Abstract
To undertake a preliminary study that uses CT texture analysis (CTTA) to quantify heterogeneity in gliomas on contrast-enhanced CT and to assess the relationship between tumour heterogeneity and grade. Retrospective analysis of contrast enhanced CT images was performed in 44 patients with histologically proven cerebral glioma between 2007 and 2010. 11 tumours were low grade (Grade I = 3; Grade II, = 8) and 33 high grade (Grade III = 10, Grade IV = 23). CTTA assessment of tumour heterogeneity was performed using a proprietary software algorithm (TexRAD) that selectively filters and extracts textures at different anatomical scales between filter values 1.0 (fine detail) and 2.5 (coarse features). Heterogeneity was quantified as standard deviation (SD) with or without filtration. Tumour heterogeneity, size and attenuation were correlated with tumour grade. For each parameter, receiver operating characteristics characterised the diagnostic performance for discrimination of high grade from low grade glioma and of grade III tumours from grade IV. Further the CTTA was compared to the radiological diagnosis. Tumour heterogeneity correlated significantly with grade (SD without filtration rs = 0.664, p < 0.001, SD with coarse filtration (rs = 0.714, p < 0.001). Tumour size and attenuation showed only moderate correlations with tumour grade (rs = 0.426, p = 0.004 and rs = 0.447, p = 0.002 respectively). Coarse texture was the best discriminator between high and low grade tumours (AUC 0.832, p < 0.0001) and between grade III and grade IV gliomas (AUC = 0.878 p = 0.0001). Compared to the radiological diagnosis, CTTA further characterised the indetermined cases. By quantifying tumour heterogeneity, CTTA has the potential to provide a marker of tumour grade for patients with cerebral glioma. By differentiating between high and low grade tumours, CTTA could possibly assist clinical management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gliomas are the most common type of primary brain tumour. The World Health Organisation (WHO) classifies gliomas into grades I–IV, where I and II are low grade and III and IV are high grades [1]. Determining the correct grade of the tumour is of great importance as this dictates the management and prognosis for the patient. High grade gliomas (HGGs) are managed with radical resection and with adjuvant radiotherapy and/or chemotherapy, whereas low grade gliomas (LGGs) are very slow growing and can undergo curative resection and have considerably better prognosis [2]. Along the same lines, differentiating between grade III and IV tumours is of interest as their treatment and prognosis differ; patients with grade IV tumours undergo a treatment regime that includes radiotherapy and chemotherapy, while patients with grade III tumours, there is still uncertainty as to which chemotherapy regime is helpful, if any [3, 4]. The current gold standard for grading gliomas is histopathological assessment by stereotactic brain biopsy which is an invasive procedure and has limiting factors, in particular sampling error [2]. Particularly in gliomas, the potential to increase clinical utility of imaging as a non-invasive technique to accurately ascertain tumour grade is gaining a lot of attention [5].
Tumour heterogeneity is a well recognised feature of malignancy, especially within gliomas, where HGG generally appear visually more ‘heterogeneous’ than LGG [6]. A method that quantifies tumour heterogeneity on routinely available images could therefore offer a complementary tool to existing radiological practice in differentiating HGGs from LGGs. As computed tomography (CT) is commonly the first imaging investigation for patients with glioma and is frequently used to guide biopsy and assess treatment response [7]. The aims of this preliminary study are therefore to use CT texture analysis (CTTA) to quantify heterogeneity in gliomas on contrast-enhanced (CE) CT and to assess the relationship between tumour heterogeneity and grade.
Materials and methods
This single institution retrospective pilot study comprised archived CE CT images of patients with cerebral glioma. Ethical approval was waived by the local institutional review board as archived anonymised data was used.
Patients
In the period between November 2007 and June 2010, 125 patients presented to our institution with cerebral gliomas. 81 patients were excluded due to previous tumour resection/debulking with no archived pre operative CE CT images of the head. The remaining 44 patients included in this study had gliomas confirmed by histology from brain biopsy or tumour resection. All 44 patients had CE CT head prior to tumour biopsy and/or resection and these images were employed for further analysis.
