BC and RCC. Subsequently, we tested the validity of this index along with the original RPA classes I and III for BC patients with BM treated by SRS. As we reported very recently [7], we found median survival times (MSTs) to be very similar between classes I and IIa (p = 0.51) as well as between classes IIb and III (p = 0.34). Thus, we proposed re-grading the entire patient population into three groups, i.e. RPA I+IIa, IIb and IIc+III (Modified RPA).
As Sperduto et al. [8] encouraged us to perform this work in a Letter to the Editor of the IJROBP, the goal of this retrospective cohort study, based on our SRS-treated BM patients with NSCLC, SCLC, GIC, BC and RCC, was to reappraise whether these two prognostic indices, the DS-GPA and the Modified RPA, are generally applicable or can be recommended for historical comparison.
Patients and methods
Patient population
This was a two-institution, institutional review board (IRB)-approved, retrospective cohort study using two prospectively accumulated databases including 4,608 BM patients: the first author’s series included 2,341 consecutive patients (Tokyo Women’s Medical University, IRB; #1981), the second author’s 2267 (Chiba University Graduate School of Medicine, IRB: #SE363). These 4,608 consecutive patients underwent SRS, without the combination of WBRT, for BMs using a gamma knife (GK) during the 13-year-period from 1998 through 2011. The primary tumors were NSCLC (2,827 patients), SCLC (460), GIC (582), BC (547) and RCC (192). Among the 582 patients with GICs, the most common primary site was the colon (274 patients) followed by the rectum (115), stomach (107), esophagus (74), duodenum (6) and small intestine (5). Our databases included only 13 melanoma patients. Thus, we could not test the validity of the two indices in patients with melanoma. Table 2 summarizes clinical characteristics along with primary cancer categories. The KPS score was used to evaluate general and neurological conditions of patients [9].
In our facilities, all patients had been referred to us for SRS by their primary physicians. Therefore, patient selections, including previous treatments, i.e., neurosurgical intervention, WBRT, chemo-/targeting-therapy, or combinations of these, had mostly been made outside of our facilities. Patient selection criteria may well have differed somewhat among referring physicians. Therefore, in all cases, the first (MY) or second (TS) author ultimately decided whether or not a patient would be accepted for SRS. Our patient selection criteria were described in our previous report [2].
The treatment strategy was explained in detail to each patient and at least one adult relative by the first (MY) or second (TS) author, and written informed consent was obtained from all patients before SRS. As our radiosurgical techniques are described in detail in our previous report, they are not repeated herein [2]. Briefly, standard SRS procedures were performed using a Leksell gamma unit Model B before June, 2003, and thereafter a Leksell gamma unit Model C (Elekta AB, Stockholm, Sweden) in the first author’s series. The change from GK Model B to C was in October of 2003 in the second author’s series.
Statistical analysis
All data were analyzed according to the intention-to-treat principle. For the baseline variables, summary statistics were constructed employing frequencies and proportions for categorical data, and means and standard deviations (SD) for continuous variables. Overall survival (OS) time was defined as the interval between the first SRS and death due to any cause or the day of the last follow-up. For time-to-event outcomes, the Kaplan–Meier method [10] was used to estimate OS for each group, and the differences in survival between groups were analyzed with the log-rank test. The hazard ratios (HRs) and 95 % confidence intervals (CIs) were estimated employing the Cox proportional hazards model [11].
All comparisons were planned and the tests were two-sided. A p value of less than 0.05 was considered to indicate a statistically significant difference. All statistical analyses were conducted by two of the authors (YH, YS), neither of whom was involved in either SRS treatment or patient follow-up. One of the authors (YH) initially analyzed the data using JMP, Japanese version 9.0 for the Windows system (SAS Institute Inc., Cary, NC, USA) and, thereafter, the other (YS) independently reconfirmed the results using the SAS software program, version 9.3 (SAS Institute Inc).