CT image acquisition
Axial CT (Lightspeed scanner, GE-Healthcare Technology, Waukesha, WI, USA) images of the head had been acquired following administration of contrast material (NIOPAM 300, 300 mg iodine per ml, Bracco, Milan, Italy) with an image duration of 1 s (120 KVp, 270 mAs, 5 mm slice thickness and pixel spacing of 0.4492 mm × 0.4492 mm). Routine CT quality assurance and control procedures had been carried out in accordance with manufacturer’s recommendations.
The CT image with the largest tumour cross-section was selected for CTTA and the corresponding image in DICOM (Digital Imaging and Communications in Medicine) format was retrieved.
CT texture analysis (CTTA)
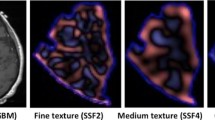
CTTA was conducted on the selected images using TexRAD, an in-house proprietary computer software algorithm, developed and validated by Ganeshan et al. [8–13]. This technique initially selectively filters and extracts textures at different anatomical, spatial scales, by using Laplacian of Gaussian (LoG) spatial band-pass filters. LoG filter values of 1.0, 1.5, 1.8, 2.0 and 2.5 extracts and enhances physical features (structures) of approximate size (width) of 4 pixels or 1.8 mm, 6 pixels or 2.7 mm, 8 pixels or 3.6 mm, 10 pixels or 4.5 mm and 12 pixels or 5.4 mm respectively. In this study we have assigned fine features to a physical scale of 4 pixels or 1.8 mm, medium features range from 6 to 10 pixels or 2.7–4.5 mm and coarse features to physical a scale of 12 pixels or 5.4 mm (Fig. 1). Texture features finer than 4 pixels were not evaluated as these would mostly represent image noise.
Texture analysis was supervised by a researcher with 6 years experience in texture analysis of radiographic images. Tumour region of interests (ROI) were constructed using only CT images for guidance by a blinded experienced neuroradiologist who leads the local brain tumour MDM, peripheral tumour oedema was not included only the area within the contrast enhanced tumour. The operator had access to all data and was not blinded, however all other authors were. The ROI was further refined by the exclusion of areas of fat with a thresholding procedure where any pixels with attenuation values below −50 Hounsfield Units (HU) were ignored from the analysis. The ROI size and mean tumour attenuation were recorded.
Heterogeneity within this ROI was quantified with and without image filtration. The histograms of tumour pixel values with and without image filtration were characterized by standard deviation (degree of variation from the mean pixel value, SD); generally a higher standard deviation would indicate increased heterogeneity [14]. An example histogram displaying the range and frequency of pixel values within the coarse filtered (filter value 2.5) image (Fig. 2).
The CTTA results were compared to the radiological and histological diagnosis. For the radiological diagnosis the neuroradiologist had access to the CT and MRI (where available) scan. MRI images and reports were available to the neuroradiologist on patients who came from peripheral hospitals prior to reporting on the pre operative CT scan on these patients. The reports were divided into LGGs, HGGs and indeterminate/unspecified. LGGs and HGGs were differentiated by their radiological characteristics, where generally homogeneous non enhancing lesions are LGGs and heterogeneous, peripherally and irregularly enhancing and centrally necrotic are HGGs. The indeterminate and unspecified group were reports were where no grade was given (e.g. Intrinsic tumour or tumour consistent with glioma) or a different diagnosis all together was given (e.g. Abscess or stroke).
Statistical analysis
Tumour texture measured as SD with and without filtration, tumour size (total number of pixels) and attenuation (HU) were correlated against tumour grade using the non-parametric two-tailed Spearman’s Rank Correlation. For all parameters that correlated significantly with tumour grade, receiver-operating characteristics (ROC) analysis was used to characterise the diagnostic performance for discrimination of HGGs (grades III and IV) from LGGs (grades I and II) and, within HGGs, grade IV from grade III. All statistical analyses were performed using SPSS for Windows version 16.0 and MedCalc for Windows, version 9.2.0.0 (MedCalc software, Mariakerke, Belgium) with statistical significance set at 5 %.
Results
Based on the results of histological examination, 11 patients had LGGs (grade I, n = 3 and grade II, n = 8) and 33 had HGGs (grade III, n = 10 and grade IV, n = 23). These were all supratentorial gliomas and the mean age for LGGs were 48 and for HGGs were 54. Table 1 summarises the correlations of tumour texture, size and attenuation with tumour grade. Tumour texture measurements correlated significantly with grade. Although a correlation between SD and grade was observed without image filtration (rs = 0.664, p < 0.001), the strongest correlation was found for SD with coarse filtration (filter value 2.5, rs = 0.714, p < 0.001, Fig. 3). This graph also shows higher intrinsic variation in SD within grade 4 tumours. Tumour size and attenuation showed only moderate correlations with tumour grade (rs = 0.426, p = 0.004 and rs = 0.447, p = 0.002 respectively).
The results of ROC analysis are shown in Tables 2, 3. For discrimination of HGGs from LGGs, the greatest area under the curve (AUC) was observed for coarse texture (0.832, p < 0.0001). A coarse SD greater than 11.62 was diagnostic of HGG with 70 % sensitivity and 91 % specificity. Coarse texture was also the best discriminator between grade III and grade IV gliomas (AUC = 0.878 p = 0.0001). A coarse SD of greater than 12.28 had 91 % sensitivity and 90 % specificity of for diagnosis of grade IV tumour.
Radiological, CTTA and histological diagnosis is compared in Table 4. Radiologically 16 of the 33 HGGs were reported as high grade, 15 tumours were reported as LGGs, 7 of these were histologically high grade. 13 were indeterminate or unspecified (6 with glioma as the main diagnosis no grade given, 4 reported as intrinsic tumour, 1 astrocytoma, 1 abscess and 1 stroke) comprising 10 HGGs and 3 LGGs. When the radiological diagnosis was HGG, there was no additional value from adding CTTA. However when the radiological diagnosis indicated LGGs or indeterminate/unspecified, CTTA identified 8 additional HGGs.
Discussion
The results from this preliminary study demonstrate the potential for CTTA to quantify tumour heterogeneity in gliomas and show a correlation between tumour heterogeneity and tumour grade. The correlations between tumour heterogeneity and grade were closer than those found between tumour grade and simple measurements of tumour size and attenuation. These findings add to the accumulating body of evidence to show that increased tumour heterogeneity demonstrated by CTTA is associated with adverse biological features and poor outcomes for a range of tumours [8–11, 15].
The heterogeneity detectible by CTTA of gliomas is most likely related to tumour necrosis and irregular tumour vascular proliferation, processes which are intimately related. A heterogeneous tumour vasculature leads to localised regions of hypoxia which, if sufficiently severe or prolonged, results in tumour cell death. Necrosis is associated with lower x-ray attenuation values on CT as compared to viable tumour whereas areas of more intense vascularisation results in local increases in attenuation after contrast enhancement due to increased vascular volume and permeability [16]. Although in some cases these image features may be visible to the reporting radiologist, CTTA benefits from enabling these appearances to be quantified. There is a marginal benefit as indicated in Tables 1, 2, and 3. It indicates that texture features on the conventional image (which looks at all texture scales) is similar in diagnostic performance to the texture features quantified independently at fine, medium and coarse scales, with the best diagnostic performance attained at coarse texture scale (which is better than the conventional image). This does indicate there is some subtle additional diagnostic information extracted by coarse texture filtration which is lost while analysing the conventional image. As these values are all obtained from the same ROI and requires no more time, the marginal benefit of CTTA should be used. Furthermore CTTA correctly identified several cases where the radiological diagnosis was unspecified and similarly identified LGGs better where the radiological diagnosis over estimated. Hence CTTA can be a useful tool to assist the radiologist in classifying the unspecified or indeterminate cases and LGGs.