Results
Four (0.2 %) patients were lost to follow-up, three with SCLC and one with NSCLC. The median post-SRS follow-up time among 519 censored observations was 14.9 (range; 0.1–138.3) months, with 4,085 patients (81.1 %) deceased as of the end of December 2011. The overall median survival time (MST) after SRS was 7.7 (95 % CI; 7.4–8.0) months. Actuarial post-SRS survival rates were 58.6 % at six, 34.7 % at 12, 15.9 % at 24, 8.9 % at 36 and 4.2 % at 60 months after SRS. Causes of death could not be determined in 99 patients, but were confirmed in the remaining 3986 to be non-brain diseases in 3480 (87.3 %) and brain diseases in 506 (12.7 %). Among the 4608 patients, 1689 (36.7 %) underwent salvage SRS mostly for new lesions (88.3 %) and, uncommonly (11.7 %), recurrence of the treated lesions. Salvage WBRT was necessary in 194 patients (4.2 %) and surgical removal in 69 (1.5 %). Detailed post-SRS treatment results for each of the four primary cancer categories are presented in Table 3.
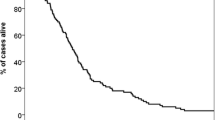
Figure 1 shows Kaplan–Meier plots of our patient series according to the DS-GPA system for the five primary cancer categories (a; NSCLC, b; SCLC, c; GIC, d; BC and e; RCC). Patient numbers were roughly equal among the four groups in three of the primary cancer categories, GIC, BC and RCC. However, there were large patient number discrepancies in the other two, NSCLC and SCLC, i.e., the patient number in the NSCLC category was very small in the 3.5–4.0 group as compared with the other three groups and those in the SCLC category in the 3.5–4.0 and 3.0 groups differed remarkably from those of the two other groups. A higher DS-GPA score is associated with a longer MST, and the survival difference by four-group stratification is statistically significant in all five primary cancer categories (p < 0.001). In the NSCLC category, there were statistically significant MST differences among the four groups without overlapping of 95 % CIs between any two pairs of groups (Fig. 1a). However, in the SCLC, GIC and RCC categories, the MST difference between the two DS-GPA groups with scores of 3.5–4.0 and 3.0 and between those with DS-GPA scores of 3.0 and 1.5–2.5 did not reach statistical significance (Fig. 1b, c, e). Also, in the BC category, the MST difference between the two DS-GPA groups with scores of 3.0 and 1.5–2.5 did not reach statistical significance (Fig. 1d).
Overall survival according to Diagnosis-Specific Graded Prognostic Assessment (DS-GPA) [5] in the five original cancer categories; NSCLC (a), SCLC (b), GIC (c), BC (d) and RCC (e). n numbers of patients, MST median survival time (months), CI confidence interval, HR hazard ratio, SRS gamma knife radiosurgery
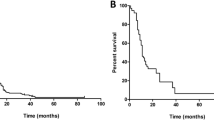
Figure 2 shows Kaplan–Meier plots of our patient series according to the modified RPA system for the five primary cancer categories (a; NSCLC, b; SCLC, c; GIC, d; BC and e; RCC). There were statistically significant MST differences (p < 0.001) among the three groups with no overlapping of 95 % CIs between any two pairs of groups. Furthermore, there were no large patient number discrepancies among the three subgroups in the five primary tumor categories.
Overall survival according to the Modified RPA system [7] in the five original cancer categories; NSCLC (a), SCLC (b), GIC (c), BC (d) and RCC (e). n numbers of patients, MST median survival time (months), CI confidence interval, HR hazard ratio, SRS gamma knife radiosurgery
Discussion
Due to the uniqueness of each primary cancer category, Sperduto et al. [4, 5] recently proposed DS-GPA, a modification of their original GPA system. Our retrospective cohort study of SRS-treated patients at two institutions confirmed the validity of DS-GPA. Although this system was shown to be applicable to patients with NSCLC, it failed to yield statistically significant MST differences for patients with SCLC, GIC, BC and RCC. Furthermore, a weakness of this system is that there were large patient number discrepancies among subgroups in patients with NSCLC and SCLC. Particularly, in the SCLC category, 59.8 % of all patients had group 1.5–2.5 DS-GPA scores while only 1.7 % had group 3.5–4.0 DS-GPA scores, the dissociation rate being 35.2 versus 1.0. In the same way, dissociation rates between the two groups, those with the highest and lowest numbers of patients, were 16.6 versus 1.0 in the NSCLC category. A prognostic index with such large patient number discrepancies among subgroups would, in our view, be fundamentally inadequate. Therefore, we believe that the scoring system of the DS-GPA should be modified for the NSCLC and SCLC categories.