Vascular proliferation and necrosis are believed to significantly influence the biological behaviour of malignant astrocytomas (HGGs) and signals the beginning of an aggressive growth phase. Patients with tumours that lack vascular proliferation and pseudopalisading necrosis on histological examination typically survive twice as long as the patients with these characteristics. The presence of vascular proliferation and pseudopalisading necrosis in malignant tumours heralds the onset of rapid tumour growth or side-effect for chaotic and excessive signalling events that initiates clinical progression and the development of glioblastoma. [17] Our finding of a correlation between CTTA measurements of tumour heterogeneity and glioma grade is in keeping with the above histological evidence indicating that irregular vascular proliferation and necrosis are associated with more advanced disease.
Owing to the invasiveness and sampling error associated with stereotactic brain biopsy, it has recently been suggested that more emphasis should be placed to grade gliomas via non-invasive imaging techniques [5]. Quantifying heterogeneity via CTTA (e.g. TexRAD, a clinically userfriendly software) could play a central role in assisting tumour grading and providing additional tumour risk-stratification to further map the management of patients with gliomas. Our preliminary assessment of the diagnostic performance indicates that CTTA has a sensitivity of 70 % and specificity of 91 % for differentiation of HGGs from LGGs as well as a sensitivity of 91 % and specificity of 90 % for distinguishing grade IV from grade III gliomas. This diagnostic performance is comparable to that previously reported for Magnetic resonance imaging (MRI) [18–21]. It is also interesting to observe the higher intrinsic variation in texture values amongst grade IV tumours (Fig. 3), which may reflect a corresponding pathological variability within this sub-group.
MRI is the imaging modality of choice in the management of gliomas on account of its ability to clearly delineate the lesion. Anatomical images supplemented by functional information (cellularity and blood flow) from diffusion and perfusion techniques have been shown to complement histopathological assessment [21, 22]. MR has also been used to assess heterogeneity of gliomas [23–29]. The method we have used for quantifying heterogeneity on CT differs from those used previously for MR in that we have used image filtration (LoG filter is similar to a non-orthogonal wavelet—providing the flexibility to tune the filter value to extract and separately evaluate fine, medium and coarse texture features). The physical size depends on the filter tuning ability (filter width) and the filter width is measured as total number of pixels. As the pixel resolution on CT may vary from one scanner to another, to make this CTTA more generally applicable across multi-centres, the fine, medium and coarse textures should reflects features of a physical size independent of the pixel-size (for example directly in ‘mm’). Further development in the filter tuning ability to extract and enhance features directly in ‘mm’ is therefore required. This filtration proved to be useful in our study as the best significant correlations with grade was obtained for filtered texture parameters (in comparison to texture quantification of conventional images without filtration).
Also the previous MR texture studies in gliomas have employed second-order direction-oriented statistical parameters (i.e. statistical methods based on joint probability distributions of pairs of pixels such as grey-level run length or co-occurrence matrices) and artificial neural network (ANN) based classifiers. By using image filtration in our texture analysis, we were able to use first order histogram parameters such as standard-deviation (degree of variation from the mean pixel value, SD). This parameter in addition of being easy to comprehend in clinical practice, has the advantage of being directionally independent and less computationally expensive. There is no prior reason to indicate that changes in gliomas should be associated with any particular direction and indeed are more likely to be non-directional. Also the use of ANN based classifiers combine parameters in a non-intuitive manner lacking biological rationale (considered like a ‘black-box’) and hence is difficult to employ in clinical practice. Therefore, our employment of a directionally independent filter, first order histogram statistical parameter and use of non-parametric Spearman’s rank correlation can be considered appropriate. Measurements of tumour heterogeneity with texture analysis are sensitive to variations in voxel size and accuracy of pixel values. Although easily controllable in research protocols, for images acquired in routine clinical practice these image characteristics are likely to be constant for CT images but may differ significantly with the variable image matrix size and gain often adopted in clinical MR acquisitions. CT images also benefit from their low cost and wide-availability.
From this pilot study, CTTA will not have the potential to substitute MRI. However CTTA is a clinically userfriendly method for a radiologist to retrieve additional information from the initial CT image on patients with glioma, which can assist in reporting a more accurate diagnosis. And further CTTA can aid to assign tumour grade combined with CT, MRI and biopsy.
Our study is limited by being a pilot evaluation of CTTA in a single centre and requires replicating in a larger prospective multi-centre study to confirm the above findings. Direct comparison of CTTA and MR methods for assessing glioma grade in the same cohort of patients are also required. The measurements of tumour heterogeneity in our study were performed by a single non blinded operator which potentially could introduce bias. Further studies should evaluate the inter-operator and intra-operator test–retest variability in CTTA measurements, which is a limitation in this study. As this is a retrospective pilot study, CTTA was performed on a single CT slice displaying largest cross-section of the tumour, not the point where the biopsy/resection was done. Assessment of heterogeneity across the whole tumour volume and/or at the specific slice corresponding to the biopsy site could potentially correlate even more closely with tumour grade. A volumetric CTTA approach, although being computationally more expensive, has been evaluated in non-cancer applications [30] and is currently being evaluated in oncology [31]. An interesting prospect that warrants assessment in future studies is the ability of CTTA to identify transition from LGGs to HGGs. This application would be of great clinical value because management of these patients changes dramatically when this transition occurs. Due to lack of availability of prior imaging data from our local patient database, sequential changes in tumour heterogeneity could not be assessed for the patients included in our study.
Conclusion
By quantifying tumour heterogeneity, CTTA has the potential to provide a marker of tumour grade for patients with cerebral glioma. By differentiating between high and low grade tumours, CTTA could possibly assist clinical management.
References
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropahtol 114(2):97–109
Weller M (2011) Novel diagnostic and therapeutic approaches to malignant glioma. Swiss Med Wkly. 24(141):w13210. doi:10.4414/smw.2011.13210
Siker ML, Chakravarti A, Mehta MP (2006) Should concomitant and adjuvant treatment with temozolomide be used as standard therapy in patients with anaplastic glioma? Critical reviews in Oncology-Haematology 60(2):99–111
Theeler BJ, Groves MD (2011) High-grade gliomas. Curr Treat Options Neurol. 13(4):386–399
Server A, Kulle B, Gadmar OB et al (2010) Measurements of diagnostic examination performance using quantitative apparent diffusion coefficient and proton MR spectroscopic imaging in the preoperative evaluation of tumor grade in cerebral gliomas. Eur J Radiol. doi: 10.1016/j.ejrad.2010.07.017
Nelson DA, Tan TT, Rabson AB, Anderson D, Degenhardt K, White E (2004) Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes Dev 18:2095–2107
Arogundade RA, Awosanya GO, Ariqbabu SO (2006) Role of computer tomography in the management of adult brain tumours. Niger Postgrad Med J. 13(2):123–127
Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles KA (2012) Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol 22(4):796–802
Ganeshan B, Skogen K, Pressney I, Coutroubis D, Miles KA (2012) Tumour heterogeneity in oesophageal cancer assessed by CT Texture Analysis: preliminary evidence of an association with tumour metabolism, stage and survival. Clin Radiol 67(2):157–164
Goh V, Ganeshan B, Nathan P, Juttla J, Vinayan A, Miles KA (2011) Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology 261(1):165–171
Ganeshan B, Abaleke SC, Young RCD, Chatwin CR, Miles KA (2010) Texture analysis of non-small cell lung cancer on unenhanced computed tomography: initial evidence for a relationship with tumour glucose metabolism and stage. Cancer Imaging 6(10):137–143
Miles KA, Ganeshan B, Griffiths MR, Young RC, Chatwin CR (2009) Colorectal cancer: texture analysis of portal phase hepatic CT images as a potential marker of survival. Radiology 250(2):444–452
Ganeshan B, Miles KA, Young RC, Chatwin CR (2007) In search of biologic correlates for liver texture on portal-phase CT. Acad Radiol. 14(9):1058–1068