The DS-GPA concept is that different indices should be applied to different primary tumors, really a very scientific approach but rather complicated for routine cancer patient management. While this system is not complicated for physicians who manage patients with one primary cancer category, a single prognostic grading system is much more convenient for those managing patients with various primary cancer categories. From this viewpoint, our recently-reported Modified RPA system is considered to be more widely applicable as it compensates for the major weakness of the RPA system [7]. Namely, as we reported previously [2], most patients fall into RPA class II. In fact, more than 84 % of all patients were class II in the present study. These large discrepancies in patient numbers among the three classes might reflect clinical factors. The survival periods vary markedly within the group comprising class II patients.
After publication of the index based on RPA by Gaspar et al. [1], two other indices were proposed [12, 13]. These are: (1) Score Index for Radiosurgery (SIR); the sum of scores (0–2) for each of five prognostic factors (age, KPS, systemic disease status, number of lesions, and volume of the largest lesion) [12]. (2) Basic Score for Brain Metastases (BSBM); the sum of scores (0–1) for three prognostic factors (KPS, controlled primary tumor and extracranial metastases) [13]. As we discussed in our previous publication [2] and Sperduto et al. [4] pointed out, the two systems have limitations although there were statistically significant MST differences (p < 0.001) among the three groups in the SIR system or four groups in the BSBM. The SIR requires volume estimation of the largest lesion for scoring [12]. Volume estimation is commonly performed at the time of SRS or SRT, but not always at the time of WBRT or when treatment options are considered by most physicians. Therefore, this index is rarely used clinically because treatments must be selected for most patients without knowing the actual tumor volume. The BSBM does not incorporate BM-related factors [13]. Most notably, tumor number is widely accepted as one of the major factors influencing patient survival periods.
As mentioned in our previous articles [2, 7], the major weakness of this study might be that clinical factors are obviously heterogeneous since our cohort included all treated patients. Greater patient group homogeneity makes a study more scientific. However, heterogeneity actually reflects clinical settings rather closely as we physicians often deal with inhomogeneous clinical factors. Particularly, our two databases included some patients whose BMs were not newly diagnosed tumors. However, proportions of such patients in our database were very small. Thus, this heterogeneity minimally impacted our results, as we very recently commented in our reply to the aforementioned Letter-to-the-Editor from Sperduto et al. [14].
The second potential weakness of the present study is that our series included considerable numbers of patients with five or more BMs. SRS alone for patients with four to five tumors, or even more, is not a standard treatment in any industrialized nations [15]. Nevertheless, as we reported elsewhere [16, 17] and as described by Knisely et al. [18], a trend to treat more BMs became apparent in the recent years [19–25]. In fact, Tsao et al. [26] recently stated in the American Society for Radiation Oncology evidence-based guideline, that “when new BMs are seen on the planning scan the day of SRS, it may be reasonable to proceed and complete the SRS procedure for all of the lesions visualized even if they exceed a total of 4 BMs”. Furthermore, Grandhi et al. [27] very recently reported that SRS can be used to safely and effectively treat intracranial disease with a high rate of local control in patients with ≥10 brain tumors.
The present study included patients who had undergone SRS no earlier than 1998. For both diagnosis and dose planning, 1.5 Tesla MR units with gadolinium administration were employed except in a very small number of patients who had contra-indications for MR imaging or gadolinium administration. Furthermore, although patient demographic features were heterogeneous, GKRS techniques were quite consistent. In both databases, SRS was performed under full supervision of the senior neurosurgeon, either the first or the second author in each patient series. Patient selection criteria, dose-planning techniques, dose selection and the follow-up protocol were consistently maintained in both series. Serizawa et al. [28] previously demonstrated that there were no significant differences in GKRS treatment results for BM patients between these two facilities.
With any prognostic index, certain revisions may be required in the future probably due to the development of novel treatments for one of the cancer types. Given such circumstances, the DS-GPA can readily be modified, as was, in fact, very recently reported by Sperduto et al. [29]. The DS-GPA would thereby remain relevant in the future while a weakness of our modified RPA system is that it may lack such flexibilities. The second potential weakness of the Modified RPA is that this system requires assessment of primary tumor control which is dependent on the type and timing of imaging studies, as pointed out by Sperduto et al. [8]. In this study, primary tumor status was judged by a referring physician based on clinical and/or imaging examinations, and thus was not very scientific. We usually judge tumor status as well controlled for patients whose primary tumors were deemed by referring physicians to be in a stable disease state, showing a partial response or complete response and whose anticipated survival times were at least 6 months, as we reported very recently [14].