Kojima S, YoshitomiY Yano M et al (2000) Heterogeneity of renal cortical circulation in hypertension assessed by dynamic computed tomography. Am J Hypertens 13(4 PT 1):346–352
Ganeshan B, Ziauddin Z, Goh VJ, Rodriguez-Just0 M, Engledow A, Taylor S, Halligan S, Miles KA 2012 Quantitative imaging biomarkers from PET–CT as potential correlates for angiogenesis and hypoxia in colorectal cancer. In: European Society of Radiology Conference 2012, Vienna, Austria
Zagzag D, Goldenberg M, Brem S (1989) Angiogenesis and blood-brain barrier breakdown modulate CT contrast enhancement: an experimental study in a rabbit brain-tumor model. Am J Roentgenol 153:141–146
Tervonen O, Forbes G, Scheithauer BW et al (1992) Diffuse “fibrillary” astrocytomas: correlation of MRI features with histopathologic parameters and tumour grade. Neuroradiology 34:173–178
Moller-Hartmann W, Herminghaus S, Krings T et al (2002) Clinical application of proton magnetic resonance spectroscopy in the diagnosis of intracranial mass lesions. Neuroradiology 44:371–381
Dean BL, Drayer BP, Bird CR et al (1990) Glioma classification with MR imaging. Radiology 174:411–415
Watanabe M, Tanaka R, Takeda N (1992) Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology 34:463–469
Kondziolka D, Lunsford LD, Martinez AJ (1993) Unreliability of contemporary neurodiagnostic imaging in evaluating suspected adult supratentorial (low Grade) astrocytoma. J Neurosurg 79(4):533–536
Christofordis GA, Grecula JC, Newton HB et al (2002) Visualization of microvascularity in glioblastoma multiforme with 8-T high-spatial-resolution MR imaging. AM J Neuroradiol 23:1553–1556
Assefa D, Keller H, Ménard C, Laperriere N, Ferrari RJ, Yeung I (2010) Robust texture features for response monitoring of glioblastoma multiforme onT1-weighted and T2-FLAIR MR images: a preliminary investigation in terms of identification and segmentation. Med Phys 37(4):1722–1736
Drabycz S, Roldán G, de Robles P, Adler D, McIntyre JB, Magliocco AM, Cairncross JG, Mitchell JR (2010) An analysis of image texture, tumor location, and MGMT promoter methylation in glioblastoma using magnetic resonance imaging. Neuroimage 49(2):1398–1405
Levner I, Drabycz S, Roldan G, De Robles P, Cairncross JG, Mitchell R (2009) Predicting MGMT methylation status of glioblastomas from MRI texture. Med Image Comput Comput Assist Interv. 12(Pt 2):522–530
Mahmoud-Ghoneim D, Alkaabi MK, de Certaines JD, Goettsche FM (2008) The impact of image dynamic range on texture classification of brain white matter. BMC Med Imaging 23(8):18
Georgiadis P, Cavouras D, Kalatzis I, Glotsos D, Athanasiadis E, Kostopoulos S, Sifaki K, Malamas M, Nikiforidis G, Solomou E (2009) Enhancing the discrimination accuracy between metastases, gliomas and meningiomas on brain MRI by volumetric textural features and ensemble pattern recognition methods. Magn Reson Imaging 27(1):120–130
Mahmoud-Ghoneim D, Toussaint G, Constans JM, de Certaines JD (2003) Three dimensional texture analysis in MRI: a preliminary evaluation in gliomas. Magn Reson Imaging 21(9):983–987
Schad LR, Blüml S, Zuna I (1993) MR tissue characterization of intracranial tumors by means of texture analysis. Magn Reson Imaging 11(6):889–896
Ganeshan B, Miles KA, Young RC, Chatwin CR (2008) Three-dimensional selective-scale texture analysis of computed tomography pulmonary angiograms. Invest Radiol 43(6):382–394
Ng F, Ganeshan B, Miles KA, Goh V 2012 Assessment of tumor heterogeneity by CT texture analysis: Comparison of the largest cross-sectional area versus whole tumor analysis. In: The European Society of Radiology Conference 2012, Vienna, Austria
Conflict of interests
Balaji Ganeshan and Kenneth Miles have a commercial interest in the tumor textural analysis software (‘TexRAD’) described in this manuscript. There are no other author disclosures. All other authors had control of the data and information submitted for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Skogen, K., Ganeshan, B., Good, C. et al. Measurements of heterogeneity in gliomas on computed tomography relationship to tumour grade. J Neurooncol 111, 213–219 (2013). https://doi.org/10.1007/s11060-012-1010-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-012-1010-5