Conclusions
The DS-GPA system is not applicable to our set of patients with SCLC, GIC and RCC for comparing survivals after SRS while it is applicable to patients with NSCLC. The Modified RPA system was shown to be applicable to patients with five primary cancer categories. This index should be considered when designing future clinical trials involving BM patients.
According to the Modified RPA system, RPA class I+IIa as well as IIb patients can be regarded as good candidates for aggressive treatment of BMs, while the treatment decision remains difficult in RPA class IIc+III patients because of their poorer outcomes. This study showed that, if we select reasonable SRS candidates and treat aggressively, only 9.1–15.4 % of such cancer patients with BMs die of brain disease regardless of primary cancer categories. Furthermore, in this era of SRS, patients with a few BMs are rather unlikely to die of brain disease.
References
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37(4):745–751
Yamamoto M, Sato Y, Serizawa T, Kawabe T, Higuchi Y, Nagano O, Barfod BE, Ono J, Kasuya H, Urakawa Y (2012) Sub-classification of Recursive Partitioning Analysis class II patients with brain metastases treated radiosurgically. Int J Radiat Oncol Biol Phys 83:1399–1405. doi:10.1016/i.ijrobp.2011.10.018
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ Jr (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W (2008) A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 70:510–514. doi:10.1016/i.ijrobp.2007.06.074
Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, Bhatt A, Jensen AW, Brown PD, Shih H, Kirkpatrick J, Schwer A, Gaspar LE, Fiveash JB, Chiang V, Knisely J, Sperduto CM, Mehta M (2010) Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 77:655–661. doi:10.1016/j.ijrobp.209.08.025
Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JP, Sperduto CM, Lin N, Mehta M (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30:419–425. doi:10.1200/JCO.2011.38.0527
Yamamoto M, Kawabe T, Higuchi Y, Sato Y, Barfod BE, Kasuya H, Urakawa Y (2012) Validity of three recently-proposed prognostic grading indexes for breast cancer patients with radiosurgically-treated brain metastases. Int J Radiat Oncol Biol Phys 84:1110–1115. doi:10.1016/j.ijrobp.2012.02.040
Sperduto PW, Sneed PK, Roberge D, Shanley R, Luo X, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LR, Fiveash JB, Knisely JPS, Lin N, Mehta M (2012) Letter to editor: comments in response to a modified recursive partitioning analysis for brain metastases. Int J Radiat Oncol Biol Phys 84:875–876. doi:10.1016/j.ijrobp.2012.03.050
Karnofsky DA, Buechenal JH (1949) The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM (ed) Evaluation of chemotherapeutic agent. Columbia Univ Press, New York, pp 191–205
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Cox DR (1972) Regression models and life tables. J Royal Stat Soc ser B 34:187–220
Weltman E, Salvajoli JV, Brandt RA, de Morais Hanriot R, Prisco FE, Cruz JC, de Oliveira Borges SR, Wajsbrot DB (2000) Radiosurgery for brain metastases: a score index for predicting prognosis. Int J Radiat Oncol Biol Phys 46:1155–1161
Lorenzoni J, Devriendt D, Massager N, David P, Ruiz S, Vanderlinden B, Van Houtte P, Brotchi J, Levivier M (2004) Radiosurgery for treatment of brain metastases: estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys 60:218–224
Yamamoto M, Sato Y, Serizawa T, Kawabe T, Higuchi Y, Nagano O, Barfod BE, Ono J, Kasuya H, Urakawa Y (2012) Reply to Dr. Sperduto and colleagues. Int J Radiat Oncol Biol Phys 84:876–877. doi:10.1016/j.ijrobp.2012.03.050
Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka D, Robinson PD, Ammirati M, Cobbs CS, Gaspar LE, Loeffler JS, McDermott M, Mehta MP, Mikkelsen T, Olson JJ, Paleologos NA, Patchell RA, Ryken TC, Kalkanis SN (2010) The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96:45–68. doi:10.1007/s11060-009-0073-4
Yamamoto M, Ide M, Nishio S, Urakawa Y (2002) Gamma knife radiosurgery for numerous brain metastases: is this a safe treatment? Int J Radiat Oncol Biol Phys 53:1279–1283
Yamamoto M, Kawabe T, Barfod BE (2012) How many metastases can be treated with radiosurgery? Prog Neurol Surg 25:261–272
Knisely JPS, Yamamoto M, Gross CP, Castrucci WA, Jokura H, Chiang VLS (2010) Radiosurgery alone for five or more brain metastases: expert opinion survey. J Neurosurg 113(Suppl):84–89. doi:10.3171/2010.8.GKS10999
Chang WS, Kim HY, Chang JW, Park YG, Chang JH (2010) Analysis of radiosurgical results in patients with brain metastases according to the number of brain lesions: is stereotactic radiosurgery effective for multiple brain metastases? J Neurosurg 113(Suppl):73–78. doi:10.3171/2010.8.GKS10994
Hunter GK, Suh JH, Reuther AM, Vogelbaum MA, Barnett GH, Angelov L, Weil RJ, Neyman G, Chao ST (2012) Treatment of five or more brain metastases with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2011.10.026
Kim CH, Im YS, Nam DH, Park K, Kim JH, Lee JI (2008) Gamma knife radiosurgery for ten or more brain metastases. J Korean Neurosurg Soc 44:358–363. doi:10.3340/jkns.2008.44.6.358
Serizawa T, Hirai T, Nagano O, Higuchi Y, Matuda S, Ono J, Saeki N (2010) Gamma knife surgery for 1–10 brain metastases without prophylactic whole-brain radiation therapy: analysis of case meeting the Japanese prospective multi-institutional study (JLGK0901) inclusion criteria. J Neurooncol 98:163–167. doi:10.1007/s11060-010-0169-x
Serizawa T, Yamamoto M, Sato Y, Higuchi Y, Nagano O, Kawabe T, Matsuda S, Ono J, Saeki N, Hatano M, Hirai T (2010) Stereotactic radiosurgery alone using gamma knife for patients with multiple brain metastases: retrospective review in two gamma knife centers of 1,508 eligible cases meeting the multi-institutional prospective study (JLGK0901) inclusion criteria. J Neuorosurg 113(Suppl):48–52. doi:10.3171/2010.8.GKS10838
Shuto T, Fujino H, Inomori S, Nagano H (2004) Repeated gamma knife radiosurgery for multiple metastatic brain tumours. Acta Neurochir (Wien) 146:989–993
Suzuki S, Omagari J, Nishio S, Nishiye E, Fukui M (2000) Gamma knife radiosurgery for simultaneous multiple metastatic tumors. J Neurosurg 93(Suppl 3):30–31
Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE, Sperduto PW, Vogelbaum MA, Radawski JD, Wang JZ, Gillin MT, Mohideen N, Hahn CA, Chang EL (2012) Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. doi: 10.1016/j.prro.2011.12.004
Grandhi R, Kondziolka D, Panczykowski D, Monaco EA 3rd, Kano H, Niranjan A, Flickinger JC, Lunsford LD (2012) Stereotactic radiosurgery using the Leksell Gamma Knife Perfexion unit in the management of patients with 10 or more brain metastases. J Neurosurg 117:237–245. doi:10.3171/JNS/2012.1.JNS12103
Serizawa T, Yamamoto M, Nagano O, Higuchi Y, Matsuda S, Ono J, Iwadate Y, Saeki N (2008) Gamma knife surgery for metastatic brain tumors: a 2-institute study in Japan. J Neurosurg 109(Suppl):118–121. doi:10.3171/JNS/2008/109/12/S18
Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JP, Sperduto CM, Lin N, Mehta M (2012) Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys 82:2111–2117. doi:10.1016/j.ijrobp.2011.02.027
Conflict of interest
No actual or potential conflicts of interest exist.
Ethical standard
Written informed consent was obtained from all patients.
Author information
Authors and Affiliations
Corresponding author
Additional information
Masaaki Yamamoto and Toru Serizawa contributed equally to this study.
Rights and permissions
About this article
Cite this article
Yamamoto, M., Serizawa, T., Sato, Y. et al. Validity of two recently-proposed prognostic grading indices for lung, gastro-intestinal, breast and renal cell cancer patients with radiosurgically-treated brain metastases. J Neurooncol 111, 327–335 (2013). https://doi.org/10.1007/s11060-012-1019-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-012-1019-9